Abstract
To determine whether temozolomide is an active agent in the treatment of children with high-grade astrocytomas and whether survival is influenced by the expression of the O6-methylguanine-methyltransferase gene (MGMT) in these patients. In the Children's Oncology Group study ACNS0126, 107 patients with a diagnosis of anaplastic astrocytoma (AA), glioblastoma multiforme (GBM), or gliosarcoma were enrolled. All patients underwent concomitant chemoradiotherapy with temozolomide, followed by adjuvant chemotherapy with temozolomide. The outcomes were compared with those of children treated in Children's Cancer Group (CCG) study CCG-945. Formalin-fixed, paraffin-embedded tumor tissue was available in 71 cases for immunohistochemical analysis of MGMT expression. Ninety patients were deemed eligible, 31 with AA, 55 with GBM, and 4 with other eligible diagnoses. The 3-year event-free survival (EFS) and overall survival (OS) rates were 11 ± 3% and 22 ± 5%, respectively. There was no evidence that temozolomide given during radiation therapy and as adjuvant therapy resulted in improved EFS compared with that found in CCG-945 (p = 0.98). The 3-year EFS rate for AA was 13 ± 6% in ACNS0126 compared with 22 ± 5.5% in CCG-945 (p = 0.95). The 3-year EFS rate for GBM was 7 ± 4% in ACNS0126 compared with 15 ± 5% in CCG-945 (p = 0.77). The 2-year EFS rate was 17 ± 5% among patients without MGMT overexpression and 5 ± 4% among those with MGMT overexpression (p = 0.045). Temozolomide failed to improve outcome in children with high-grade astrocytomas. MGMT overexpression was adversely associated with survival.
Keywords: High-grade glioma, temozolomide, pediatric brain tumors
The treatment of children with high-grade glioma (HGG) remains challenging. Similar to that in adults, the outcome in children with anaplastic astrocytoma (AA) or glioblastoma multiforme (GBM) is generally poor. Results from the Children's Cancer Study Group (CCG) study CCG-9451 demonstrated a 5-year progression-free survival (PFS) rate of approximately 23% for children with centrally reviewed AA and 16% for children with GBM.2 Outcomes were most favorable in children with near or gross total resection, followed by multimodality therapy with radiotherapy (XRT) and chemotherapy.
Temozolomide (TMZ) is an alkylating agent whose primary mechanism of cytotoxicity involves alkylation at the O6 position of guanine, with additional contributions at the N3 and N7 positions of adenine. TMZ is now widely recommended for the treatment of adults with HGG, given concomitantly with XRT and then in a series of adjuvant courses. Numerous studies3–7 have reported that TMZ/XRT is beneficial in this population, with the seminal report demonstrating a 2-year overall survival (OS) rate of 26% in adults with GBM versus 10% for XRT alone.8 The survival benefit was most apparent in patients whose tumors had methylation of the methylguanine DNA methyltransferase (MGMT) promoter, which likely results in diminished MGMT expression.9
The results of previous studies have indicated that pediatric malignant gliomas are molecularly different from adult lesions, demonstrating a substantially lower frequency of EGFR amplification, PTEN mutations, and IDH gene alterations.10,11 Accordingly, there is a strong rationale to independently validate therapeutic observations from the adult setting in the pediatric setting. Therefore, to test the utility of TMZ in the treatment of children with HGG and to determine the association between MGMT expression and outcome,12 the Children's Oncology Group (COG) undertook a single-arm phase II study of chemoradiotherapy with TMZ followed by adjuvant TMZ in children with newly diagnosed AA, GBM, or gliosarcoma.
Patient and Methods
Eligibility
Children were registered on-study after informed consent. All children were at least 3 and less than 22 years old at diagnosis. Patients were previously untreated, with a Lansky or Karnofsky performance status ≥50, adequate organ function, and no MRI evidence of neuraxis dissemination. All centers were required to have institutional review board approval prior to enrolling children in the study.
Surgery
Maximal surgical resection was encouraged but was limited in some cases based on tumor location or patient comorbidities. Postoperative MRI was generally obtained 24 to 48 hours after surgery. Extent of resection was coded as gross total resection, extensive subtotal resection (≥90% resected), subtotal resection (≥50% but <90% resected), partial resection (≥10% but <50% resected), or biopsy (<10% resected).
