Abstract
Study Objectives:
To determine whether sublaterodorsal tegmental nucleus (SLD) neurons triggering paradoxical (REM) sleep (PS) are glutamatergic.
Design:
Three groups of rats were used: controls, rats deprived of PS for 72 h, and rats allowed to recover for 3 h after deprivation. Brain sections were processed for double labeling combining Fos immunohistochemistry and vesicular glutamate transporter 2 (vGLUT2) in situ hybridization.
Measurements and Results:
The number of single Fos+ and Fos/vGLUT2+ double-labeled neurons was counted for each experimental condition. A very large number of Fos+ neurons expressing vGLUT2 mRNA specifically after PS hypersomnia was counted in the SLD. These double-labeled cells accounted for 84% of the total number of Fos+ cells.
Conclusions:
This finding adds further evidence to the concept that PS-on neurons of the SLD generating PS are of small size and glutamatergic in nature. By means of their descending projections to medullary and/or spinal glycinergic/GABAergic premotoneurons, they may be especially important for the induction of muscle atonia during PS, a disturbed phenomenon in narcolepsy and REM sleep behavior disorder.
Citation:
Clément O; Sapin E; Bérod A; Fort P; Luppi PH. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. SLEEP 2011;34(4):419-423.
Keywords: REM sleep behavior disorder, Parkinson, brainstem reticular formation, cataplexy, hypocretin
INTRODUCTION
Based on lesion, local pharmacological unit recordings, and Fos staining studies, it is now well accepted that paradoxical sleep (PS, also known as REM sleep) induction and maintenance are due to the activation of neurons located in a small pontine nucleus named sublaterodorsal tegmental nucleus (SLD) in rats.1,2 However, the neurochemical nature of these PS-generating neurons is still a matter of debate. It was long thought that they were cholinergic,3 and it was more recently proposed that some of them might be GABAergic.4 However, we found that SLD neurons activated after PS hypersomnia express neither choline acetyltransferase, the enzyme of synthesis of acetylcholine,5 nor glutamate decarboxylase, that of GABA.6 A few studies suggested that SLD PS-on neurons might in fact be glutamatergic. Indeed, neurons expressing the vesicular glutamate transporter 2 (vGLUT2), a specific marker of glutamatergic neurons, were observed in the SLD.4 It was also shown that glutamate release is specifically increased during PS in the nucleus reticularis magnocellularis (Mc),7 known to receive projections from the SLD and to contain glycinergic premoto-neurons responsible for muscle atonia during PS.1 Moreover, Lai and Siegel showed in cats that local application of gluta-mate into the Mc induces muscle atonia in a dose-dependent manner. Finally, blockade of glutamatergic transmission by application of kainate or NMDA antagonists into the Mc impaired muscle atonia induced by carbachol infusion into the SLD region.8 To definitely resolve the issue of the chemical nature of PS-generating neurons, we compared the number of glutamatergic neurons activated in the SLD in control rats, rats deprived of PS for 72 h, and rats allowed to recover during 3 h from that deprivation. To this aim, we developed a new method combining immunodetection of Fos, a marker a neuronal activation, with in situ hybridization of the mRNA coding for vGLUT2.
METHODS
All experiments were conducted in accordance to the French and European Community guidelines for the use of research animals and approved by the institutional animal care and use committee of the University of Lyon 1 (protocols BH 2006-09 and BH 2006-10). Sprague-Dawley male rats were housed individually in recording barrels under a constant 12h light-dark cycle (light on at 07:00). Room temperature was maintained at 21 ± 1°C, and standard rodent food and water were available ad libitum throughout the experiment.
Surgery and Polygraphic Recordings
As previously described,6 12 rats (240-260g, Charles River, France) were implanted for electroencephalographic (EEG) and electromyographic (EMG) recordings. After 5 days recovery from surgery and 4 days habituation to the recording conditions, rats were connected to a cable attached to a rotating connector (Plastics One Inc., CT) to allow free movements of the animal within its home cage.
