Abstract
Amiodarone is a widely used anti-arrhythmic drug that inhibits diverse ion channels, including the Na+/Ca2+ exchanger (NCX), L-type Ca2+ channels, and Na+ channels. Here, we report that subtoxic doses of amiodarone and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) synergistically induced apoptosis of various glioma cells. Treatment of U251MG glioma cells with amiodarone increased intracellular Ca2+ levels and enhanced the expression of the endoplasmic reticulum (ER) stress-inducible transcription factor C/EBP homologous protein (CHOP). This upregulation of CHOP was followed by marked upregulation of the TRAIL receptor, DR5. Suppression of DR5 expression by small interfering (si) RNAs almost completely blocked amiodarone/TRAIL-induced apoptosis in U251MG glioma cells, demonstrating that DR5 is critical to this cell death. siRNA-mediated CHOP suppression reduced amiodarone-induced DR5 upregulation and attenuated the cell death induced by amiodarone plus TRAIL. In addition, omitting Ca2+ from the external medium using ethylene glycol tetraacetic acid markedly inhibited this cell death, reducing the protein levels of CHOP and DR5. These results suggest that amiodarone-induced influx of Ca2+ plays an important role in sensitizing U251MG cells to TRAIL-mediated apoptosis through CHOP-mediated DR5 upregulation. Furthermore, subtoxic doses of bepridil and cibenzoline, two other anti-arrhythmic drugs with NCX-inhibitor activity, also sensitized glioma cells to TRAIL-mediated apoptosis, via the upregulation of both CHOP and DR5. Notably, amiodarone/TRAIL cotreatment did not induce cell death in astrocytes, nor did it affect the expression of CHOP or DR5 in these cells. These results collectively suggest that a combined regimen of amiodarone plus TRAIL may offer an effective therapeutic strategy for safely and selectively treating resistant gliomas.
Keywords: TRAIL, apoptosis, amiodarone, glioma, astrocytes
The tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a member of the TNF family, is an attractive anticancer agent due to its ability to induce apoptosis in a variety of tumor cell types while having negligible effects on normal cells.1 The binding of TRAIL to cell surface–death receptors can kill some cancer cells that show resistance to conventional anticancer treatments.2
The most common brain tumors, malignant gliomas, have a high mortality rate and remain largely incurable despite the use of multimodal treatments that involve surgical resection, radiotherapy, and chemotherapy.3,4 Thus, researchers are currently attempting to develop novel therapeutic strategies against malignant gliomas. Previous studies have shown that many malignant glioma cells express TRAIL receptors but are resistant to TRAIL-induced apoptosis.5 Therefore, it is hoped that the identification of safe and effective agents capable of recovering TRAIL sensitivity in glioma cells may provide a means for improving the efficacy of TRAIL-based cancer therapeutics.
Amiodarone is an anti-arrhythmic drug that is widely used in clinical practice. It inhibits diverse ion channels, including the sodium-calcium exchanger (NCX), L-type Ca2+ channels, and Na+ channels,6 and has been reported to possess anti-inflammatory and antioxidative properties.7,8 Furthermore, amiodarone treatment has been shown to reverse multidrug resistance in several types of cancer cells9–11 and potentiate the growth-inhibitory effects of tamoxifen in various tumor cells.12 Recent studies using an amiodarone-containing treatment cocktail have shown promise in the treatment of prostate carcinomas and unresectable hepatocellular carcinomas.13,14
Here, we examined whether amiodarone could recover TRAIL sensitivity in malignant glioma cells. We report for the first time that amiodarone induced C/EBP homologous protein (CHOP)–dependent DR5 upregulation selectively in glioma cells. Furthermore, combined treatment with subtoxic doses of amiodarone and TRAIL synergistically induced apoptosis in human glioma cells, but not in normal astrocytes. Thus, amiodarone/TRAIL cotreatment may offer an attractive strategy for the treatment of malignant gliomas.