Pathological Analysis
Patients were eligible if the institutional pathologist diagnosed an AA, GBM, or gliosarcoma and if the diagnosis was validated upon central review. Because of the high incidence of reclassification from HGG to low-grade glioma (LGG) in CCG-945 upon central neuropathologic review,2,13 an early review of all specimens was undertaken to be certain that the institutional diagnosis was consistent with the centrally reviewed diagnosis. All evaluable patients underwent central neuropathologic review (P.C.B., M.K.R., or D.J.B.). If there was a discordant diagnosis between 2 central reviewers, the third pathologist served as the tie-breaker for determination of the final central review diagnosis.
Treatment
Chemoradiotherapy began within 42 days of surgery. XRT for intracranial tumors consisted of 54.0 Gy to the preoperative tumor volume plus a 2-cm margin in 1.8 Gy fractions delivered once daily, with an additional 5.4-Gy boost in 3 fractions to any residual disease on postoperative MRI scan plus a 1-cm margin. If a gross total resection was achieved, no boost dose was given. For patients with a primary tumor within the spinal cord, an XRT dose of 45–54 Gy in 1.8-Gy daily fractions was delivered in consultation with the study radiation oncologist.
Chemoradiotherapy consisted of 90 mg/m2/day of oral TMZ for 42 days.14 Four weeks after the completion of chemoradiotherapy, adjuvant TMZ was given at a dosage of 200 mg/m2/day orally for 5 days, administered for up to 10 cycles. Cycles were repeated every 28 days upon bone marrow recovery. Pneumocystis jiroveci pneumonia prophylaxis was mandatory, with pentamidine as the recommended agent. Trimethoprim-sulfamethoxazole prophylaxis could be substituted during adjuvant therapy.
Evaluation of Toxicity
Patients were assessed by medical history, physical examination (including performance status), and laboratory evaluations at least monthly, with certain laboratory parameters assessed weekly during XRT. Targeted toxicities included all hematologic parameters, nausea, vomiting, liver dysfunction, infection, and selected neurologic findings. All remaining toxicities were analyzed as “other” toxicities.
Evaluation of Response and Off-Protocol and Off-Study Criteria
MRI was used to assess response, with the largest measurable lesion considered the target lesion. Post-contrast T1 imaging or T2 imaging was used for longitudinal assessment of response, with the same sequence being used throughout treatment for each subject. Postoperative MRI prior to chemoradiotherapy was required, with subsequent MRI evaluations prior to each odd-numbered cycle of adjuvant therapy. Patients were removed from therapy for progressive disease, defined as a 25% or more increase in the cross-sectional area of the largest 2 diameters of the target lesion, taking as the reference the smallest product observed since the start of treatment or the appearance of 1 or more new lesions. Additional criteria for removal included parent or patient request, completion of chemoradiotherapy and maintenance therapy, hypersensitivity to TMZ, noncompliance, and physician's choice. Off-study criteria included death, loss to follow-up, entry into another therapeutic study, and failure to submit pathology specimens.
Expression of MGMT
Paraffin-embedded specimens were available for 71 eligible patients. Adjacent sections were subjected to immunohistochemical analysis of MGMT expression. Tumor-containing sections were baked at 60°C for 30 minutes, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Endogenous peroxidase activity was quenched by incubation in 0.3% hydrogen peroxide/methanol solution. Antigen retrieval15 was performed by heating the slides in 10 mmol citrate buffer (pH 6.0) for 20 minutes. Nonspecific antibody binding was blocked by incubation for 20 minutes in protein-blocking reagent (Thermo Corp). Sections were incubated with mouse anti-MGMT antibody (mT23.2, Zymed Laboratories, 1:100)16,17 in common antibody diluent (BioGenex) at ambient temperature for 2 hours.18 Antibody binding was localized using a universal labeled streptavidin-biotin 2 system (LSAB 2-HRP, Dako) and visualized using 3,3′-diaminobenzidine.19 Slides were counterstained with Mayer's hematoxylin, dehydrated through graded concentrations of ethanol, and cleared in xylene. Specimens were examined independently for immuno-reactivity using an antibody against a histologically verifiable internal positive control antigen (i.e., MIB1 staining of the KI67 antigen in tumor mitotic figures) to eliminate cases in which lack of immunoreactivity for MGMT might indicate problems in tissue preservation rather than lack of protein expression.