Paradoxical Sleep Deprivation and Recovery
PS deprivation was performed using the inverted flowerpot technique. Rats were divided in 3 groups: controls (PSC), deprived of PS (PSD), and PS hypersomniac (PSR) (n = 4 in each group). PSC animals remained in their standard cage throughout the experiment. After a 48 h baseline recording, PSD and PSR rats were placed (10:00) on a platform surrounded by 2 cm of water for 72 h preventing rats from entering PS. The last day, PSR animals were removed from the platform (10:00) and were replaced on a dry bed of woodchips to allow PS recovery. All animals were anesthetized for perfusion at approximately 13:00. PSR rats were anesthetized 150 min after the appearance of the first PS episode, occurring approximately 30 min after the animals were put back in their home cage.
Perfusion, Fixation, and Section
Rats were perfused with a Ringer's lactate solution containing 0.1% heparin, followed by 500 ml of a fixative solution composed of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were removed and stored at 4°C for 1 night in the fixative solution and then for 3 days at 4°C in 30% sucrose in 0.1 M PB. Brains were rapidly frozen in methyl-butane cooled with dry ice, and 30-μm-thick coronal sections were cut on a cryostat (Microm, France). Free-floating sections were collected and stored at −20°C in an RNase free cryoprotectant solution.
Fos Immunohistochemistry Combined with vGLUT2 mRNA In Situ Hybridization
The antisense and sense digoxigenin-labeled probe against vGLUT2 mRNA were synthesized from a recombinant linearized plasmid containing the vGLUT2 cDNA using a nonradio-active RNA labeling kit (Roche Diagnostic, Switzerland). As described before,6,9 brain sections were successively incubated with a rabbit antiserum to Fos (1:3000; Merck, Germany), a biotinylated goat anti-rabbit IgG solution (1:1000; Vector Laboratories, Burlingame, CA) and an ABC-HRP solution (1:1000; Elite kit, Vector Laboratories). Then sections were immersed for around 15 min in 3,3-diaminobenzidine-4 HCl (DAB, Sigma-Aldrich, St. Louis, MO) and 0.003% H2O2. They were then rinsed in PBST containing 10 mM dithio-threitol (DTT, Sigma-Aldrich) and in standard saline citrate solution (SSC 2X). Sections were then placed overnight at 65°C in the hybridization buffer containing 0.5 μg/mL of the digoxigenin-labeled probe. Sections were washed in SSC 1X, 50% formamide, 0.1% Tween-20 and treated with RNase A (USB Corporation, Cleveland, OH). Sections were then incubated with an anti-digoxigenin antibody conjugated to alkaline phosphatase (1:2000, Roche Diagnostic). Staining was revealed using nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Roche Diagnostic). Controls in the absence of primary antibodies (anti-Fos and anti-digoxigenin) or with the sense probe were run to ensure the specificity of the labeling.
Analysis of Sleep-Wake State Data
Vigilance states were discriminated using EEG and EMG data as previously described.6 For each rat, the last 150 min of EEG/EMG recordings before perfusion were analyzed by 5 sec epochs in order to determine quantities of W, SWS, and PS.
Analysis of Double Labeling
Drawings of double-labeled sections were made with an Axioscope microscope (Carl Zeiss AG, Germany) connected to a computerized image analysis system (Mercator; ExploraNova, France). Single- and double-labeled neurons were plotted on sections taken at 300 μm intervals (between AP -8,7 and -9,3 from bregma). The number of Fos+ and Fos/vGLUT2+ plotted neurons per brainstem structure was automatically counted and exported using Mercator software (ExploraNova). For structures present on several sections, neurons counted on all sections were summed.
Statistical Analysis
Nonparametric analyses of variance (Kruskal-Wallis test) were performed on the vigilance states and on the number of labeled neurons for each structure across experimental conditions (PSC, PSD, and PSR). Post hoc PLSD Mann-Whitney tests were used to identify significant pairwise differences (PSR or PSD vs PSC; PSR vs PSD). All statistics were performed using StatView software.