Materials and Methods
Chemicals and Antibodies
Amiodarone was purchased from Sigma Chemical Corporation. Recombinant human TRAIL/Apo2 ligand (the nontagged 19 kDa protein, amino acid 114-281) was from KOMA Biotech. Calcein acetoxymethyl ester (calcein-AM) and ethidium homodimer-1 (EthD-1) were from Invitrogen. Caspase inhibitors benzyloxy-carbonyl-Val-Ala-Asp-(OMe)-fluoromethyl ketone (z-VAD-fmk), benzyloxy-carbonyl-Ile-Glu-(OMe)-Tyr-Asp-(OMe)-fluoromethyl ketone (z-IETD-fmk), benzyloxy-carbonyl-Leu-Glu-(OMe)-His-Asp-(OMe)-fluoromethyl ketone (z-LEHD-fmk), and benzyloxy-carbonyl-Asp-(OMe)-Glu-(OMe)-Val-Asp-(OMe)-fluoromethyl ketone (z-DEVD-fmk) were from R&D systems. Ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 1,2-bis-(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra-(actoxymethyl) ester (BAPTA-AM), and CaCl2 were from Sigma. The following antibodies were used: anti-caspase-8, anti-caspase-9, anti-caspase-3, and anti-KDEL; anti-Bid, anti-phospho-eIF2α, and anti-eIF2α (Cell Signaling Technology); anti-PARP (Upstate Biotechnology); anti-DR5 (KOMA Biotech); anti-ATF4, anti-DR5, and anti-CHOP (Santa Cruz Biotechnology); anti-α-tubulin (Calbiomchem); horseradish peroxidase–conjugated anti-rabbit immunoglobulin G (IgG) and horseradish peroxidase–conjugated anti-mouse IgG (Invitrogen).
Culture of Glioma Cell Lines and Normal Human Astrocytes
Cells from the human malignant glioma cell lines U251MG, U87MG, U343, and U251N were cultured in Dulbecco's modified Eagle's medium (DMEM) (GIBCO BRL, Life Technologies) supplemented with 10% fetal bovine serum (FBS) and antibiotics (GIBCO-BRL, Life Technologies). The cells were incubated in 5% CO2 at 37°. The primary cultures of normal human astrocytes were prepared from 14-week gestation of fetal cerebrum tissues as described previously.15 Human astrocyte cultures were grown in DMEM with high glucose supplemented with 10% FBS and 20 mg/ml gentamicin, subcultured every 2 weeks, and cell culture passage numbers of <5 were used in the present study.
Measurement of Cell Viability
Cell viability was assessed by double labeling of cells with 2 µM calcein-AM and 4 µM EthD-1. The calcein-positive live cells and EthD-1–positive dead cells were visualized using a fluorescence microscope (Axiovert 200 M, Carl Zeiss).
Immunoblotting
Cell were washed in phosphate-buffered saline and lysed in boiling sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (62.5 millimolar Tris [pH6.8], 1% SDS, 10% glycerol, and 5% β-mercaptoethanol). The lysates were boiled for 5 min, separated by SDS-PAGE, and transfected to an Immobilon membrane (Millipore). After blocking nonspecific binding sites for 1 h by 5% skim milk, membranes were incubated for 2 h with specific antibodies. Membranes were then washed three times with TNET buffer (50 mM Tris-HCl [pH7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and incubated further for 1 h with horseradish peroxidase–conjugated anti-rabbit or mouse. Visualization of protein bands was accomplished using enhanced chemiluminescence (Amersham Life Science).
Measurement of Intracellular Calcium Level
Cells were plated at a density of 5 ×104 in 60-mm plates, allowed to attach overnight, and exposed to 20 µM amiodarone for different time points. The cells were stained with 2.5 µM fluo-3 for 20 min at 37° in Hank's balanced salt solution (HBSS), and Ca2+-dependent fluorescence intensity was measured using the flow cytometer (Becton Dickinson) in the fluorescence channel FL-1 with an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Reverse Transcription-PCR Analysis
Total RNA was extracted from U251MG cells using the TRIzol reagent (Invitrogen). Reverse transcription–PCR (RT-PCR) was done, following the manufacturer's protocol (Takara Shuzo Co.). The cDNAs were amplified by PCR (94° for 30 sec, 60° for 30 sec, and 72° for 1 min) with Taq DNA polymerase (Takara Shuzo Co.). Conditions for final analysis were chosen when amplification of mRNA was in the middle of the exponential amplification phase for 20 µM amiodarone.