MGMT labeling was assessed semiquantitatively by examining stained and unstained cells in 5 to 10 high-power fields that incorporated the most anaplastic regions of the specimen; this assessment was performed by an observer (R.L.H.) who was masked to diagnoses and outcomes. Only cells with dense nuclear staining were graded positive. Tumors were categorized as exhibiting little or no expression (0/1) or scattered positive cells (2), similar to that found in normal brain tissue, versus overexpression, in which staining was observed in most or nearly all cells (3/4), as previously described.18 This approach was used for MGMT assessment because it allows a direct measurement of protein expression, in contrast to methylation-specific PCR, an indirect analysis approach, and because it demonstrated robust correlations with alkylator response in previous COG HGG studies.18
Statistical Considerations
The primary objective was to determine whether TMZ chemoradiotherapy and adjuvant therapy resulted in an improvement in EFS compared with that in historical controls. EFS was defined as the time from enrollment to disease progression, relapse, second malignant neoplasm (SMN), or death from any cause. The nonmixture parametric cure model of the form S(t) = πF(t), where S(t) is the EFS function, π is the long-term EFS (cure) rate, and F(t) is a log-normal distribution function, was used to describe outcome in a series of eligible randomized HGG patients aged 3 years and older who were treated in CCG-945. Maximum likelihood estimates of the parameters are π = 0.11 (95% CI: 0.05, 0.17), λ = 0.61/year (0.44, 0.88), γ = 0.84 (0.71, 1.0). This parametric cure model served as an historical comparison with ACNS0126. The observed and expected numbers of events were compared using a chi-square test.20 The number of events expected was obtained as the sum of the null cumulative hazard to time ti, where ti is the follow-up for patient i. Patients in CCG-945 with follow-up longer than 4.9 years were censored at 4.9 years (the maximum follow-up among nonfailures in ACNS0126).
The comparison of outcome by MGMT and extent of resection was based on the cure model method.21 A nonparametric OS curve was computed using the product-limit (Kaplan–Meier) estimate, with standard error via the Greenwood formula. OS was defined as the time from enrollment to death from any cause. p-Values were based on a 1-sided test. Analyses were based on data available as of July 2009.
Results
Between December 2002 and October 2004, 107 subjects were enrolled. Seventeen children were ineligible because of ineligible diagnosis (7; see below), inability to confirm diagnosis (5), pathologic findings not submitted (1), inadequate informed consent (1), and not meeting other eligibility criteria (3). The clinical characteristics of the 90 eligible patients are listed in Table 1. Thirty-one had AA, 55 had GBM, and 4 had other eligible diagnoses.
Table 1.
Baseline Patient Characteristics
| Patient Characteristic |
Frequency | Percent | |
|---|---|---|---|
| Sex | |||
| Male | 46 | 51.1 | |
| Female | 44 | 48.9 | |
| Age | |||
| <5 years | 3 | 3.3 | |
| 5–10 | 28 | 31.1 | |
| 10–15 | 32 | 35.6 | |
| ≥15 years | 27 | 30.0 | |
| Race | |||
| White | 64 | 71.1 | |
| Black | 15 | 16.7 | |
| Other/unknown | 11 | 12.2 | |
| Extent of resection | |||
| Biopsy | 19 | 21.1% | |
| Partial (10–50%) | 11 | 12.2% | |
| Subtotal (50–90%) | 15 | 16.7% | |
| Extensive subtotal (>90%) | 12 | 13.3% | |
| Gross total resection | 27 | 30.0% | |
| Unknown | 6 | 6.7% | |
| Pathologic diagnosis (composite) | |||
| Anaplastic astrocytoma | 31 | 34.4 | |
| Glioblastoma multiforme | 55 | 61.1 | |
| Other eligible diagnosis | 4 | 4.4 | |
| Primary site | |||
| Cerebral hemisphere | 49 | 54.4 | |
| Basal ganglia–thalamus | 25 | 27.8 | |
| Midbrain, brainstem | 2 | 2.2 | |
| Cerebellar hemisphere | 5 | 5.6 | |
| Spinal cord | 9 | 10.0 | |
Pathologic Concordance
In contrast to CCG-945, in which almost 30% of samples were reclassified as LGG, only 7 of 107 patients (6.5%) in the current study had an ineligible diagnosis (low-grade astrocytoma [4], oligoastrocytoma [1], oligodendroglioma [1], and malignant neuronal tumor [1]). A subset of samples diagnosed as AA by the institutional pathologist were reclassified as GBM (17%), and a subset diagnosed as GBM were reclassified as AA (9%).