RESULTS
Quantification of Sleep
During the last 150 min before sacrifice, rats deprived of PS for 72 h by the inverted flower pot method (PSD animals) showed almost no PS (2.3% ± 2.3%), whereas controls (PSC animals) and animals allowed to recover from the deprivation (PSR animals) spent respectively 7.1% ± 1.8% and 37.4% ± 3.2% of their time in PS. PSR animals spent significantly more time in PS than PSC (P = 0.0209) and PSD (P = 0.0209) animals, and PSD rats spent significantly less time in PS than PSC rats (P = 0.0433). PSR animals displayed less waking (16.1% ± 5.7%) than PSC (49.4% ± 8.5%; P = 0.0433) and PSD (66.8% ± 4.6%; P = 0.0209) animals. No difference in SWS quantities was observed between the 3 groups of animals (PSC: 43.6% ± 7.0%; PSD: 31.7% ± 3.1%; PSR 46.5% ± 4.5%) (Figure S1).
Localization of the Single-Labeled Fos+ and Fos/vGLUT2+ Double-Labeled Neurons
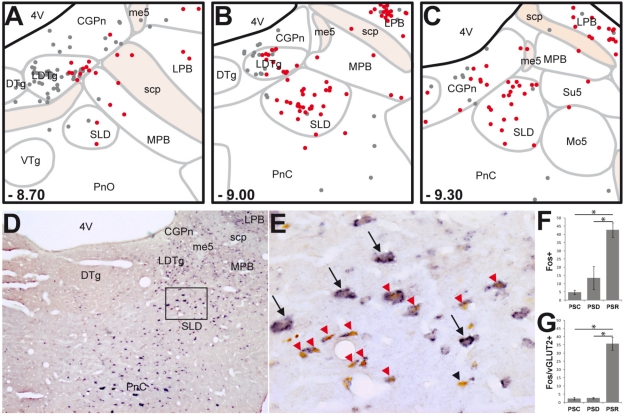
We focused our analysis on the brainstem level containing the SLD. In agreement with our previous results,5,6 the lateral parabrachial nucleus (LPB) was the only structure containing significantly more Fos+ neurons in PSD (106.6 ± 22.5) and PSR animals (82.4 ± 9.4) compared to PSC ones (33.0 ± 11.2) (Table S1). In addition, 3 structures, namely the laterodorsal tegmental nucleus (LDTg), the caudal part of the pontine reticular nucleus (PnC), and the SLD contained significantly more Fos+ neurons in PSR than in PSD and PSC animals (Figure 1F and Table S1).
Figure 1.
SLD neurons expressing Fos after PS-recovery are mostly glutamatergic. (A-C) Schematic distribution of Fos+ (gray dots) and Fos-vGLUT2+ (red dots) neurons on coronal sections taken at 300 μm intervals through the full rostro-caudal extent of the SLD in a representative PSR animal. Rostro-caudal localization of each section is indicated from Bregma at the bottom left corner of each drawing. (D-E) Photomicrographs showing Fos (brown nuclear staining) and vGLUT2 (blue diffuse cytoplasmic staining) double staining at SLD level. (E) is a higher magnification of the rectangular box in D. Note the dense cluster of double-labeled neurons into the SLD of a PSR rat. Interestingly, most of the large glutamatergic SLD neurons do not express Fos after PS hypersomnia (black arrows). Conversely, double-labeled neurons after PS hypersomnia are rather of small size (red arrowheads). Only one neuron labeled for Fos after PS-hypersomnia does not express vGLUT2 mRNA (black arrowhead). (F-G) Number of Fos+ and Fos/vGLUT2+ neurons in the SLD in control (PSC), PS deprived (PSP) and PS recovery (PSR) conditions. Values are mean ± SEM. *P < 0.05. 4V, 4th ventricle; CGPn, central gray of the pons; DTg, dorsal tegmental nucleus; LDTg, laterodorsal tegmental nucleus; LPB, lateral parabrachial nucleus; me5, mesencephalic trigeminal tract; Mo5, motor trigeminal nucleus; MPB, medial parabrachial nucleus; PnC, pontine reticular nucleus, caudal part; PnO, pontine reticular nucleus, oral part; scp, superior cerebellar peduncle; SLD, sublaterodorsal tegmental nucleus; su5, supratrigeminal nucleus; VTg, ventral tegmental nucleus.