Primer sequences used in this study were as follows (forward, reverse):
β-actin: 5′ CAGGTCATCACCATTGGCAATGAGC 3′,
5′ GATGTCCACGTCACACTTCATGA 3′ (corresponding to a 132-bp region),
CHOP: 5′ CAACTGCAGAGATGGCAGCTA 3,
5′ CTGATGCTCCCAATTGTTCAT 3′ (corresponding to a 536-bp region),
DR5: 5′ GTCTGCTCTGATCACCCAAC 3′,
5′ CTGCAAACTGTGACTCCTATG 3′ (corresponding to a 424-bp region).
Reaction products were analyzed on 2% agarose gels. The bands were visualized by ethidium bromide.
Small Interfering RNAs
The 25-nucleotide small interfering (si) RNA duplexes used in this study were purchased from Invitrogen, and their sequences are as follows:
DR5 (E9): UACAAUCACCGACCUUGACCAUCCC,
DR5 (E11): AUCAGCAUCGUGUACAAGGUGUCCC,
CHOP: UUCACCAUUCGGUCAACAGAGCUC.
BLOCK-IT Fluorescent Oligo (Invitrogen) was used as the control. Cells were transfected with siRNA oligonucleotides using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations.
Luciferase Assay
The pDR5-WT (containing DR5 promoter sequence (−605/+ 3)) was a gift from Dr. T. Sakai (Kyoto Prefectural University of Medicine). Point mutations of the CHOP-binding sites to the DR5-WT promoter were generated by a two-step PCR method (using 5′ CTTGCGGAGGAGGTAGTTGACGA to 5′ CGTCAACTACCTCCTCCGAAAG). The clone containing the mutation was sequenced to ensure the accuracy of the PCR amplification procedure, and this plasmid was named as pDR5-mCHOP. For transfection, in brief, cells were plated onto 60-mm culture dishes at a density of 3 ×105 cells and grown overnight. Cells were transfected with 1 µg of the respective luciferase reporter construct using Lipofectamine Plus reagent (Invitrogen) following the manufacturer's instructions. After incubation for 24 h, transfected cells were further treated with or without amiodarone. Luciferase activities were assayed following the manufacturer's protocol (Promega).
Statistical Analysis and Determination of Synergy
All data are presented as means ± SDs of at least three independent experiments. The statistical significance of differences was assessed using analysis of variance with Bonferroni or repeated-measures analysis of variance followed by Greenhouse-Geisser adjustment. Values of P < 0.05 were considered significant. Synergy of amiodarone and TRAIL was evaluated by using the isobologram method.16 The cells were treated with different concentrations of each agent (amiodarone or TRAIL) alone or with the two agents in combination for 24 h. The relative survival was assessed, and the half maximal inhibitory concentration (IC50) values for each drug given alone or in combination with a fixed concentration of the second agent were established from the concentration–effect curves. The IC50 values of amiodarone and TRAIL in the respective glioma cell lines are as follows: U251MG (50 µM, 500 ng/ml), U87MG (25 µM, 360 ng/ml), U343 (85 µM, 40 ng/ml), U251N (220 µM, 93 ng/ml). The IC50 values of cotreatment were divided by the IC50 value of each drug in the absence of the other drug. In a graphical presentation, the straight line connecting the IC50 values of the two agents when applied alone corresponds to additivity or independent effects of both agents. Values below this line indicate synergy, and values above this line indicate antagonism.