Toxicity
Grades 3 and 4 toxicity data are presented in Table 2. Hematologic toxicity was the most common, with 36% of patients experiencing grade 3 or 4 lymphopenia. Grade 3 or 4 nontargeted toxicities were infrequent and required transfusion of platelets (4) and peripheral red blood cells (3).
Table 2.
Summary of Targeted Toxicities for HGG Stratum Percent of Grade 3 or 4 Toxicities for Chemoradiotherapy and Maintenance Courses
| Toxicity | Chemoradiotherapy (N = 90) | Maintenance (N = 65) |
|---|---|---|
| Hemoglobin | 7% | 2% |
| Leukocytes (total WBC) | 13% | 37% |
| Lymphopenia | 36% | 44% |
| Neutrophils/granulocytes | 11% | 38% |
| Platelets | 8% | 15% |
| Nausea | 3% | 11% |
| Vomiting | 10% | 7% |
| Bilirubin | 0% | 2% |
| SGOT (AST) | 2% | 0% |
| SGPT (ALT) | 4% | 4% |
| Febrile neutropenia | 1% | 13% |
| Infection with grade 3 or 4 neutropenia | 4% | 2% |
| Infection with unknown ANC | 0% | 0% |
| Infection without neutropenia | 6% | 12% |
| Neuropathy–cranial | 2% | 12% |
| Neuropathy–motor | 6% | 24% |
| Neurology–sensory | 4% | 14% |
| Neurology–seizures | 3% | 5% |
WBC, white blood cell; SGOT, serum glutamic-oxaloacetic transaminase; AST, aspartate aminotransferase; SGPT, serum glutamic-pyruvic transaminase; ALT, alanine aminotransferase; ANC, absolute neutrophil count.
Duration of Therapy
Ninety children initially underwent chemoradiotherapy, and 65 began maintenance chemotherapy. Of the 25 patients who did not start maintenance chemotherapy, 17 experienced disease recurrence or progression during chemoradiotherapy; the remaining 8 patients underwent off-protocol therapy due to withdrawal by physician (1), withdrawal by parent or patient (1), withdrawal of consent (1), hypersensitivity to TMZ (1), noncompliance with medications (1), myelosuppression (1), nonrecovery of cell counts (1), and death (1). Only 4 of 65 children received all scheduled maintenance courses. Of the 61 who did not, 52 stopped secondary to relapse, progression, or death. Nine stopped for reasons other than progressive disease, including hypersensitivity to TMZ manifested by hives during administration (4), prolonged myelosuppression (2), noncompliance (1), physician choice (1), and unknown (1).
Event-Free and Overall Survival
Of 90 initially eligible HGG patients, 76 experienced disease relapse or progression, 66 of whom subsequently died of disease. Three deaths were reported as first events (2 were due to tumor and 1 to acute respiratory distress syndrome). Two patients developed SMNs—one patient experienced disease progression 16 months after study entry, developed an SMN (colon carcinoma) 10 months later, and died due to his brain tumor 2 months hence; and another patient developed an SMN (kidney, not otherwise specified) 35 months after the study and was still alive at last follow-up. The median follow-up duration was 4.5 years (0 to 4.9) for the 10 patients who were alive with no events; 1 patient withdrew after 1 dose of TMZ (0 years), another withdrew consent for follow-up (0.3 years), and the remaining 8 have been followed up for 4.0 to 4.9 years.