The number of Fos/vGLUT2+ double-labeled cells was significantly increased in the LPB after PS deprivation (72.0 ± 15.4) and PS recovery (55.9 ± 4.8) compared to control (17.0 ± 6.4). These double-labeled cells constituted for both conditions 68% of the total number of Fos+ cells.
In the LDTg and SLD, the number of Fos/vGLUT2+ neurons increased specifically in the PSR condition compared to the PSC and PSD conditions (Table S1). Within the SLD, the number of Fos/vGLUT2+ neurons increased from 2.3 ± 0.8 in PSC and 2.6 ± 0.4 in PSD to 35.6 ± 3.8 in PSR (Figure 1A-C, G and Table S1). Double-labeled cells constituted 84% and 46% of the total number of Fos+ neurons in the SLD and LDTg, respectively. Interestingly, as illustrated in Figure 1E, vGLUT2+ neurons of the SLD that expressed Fos after PS recovery were of small size, whereas large vGLUT2+ neurons almost never expressed Fos.
DISCUSSION
Here, we show for the first time that nearly all Fos+ neurons (84%) localized in the SLD after PS hypersomnia express vGLUT2 mRNA. These results strongly suggest that SLD neurons responsible for the generation of PS are glutamatergic in nature.
Methodological Considerations
Even if one could argue that Fos expression is not strictly correlated to neuronal discharge, we believe that our results convincingly show that SLD PS-on neurons are glutamatergic. Indeed, when unit recordings have been performed in areas reported to contain Fos+ labeled cells after PS hypersomnia,1,5,6,9 PS-on neurons have always been recorded.10–13 In particular, the presence of PS-on neurons has been reported in the SLD both in cats14 and in rats.15 However, a direct demonstration using for example juxtacellular labeling would be required. It is also well accepted that vGLUT2 is a specific marker of the glutamatergic neurons located in the brainstem.16 We performed in situ hybridization rather than immunohistochemistry of vGLUT2 because it specifically and strongly labels cells bodies' cytoplasm, whereas immunohistochemistry only labels terminals.
SLD PS-On Neurons Are Glutamatergic
The results reported here that SLD PS-on neurons are glutamatergic are in line with our previous results showing that they are neither cholinergic nor GABAergic in nature. Indeed, we previously showed that Fos+ neurons located in the SLD after PS hypersomnia are not immunoreactive to choline acetyltransferase, the enzyme of synthesis of acetylcholine.5 In addition, we previously reported that 15% of Fos+ neurons located in the SLD were GABAergic after PS hypersomnia, but also after PS deprivation and in control animals, indicating that SLD GABAergic neurons are not specifically active during PS.6 Further supporting these results, Xi et al. reported in cats that inactivation of GABAergic neurons of the SLD induces a decrease in waking and an increase in PS.17 The present results are also in agreement with Lu et al., who reported the presence within the SLD of vGLUT2 expressing neurons.4
Functional Significance
It is known that the SLD projects rostrally to the intralaminar nuclei of the thalamus, and caudally to the ventral part of the gigantocellular reticular nucleus (GiV),1 the rat equivalent of the cat Mc, and the spinal cord.4 Based on these and additional data, we previously proposed that PS-on neurons of the SLD activate the cortex by means of their ascending projections to the intralaminar thalamic nuclei and induce muscle atonia via their descending projections to glycinergic premotoneurons located in the GiV.1 Our results indicate that the neurons at the origin of these projections are likely glutamatergic (Figure 2).