Results
Amiodarone Sensitizes Human Glioma Cells to TRAIL-Mediated Apoptosis via Caspase-Dependent Apoptosis
To examine whether amiodarone can sensitize malignant glioma cells to TRAIL-mediated apoptosis, we tested the effect of amiodarone and/or TRAIL on the viability of U251MG and U87MG glioma cells. Measurement of cell viability using calcein-AM and EthD-1 demonstrated that these cells were resistant to TRAIL alone up to 100 ng/ml or amiodarone alone up to 20 µM (Fig. 1A). However, cotreatment with amiodarone and TRAIL significantly and dose-dependently increased cell death in both U251MG and U87MG cells (Fig. 1A). Also in U343 and U251N glioma cells, which are relatively sensitive to TRAIL, amiodarone cotreatment markedly enhanced TRAIL-mediated apoptosis (Fig. 1A). An isobologram analysis demonstrated that amiodarone and TRAIL synergistically induced cell death in these 4 different glioma cells (Fig. 1B). These results indicate that combined treatment with amiodarone and TRAIL effectively kills glioma cells. We next examined whether the amiodarone-facilitated TRAIL-induced cell death of glioma cells was mediated through caspases. In U251MG cells treated with 20 µM amiodarone alone, we were unable to detect processing of the caspases and the caspase substrates, PARP and Bid (Fig. 1C). In response to 100 ng/ml TRAIL alone, caspase-3 was partially processed into its p20 intermediate form, but we did not observe further cleavage into the active p17 subunit. Notably, we could not detect any processing of caspase-8, caspase-9, PARP, or Bid following treatment with TRAIL alone. However, in cells cotreated with amiodarone and TRAIL, caspase-3 was effectively processed into its active p17 subunit, and caspase-8, caspase-9, PARP, and Bid were all progressively processed. These results suggest that TRAIL resistance in U251MG cells may be associated with a proteolytic processing blockade of procaspase-3, leading to failure in the subsequent caspase amplification cascade. In these cells, cotreatment with amiodarone may help relieve this proteolytic processing blockade. To ascertain the role of the various caspases in the amiodarone-mediated potentiation of TRAIL-induced apoptosis, we tested the effects of specific caspase inhibitors. Pretreatment of U251MG cells with z-VAD-fmk (a pancaspase inhibitor), z-IETD-fmk (a caspase-8 inhibitor), z-LEHD-fmk (a caspase-9 inhibitor), or z-DEVD-fmk (a caspase-3 inhibitor) dose-dependently blocked cotreatment-induced cell death (Fig. 1D). Taken together, these results show that amiodarone sensitizes glioma cells to TRAIL-induced caspase-dependent apoptosis.
Fig. 1.
Combined treatment with amiodarone and TRAIL effectively induces TRAIL-mediated apoptosis in glioma cells. (A) Effect of amiodarone and/or TRAIL on the viability of glioma cells. U251MG, U87MG, U343, and U251N cells were treated with amiodarone for 30 min and then further treated with TRAIL for 24 h at the indicated concentrations. Cellular viability was assessed using calcein-AM and EthD-1. Columns indicate average of 3 individual experiments; bars represents ±SD; #P < 0.01, compared with untreated cells; *P < 0.001, compared with TRAIL-treated cells; **P < 0.01, compared with TRAIL-treated cells. (B) Synergistic induction of cell death by amiodarone and TRAIL. Four different glioma cells were treated for 24 h with increasing concentrations of amiodarone and TRAIL. Isobologram analysis was performed as described in Materials and Methods. IC50 for amiodarone or TRAIL in the respective glioma cells are as follows: U251MG (50 µM, 500 ng/ml); U87MG (90 µM, 360 ng/ml); U343 (85 µM, 40 ng/ml); U251N (220 µM, 93 ng/ml). (C) Activation of caspases following combined treatment with amiodarone and TRAIL. U251MG cells were treated with 20 µM amiodarone (Amio.) alone, 100 ng/ml TRAIL (T) alone, or amiodarone plus TRAIL (Amio. + T) for the indicated time points. Western blotting of the caspases, PARP, Bid, and α-tubulin was performed. (D) Effect of caspases on the cell death induced by amiodarone and TRAIL. U251MG cells were treated with the respective caspase-specific tetrapeptide inhibitors at the indicated concentrations for 30 min and further treated with 20 µM amiodarone and 100 ng/ml TRAIL for 24 h. Cellular viability was determined using calcein-AM and EhtD-1. The graph represents the result of 1 of 3 independent experiments with consistent results. Columns indicate average of 3 individual experiments; bars represents ±SD; #P < 0.001, compared with untreated cells; *P < 0.001, compared with cells treated with TRAIL alone; **P < 0.01, compared with cells treated with TRAIL alone.