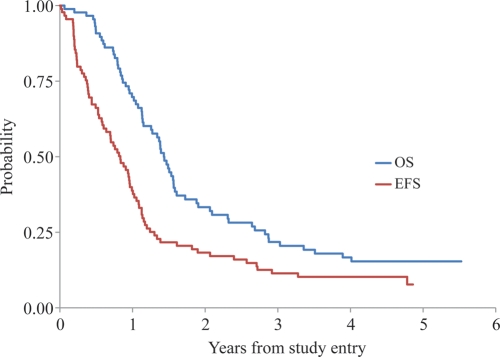
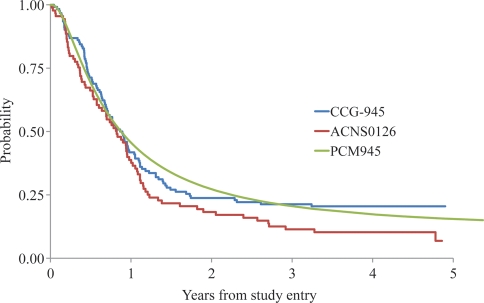
Figure 1 shows the EFS and OS rates of the 90 eligible patients. The 3-year EFS and OS rates were 11 ± 3% and 22 ± 5%, respectively. Figure 2 compares the EFS rates with those of a similar historical control cohort of 122 patients (CCG-945). For EFS, 80 events were observed in ACNS0126, and 62.8 events were expected under the historical baseline (p = 0.98).* The failure rate for AA and GBM in ACNS0126 was not significantly lower than that in CCG-945. The 3-year EFS rate for AA was 13 ± 6% for ACNS0126 compared with 22 ± 5.5% in CCG-945 (p = 0.95). The 3-year EFS rate for GBM was 7 ± 4% for ACNS0126 compared with 15 ± 5% in CCG-945 (p = 0.77). As expected, patients with gross total resection (p = 27) had a significantly better outcome than those (n = 57) without gross total resection (3-year EFS rates, 30 ± 9% vs. 4 ± 3%, respectively; p < 0.01).
Fig. 1.
EFS and OS rates of HGG patients enrolled in ACNS0126.
Fig. 2.
EFS comparison of ACNS0126 and CCG-945. PCM, parametric cure model.
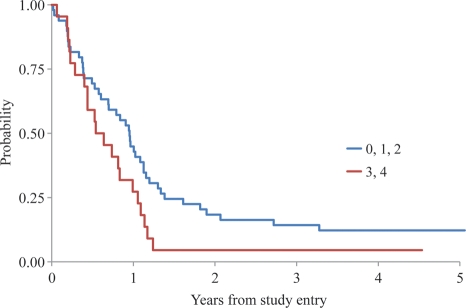
Tumor tissue for MGMT analysis was available for 71 patients, 22 of whom had MGMT overexpression. In 19 cases, despite access to hematoxylin and eosin–stained slides for central review, unstained slides were not available for MGMT analysis. The distribution of MGMT staining in relation to other clinical and molecular features is shown in Table 3; no significant differences were found based on Fisher's exact test. However, the cure model analysis shows that the failure rate for patients with MGMT overexpression was significantly higher than that for those without overexpression (p = 0.045). The 2-year EFS rate was 17 ± 5% among patients without MGMT overexpression and 5 ± 4% among those with overexpression (Figure 3). Among AAs, the 2-year EFS rate was 10 ± 10% in tumors without MGMT overexpression versus 7 + 6% in tumors with MGMT overexpression (p = 0.35). However, among GBMs, the 2-year EFS was 22 + 7% in tumors without MGMT overexpression versus 0% in those with overexpression (p = 0.04).
Table 3.
Relationship of MGMT to Other Variables: Frequency of Patients and Results of Fisher's Exact Test
| Variable | MGMT = 0–2 | MGMT = 3,4 | p-Value | |
|---|---|---|---|---|
| Age | <10 yr | 16 | 10 | 0.42 |
| ≥10 yr | 33 | 12 | ||
| Gender | Male | 28 | 11 | 0.61 |
| Female | 21 | 11 | ||
| Histology | AA | 15 | 10 | 0.30 |
| GBM | 32 | 12 | ||
| p53 | 0,1 | 22 | 6 | 0.20 |
| 2,3,4 | 27 | 16 | ||
| MIB | <18 | 20 | 9 | 0.75 |
| 18–36 | 12 | 6 | ||
| ≥36 | 10 | 7 |
MIB, Molecular Immunology Borstel. Note: Missing values were excluded.
Fig. 3.
EFS comparison as a function of MGMT expression (no overexpression: 0, 1, and 2; overexpression: 3 and 4).
Discussion
TMZ did not seem to result in an improved outcome in children with HGG compared with the therapy provided in CCG-945. This observation may lead some to question TMZ's role in the treatment of these patients22 and is in stark contrast to the consensus opinion in adults. However, it is important to put the results into a larger context before indiscriminately abandoning TMZ in childhood HGG.
First, major differences exist between the Stupp TMZ trial8 and this study. This study was a comparison with a historical control cohort and lacks the rigor of a randomized controlled comparison, making definitive conclusions about efficacy more challenging. The Stupp trial was a randomized trial between XRT alone versus XRT plus TMZ and is fundamentally different in design than this single-arm phase II trial. The last cooperative group study in the United States that randomly assigned children to XRT ± chemotherapy was CCG-943, which used adjuvant prednisone/lomustine-CCNU/vincristine (pCV).23 The 5-year EFS for children receiving XRT alone was 18% compared with 46% for those receiving combination therapy. Subsequently, adjuvant chemotherapy became the standard of practice for children with HGG, and all future trials have included XRT and chemotherapy in every study arm.