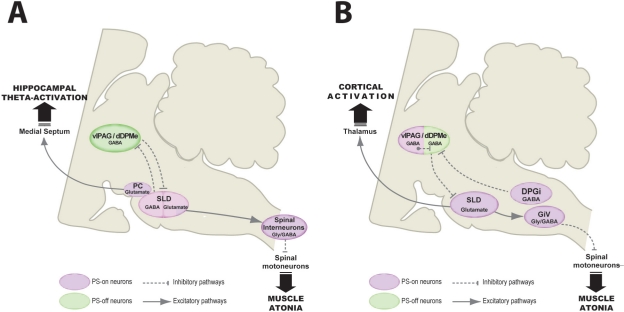
Figure 2.
Models of the pontine network responsible for paradoxical (REM) sleep generation. This figure aims to compare our model (B) and that of Lu et al.4 (A). In both models, the SLD contains PS-on glutamatergic neurons generating PS. Their activation at the onset of PS is gated by the removal of a tonic GABAergic input activated during waking and SWS and arising from vlPAG/dDpMe neurons. Lu et al. further hypothesized that the SLD also contains a population of GABAergic PS-on neurons inhibiting at the onset of and during PS the vlPAG/dDpMe PS-off neurons. They proposed that a flip-flop switch between these 2 populations of GABAergic neurons control PS occurrence. In our model, PS-on GABAergic neurons also control PS occurrence by means of their projections to the vlPAG/dDpMe PS-off GABAergic neurons, but they are localized in the vlPAG/dDpMe and a medullary nucleus named the dorsal paragigantocellular reticular nucleus (DPGi).2,3 We propose that their activation at the onset of PS is due to an intrinsic “clock like” mechanism.
Another major difference between the two models concerns the pathways by which SLD triggers the muscle atonia during PS. We propose that SLD PS-on glutamatergic neurons activate glycinergic/GABAergic neurons localized in the GiV, which in turn, hyperpolarize cranial and spinal motoneurons, leading to muscle atonia. Lu et al. proposed that SLD PS-on glutamatergic neurons inhibit spinal motoneurons via direct projections to spinal interneurons co-containing GABA and glycine. One could argue that these two hypotheses are not mutually exclusive. We finally propose that SLD PS-on neurons activate the cortex by means of their projection to intralaminar thalamic nuclei whereas Lu et al. proposed that a small nucleus close to the SLD named precoeruleus (PC) generate theta oscillations during PS via projections to the medial septum.
DPGi, dorsal paragigantocellular reticular nucleus; dDpMe, dorsal part of the deep mesencephalic nucleus; GiV, ventral gigantocellular reticular nucleus; PC, precoeruleus nucleus; SLD, sublaterodorsal tegmental nucleus; vlPAG, ventrolateral periaqueductal gray.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The work was performed in the laboratory, CNRS UMR 5167, Physiopathologie des réseaux neuronaux du cycle veillesommeil, Lyon, France. This work was supported by CNRS, Université Claude Bernard Lyon 1, Université de Lyon and the French ministry of research. Olivier Clément has a grant from the French ministry of research.
Time spent in waking, slow-wave sleep and paradoxical sleep. Percentage of time spent in waking (WK), slow-wave sleep (SWS) and paradoxical sleep (PS) scored by 5sec epochs during the last 150 min before sacrifice for control (PSC), PS-deprived (PSD) and PS-recovery (PSR) rats (n = 4 in each group). Graphs represent means with standard errors. *P < 0.05 compared to PSC, #P < 0.05 compared to PSD.
Table S1.
Number of Fos+ and Fos/vGLUT2+ neurons in controls (PSC), PS-deprived (PSD) and PS-recovery (PSR) rats
| Fos+ total |
Fos/vGLUT2+ |
||||||
|---|---|---|---|---|---|---|---|
| n | PSC | PSD | PSR | PSC | PSD | PSR | |
| CGPn | 3 | 19.8 ± 4.6 | 47.1 ± 17.8 | 55.3 ± 8.3 | 4.4 ± 1.5 | 8.5 ± 2.2 | 13.0 ± 2.7 |
| DTg | 3 | 0.5 ± 0.2 | 1.9 ± 0.7 | 9.9 ± 4.6 | 0.1 ± 0.1 | 1.0 ± 0.5 | 4.6 ± 2.7 |
| KF | 2 | 14.0 ± 6.6 | 15.9 ± 1.7 | 18.3 ± 2.3 | 5.9 ± 3.2 | 9.3 ± 1.5 | 13.4 ± 2.2 |
| LDTg | 2 | 6.5 ± 2.9 | 11.6 ± 3.2 | 33.3 ± 6.4*# | 2.6 ± 1.4 | 3.3 ± 0.8 | 15.3 ± 1.5*# |
| LPB | 3 | 33.0 ± 11.2 | 106.6 ± 22.5*# | 82.4 ± 9.4* | 17.0 ± 6.4 | 72.0 ± 15.4* | 55.9 ± 4.8* |
| MPB | 3 | 7.3 ± 3.8 | 15.6 ± 5.3 | 15.3 ± 6.1 | 3.8 ± 1.8 | 7.9 ± 1.7 | 11.0 ± 4.2 |
| PnC | 2 | 11.3 ± 4.0 | 26.4 ± 5.9 | 39.9 ± 7.7* | 3.3 ± 1.4 | 8.9 ± 2.8 | 16.4 ± 4.6 |
| PnO | 1 | 3.1 ± 0.7 | 4.9 ± 1.9 | 16.0 ± 5.2*# | 0.9 ± 0.4 | 2.4 ± 1.0 | 8.0 ± 3.6 |
| SLD | 3 | 4.8 ± 1.4 | 13.5 ± 7.0 | 42.6 ± 4.5*# | 2.3 ± 0.8 | 2.6 ± 0.4 | 35.6 ± 3.8*# |
Neurons were counted on 3 sections taken at 300μm interval through the full rostrocaudal extension of the SLD. Displayed values are mean (± sem) of 4 animals in each group of all Fos labelled neurons (Fos+ Total) and Fos/vGLUT2 double-labelled neurons (Fos/vGLUT2+) counted on one or several sections (column n) depending on the rostrocaudal extent of the structures.
P < 0.05 compared to PSC,
P < 0.05 compared to PSD.
CGPn, central gray of the pons; DTg, dorsal tegmental nucleus; KF, Kölliker-Fuse nucleus; LDTg, laterodorsal tegmental nucleus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; PnC, pontine reticular nucleus, caudal part; PnO, pontine reticular nucleus, oral part; SLD, sublaterodorsal tegmental nucleus.
REFERENCES
- 1.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–73. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 2.Fort P, Bassetti CL, Luppi PH. Alternating vigilance states: new insights regarding neuronal networks and mechanisms. Eur J Neurosci. 2009;29:1741–53. doi: 10.1111/j.1460-9568.2009.06722.x. [DOI] [PubMed] [Google Scholar]
- 3.Luppi PH, Gervasoni D, Verret L, et al. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–83. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 5.Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21:2488–504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 6.Sapin E, Lapray D, Bérod A, et al. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS ONE. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res. 1998;780:178–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–96. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapin E, Bérod A, Léger L, Herman PA, Luppi PH, Peyron C. A very large number of GABAergic neurons are activated in the tuberal hypothalamus during paradoxical (REM) sleep hypersomnia. PLoS ONE. 2010;5:e11766. doi: 10.1371/journal.pone.0011766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goutagny R, Luppi PH, Salvert D, Gervasoni D, Fort P. GABAergic control of hypothalamic melanin-concentrating hormone-containing neurons across the sleep-waking cycle. Neuroreport. 2005;16:1069–73. doi: 10.1097/00001756-200507130-00008. [DOI] [PubMed] [Google Scholar]
- 11.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–19. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 12.Goutagny R, Luppi PH, Salvert D, Lapray D, Gervasoni D, Fort P. Role of the dorsal paragigantocellular reticular nucleus in paradoxical (rapid eye movement) sleep generation: a combined electrophysiological and anatomical study in the rat. Neuroscience. 2008;152:849–57. doi: 10.1016/j.neuroscience.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. PNAS. 2009;106:2418–22. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai K, Koyama Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport. 1996;7:2449–53. doi: 10.1097/00001756-199611040-00009. [DOI] [PubMed] [Google Scholar]
- 15.Luppi PH, Boissard R, Gervasoni D, et al. Luppi PH, editor. The network responsible for paradoxical sleep onset and maintenance: a new theory based on the head-restrained rat model. Sleep: circuits and function. 2004 CRC Press. [Google Scholar]
- 16.Herzog E, Bellenchi GC, Gras C, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–19. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time spent in waking, slow-wave sleep and paradoxical sleep. Percentage of time spent in waking (WK), slow-wave sleep (SWS) and paradoxical sleep (PS) scored by 5sec epochs during the last 150 min before sacrifice for control (PSC), PS-deprived (PSD) and PS-recovery (PSR) rats (n = 4 in each group). Graphs represent means with standard errors. *P < 0.05 compared to PSC, #P < 0.05 compared to PSD.