Second, prior to the Stupp trial, the benefits of chemoradiotherapy and adjuvant chemotherapy had not been well established in adults with newly diagnosed HGG. Some have suggested that this was partly because of the difficulty adults have in tolerating protracted exposure to nitrosoureas. TMZ appears to be the first alkylating agent that is well tolerated in adults, allowing for the completion of protocol-prescribed therapy. Of note, the 1-year progression-free survival rate for adults receiving TMZ chemoradiotherapy in the Stupp trial was 26.9%; the rate was 38% in children in our study. A direct statistical comparison between these results is not possible, but they suggest that the initial response and outcome of children with HGG treated with TMZ chemoradiotherapy are not inferior to those in adults.
As always, the most common reason for stopping therapy was for progressive disease. However, some children may have been removed for presumed progressive disease when the findings reflected pseudoprogression. However, time from progression to death was relatively short, suggesting that even if pseudoprogression was incorrectly coded as progressive disease in some cases, “true” progressive disease followed shortly thereafter. The current study showed no improvement in survival for children with newly diagnosed HGG who were treated with TMZ, but it did demonstrate similar survival, with less toxicity than in studies using prior nitrosourea-based regimens.1 The toxicity profile for TMZ was relatively mild. Hematologic toxicity was the most common adverse event and was generally self-limited. No life-threatening acute toxic events occurred. This has led many to argue that TMZ is a good backbone agent to use in further combination regimens.
An interesting finding in this trial is that the outcomes in children diagnosed with AA and to a lesser extent GBM were poorer than those in CCG-945. Similarly, children treated with pCV in CCG-945 had an inferior outcome to that of children treated with pCV in CCG-943. One explanation for this observation is the evolving rigor of histopathologic criteria. Nowhere is this more evident than in the review of subjects in CCG-945: among the 250 diagnoses of HGG, almost 30% were reclassified as low-grade tumors on central neuropathology review.2 While such a review was never undertaken to confirm pathologic diagnoses in CCG-943, one can reasonably presume that a similar, if not higher, number of misdiagnoses may have occurred in the earlier era of the CCG-943 study.
The above findings suggest a change in the way astrocytic tumors are being diagnosed. If neuropathologists use low thresholds for diagnosing borderline specimens as AA versus LGG, therapeutic trials will be likely to result in more AA survivors. Conversely, if more stringency is used in borderline cases, fewer AAs will be “contaminated” by LGGs and the result will be fewer AA survivors than in earlier studies. Many have speculated that this is the primary explanation for the reduction in survival seen in each subsequent trial in the COG since the results of CCG-943 were reported. One mechanism to address this apparent change in stringency is to use randomized phase II designs that would allow contemporaneous comparisons of outcome as opposed to using historic control cohorts. This strategy is being proposed by the COG for future HGG trials.
A third observation is that MGMT expression data support the finding that high levels of MGMT expression are adversely associated with outcome, which is analogous to the results of prior adult analyses in methylguanine9,24,25 and pediatric series.18,26 A strong association with outcome was particularly apparent when attention was focused on 2-year EFS. This fits with the fact that the effect of MGMT status on the response to TMZ is most likely to manifest during and several months after the time that this alkylating agent is administered. The survival curves between the MGMT-overexpressing and non-MGMT-overexpressing subsets diverged the most between 18 and 24 months after diagnosis, which is consistent with the observations of Hegi et al.9 in a series of adult GBM patients. In agreement with our previous observations, the effect of MGMT status on outcome was more apparent in tumors centrally classified as GBM, rather than those classified as AA,18 despite the lack of an association between MGMT status and histologic findings. This observation supports the prospective evaluation of MGMT expression status in future studies of childhood gliomas as a potential correlate of outcome. It remains to be determined whether MGMT overexpression constitutes an adverse prognostic factor, independent of alkylator usage; this will be addressed in subsequent studies in which the effect of MGMT expression is assessed in parallel in patients receiving alkylator-based and non-alkylator-based treatment regimens.
Where TMZ ultimately fits in the treatment of children with HGG remains unclear. Those in favor of its continued use cite the compelling adult data and the potential for similar efficacy to prior regimens with less toxicity, allowing for the development of further combinatorial therapies. Those opposed cite the results from this trial as evidence supporting the abandonment of TMZ in future trials. Given the limitation of the historical control cohort design of this trial, randomized phase II trials are being designed that will compare TMZ-based chemoradiotherapy with alternative regimens in an effort to more definitively determine the utility of TMZ in children with HGG.
Footnotes
Note that this p-value was associated with a 1-sided test. We were interested in determining only whether the EFS rate improved with the new treatment strategy compared with the baseline from CCG-945. If we had used a 2-sided test, the result would suggest that the outcome in ACNS0126 was significantly poorer than the baseline established in CCG-945 (2-sided p = 0.03).
References
- 1.Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children's Cancer Group. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 2.Boyett J, Yates A, Gilles F, et al. When is a high-grade astrocytoma (HGA) not a HGA? Results of a central review of 226 cases of anaplastic astrocytoma (AA), glioblastoma multiforme (GBM), and other-HGA (OTH-HGA) by five neuropathologists. Proc Am Soc Clin Oncol. 1998;17:526a. [Google Scholar]
- 3.Athanassiou H, Synodinou M, Maragoudakis E, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–2377. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 4.Jalali R, Basu A, Gupta T, et al. Encouraging experience of concomitant temozolomide with radiotherapy followed by adjuvant temozolomide in newly diagnosed glioblastoma multiforme: single institution experience. Br J Neurosurg. 2007;21:583–587. doi: 10.1080/02688690701604574. [DOI] [PubMed] [Google Scholar]
- 5.Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–3867. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon HJ, Kong DS, Park KB, et al. Clinical outcome of concomitant chemoradiotherapy followed by adjuvant temozolomide therapy for glioblastomas: single-center experience. Clin Neurol Neurosurg. 2009;111:679–682. doi: 10.1016/j.clineuro.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 7.van Genugten JA, Leffers P, Baumert BG, et al. Effectiveness of temozolomide for primary glioblastoma multiforme in routine clinical practice. J Neurooncol. 2010;96:249–257. doi: 10.1007/s11060-009-9956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 10.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 11.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 13.Pollack IF, Boyett JM, Yates AJ, et al. The influence of central review on outcome associations in childhood malignant gliomas: results from the CCG-945 experience. Neuro-Oncol. 2003;5:197–207. doi: 10.1215/S1152-8517-03-00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen K, Aronson L, Siffert J, et al. Final outcome of a Phase 1 trial of low-dose temozolomide given concurrently with radiation therapy in children and adolescents with brain tumors. 2002 ISPNO Abstracts: O44. [Google Scholar]
- 15.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 16.Brent TP, von Wronski M, Pegram CN, et al. Immunoaffinity purification of human O6-alkylguanine-DNA alkyltransferase using newly developed monoclonal antibodies. Cancer Res. 1990;50:58–61. [PubMed] [Google Scholar]
- 17.McLendon RE, Cleveland L, Pegram C, et al. Immunohistochemical detection of the DNA repair enzyme O6-methylguanine-DNA methyltransferase in formalin-fixed, paraffin-embedded astrocytomas. Lab Invest. 1998;78:643–644. [PubMed] [Google Scholar]
- 18.Pollack IF, Hamilton RL, Sobol RW, et al. O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 Cohort. J Clin Oncol. 2006;24:3431–3437. doi: 10.1200/JCO.2006.05.7265. [DOI] [PubMed] [Google Scholar]
- 19.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 20.Woolson RF. Rank tests and a one-sample logrank test for comparing observed survival data to a standard population. Biometrics. 1981;37:687–696. [Google Scholar]
- 21.Sposto R. Cure model analysis in cancer: an application to data from the Children's Cancer Group. Stat Med. 2002;21:293–312. doi: 10.1002/sim.987. [DOI] [PubMed] [Google Scholar]
- 22.Lashford LS, Thiesse P, Jouvet A, et al. Temozolomide in malignant gliomas of childhood: a United Kingdom Children's Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol. 2002;20:4684–4691. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 23.Sposto R, Ertel IJ, Jenkin RD, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7:165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 24.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 25.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 26.Donson AM, Addo-Yobo SO, Handler MH, et al. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007;48:403–407. doi: 10.1002/pbc.20803. [DOI] [PubMed] [Google Scholar]