Table S1.
Number of Fos+ and Fos/vGLUT2+ neurons in controls (PSC), PS-deprived (PSD) and PS-recovery (PSR) rats
| Fos+ total |
Fos/vGLUT2+ |
||||||
|---|---|---|---|---|---|---|---|
| n | PSC | PSD | PSR | PSC | PSD | PSR | |
| CGPn | 3 | 19.8 ± 4.6 | 47.1 ± 17.8 | 55.3 ± 8.3 | 4.4 ± 1.5 | 8.5 ± 2.2 | 13.0 ± 2.7 |
| DTg | 3 | 0.5 ± 0.2 | 1.9 ± 0.7 | 9.9 ± 4.6 | 0.1 ± 0.1 | 1.0 ± 0.5 | 4.6 ± 2.7 |
| KF | 2 | 14.0 ± 6.6 | 15.9 ± 1.7 | 18.3 ± 2.3 | 5.9 ± 3.2 | 9.3 ± 1.5 | 13.4 ± 2.2 |
| LDTg | 2 | 6.5 ± 2.9 | 11.6 ± 3.2 | 33.3 ± 6.4*# | 2.6 ± 1.4 | 3.3 ± 0.8 | 15.3 ± 1.5*# |
| LPB | 3 | 33.0 ± 11.2 | 106.6 ± 22.5*# | 82.4 ± 9.4* | 17.0 ± 6.4 | 72.0 ± 15.4* | 55.9 ± 4.8* |
| MPB | 3 | 7.3 ± 3.8 | 15.6 ± 5.3 | 15.3 ± 6.1 | 3.8 ± 1.8 | 7.9 ± 1.7 | 11.0 ± 4.2 |
| PnC | 2 | 11.3 ± 4.0 | 26.4 ± 5.9 | 39.9 ± 7.7* | 3.3 ± 1.4 | 8.9 ± 2.8 | 16.4 ± 4.6 |
| PnO | 1 | 3.1 ± 0.7 | 4.9 ± 1.9 | 16.0 ± 5.2*# | 0.9 ± 0.4 | 2.4 ± 1.0 | 8.0 ± 3.6 |
| SLD | 3 | 4.8 ± 1.4 | 13.5 ± 7.0 | 42.6 ± 4.5*# | 2.3 ± 0.8 | 2.6 ± 0.4 | 35.6 ± 3.8*# |
Neurons were counted on 3 sections taken at 300μm interval through the full rostrocaudal extension of the SLD. Displayed values are mean (± sem) of 4 animals in each group of all Fos labelled neurons (Fos+ Total) and Fos/vGLUT2 double-labelled neurons (Fos/vGLUT2+) counted on one or several sections (column n) depending on the rostrocaudal extent of the structures.
P < 0.05 compared to PSC,
P < 0.05 compared to PSD.
CGPn, central gray of the pons; DTg, dorsal tegmental nucleus; KF, Kölliker-Fuse nucleus; LDTg, laterodorsal tegmental nucleus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; PnC, pontine reticular nucleus, caudal part; PnO, pontine reticular nucleus, oral part; SLD, sublaterodorsal tegmental nucleus.