Amiodarone Induces ER Stress Accompanied by CHOP Induction in Glioma Cells
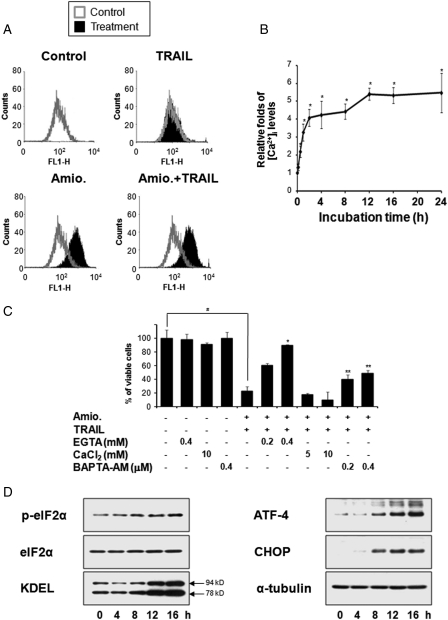
As amiodarone is known to inhibit various ion channels, including NCX, L-type Ca2+ channels, and Na+ channels,17 we examined whether amiodarone treatment could affect the intracellular Ca2+ levels in glioma cells. Flow cytometric analysis using fluo-3, a fluorescent dye capable of detecting cytosolic Ca2+, demonstrated that intracellular Ca2+ levels were dramatically increased in U251MG cells treated with 20 µM amiodarone alone or cotreated with 20 µM amiodarone and 100 ng/ml TRAIL for 24 h, but not in cells treated with TRAIL alone (Fig. 2A). Time-course experiments with amiodarone-treated U251MG cells showed that intracellular Ca2+ levels were markedly increased by 12 h posttreatment and were sustained by 24 h (Fig. 2B). Since amiodarone was previously shown to increase intracellular Ca2+ levels ([Ca2+]i) in various cell types,18−20 primarily via the influx of Ca2+ from the extracellular space,18,19 we examined whether omitting Ca2+ from the external medium could affect the cell death induced by amiodarone plus TRAIL. We found that treatment with EGTA, a chelator of extracellular Ca2+, dose-dependently inhibited the cell death induced by amiodarone and TRAIL cotreatment, inhibiting the amiodarone-induced increase in [Ca2+]i (Fig. 2C and Supplementary Fig. 1). In contrast, addition of Ca2+ to the media accelerated this cell death, slightly enhancing amiodarone-induced [Ca2+]i rise. Interestingly, treatment with BAPTA-AM (1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid), an intracellular Ca2+ chelator, also attenuated these responses induced by amiodarone plus TRAIL, but to a lesser degree than those seen following EGTA pretreatment. These results suggest that the amiodarone-induced influx of Ca2+ plays a critical role in sensitizing U251MG cells to TRAIL-mediated apoptosis. Since imbalances in the homeostasis of intracellular Ca2+ are known to induce endoplasmic reticulum (ER) stress,21 we further examined whether amiodarone could affect the ER stress-associated signaling pathways. We found that amiodarone treatment of U251MG cells progressively increased the phosphorylation of eIF2α and the protein expression levels of KDEL, ATF-4, and CHOP (Fig. 2D), indicating that ER stress is induced by amiodarone in these cells.
Fig. 2.
Amiodarone induces ER stress. (A) Effect of amiodarone and/or TRAIL on the intracellular Ca2+ levels. U251MG cells were treated with 20 µM amiodarone alone, 100 ng/ml TRAIL alone, or amiodarone plus TRAIL for 24 h. Flow cytometry using fluo-3 was performed as described in Materials and Methods. (B) Changes in the intracellular Ca2+ levels in response to amiodarone. U251MG cells were treated with 20 µM amiodarone for the indicated time points, analyzed by flow cytometry. Dots indicate average of 3 individual experiments; bars represents ±SD; *P < 0.001, compared with untreated cells. (C) Effect of EGTA, CaCl2, or BAPTA-AM on the cell death induced by amiodarone plus TRAIL. U251MG cells were treated with EGTA, CaCl2, or BAPTA-AM at the indicated concentrations and further treated with 20 µM amiodarone and 100 ng/ml TRAIL for 24 h. Cellular viability was determined using calcein-AM and EhtD-1. The graph represents the result of 1 of 3 independent experiments with consistent results. Columns indicate average of 3 individual experiments; bars represents ±SD; #P < 0.001, compared with untreated cells; *P < 0.001, compared with amiodarone plus TRAIL-treated cells; **P < 0.01, compared with amiodarone plus TRAIL-treated cells. (D) Changes in the protein levels associated with ER stress following amiodarone treatment. U251MG cells were treated with 20 µM amiodarone for the indicated times and Western blotting was performed. Western blotting of α-tubulin served as a loading control of the samples.
CHOP Directly Mediates Amiodarone-Induced DR5 Upregulation
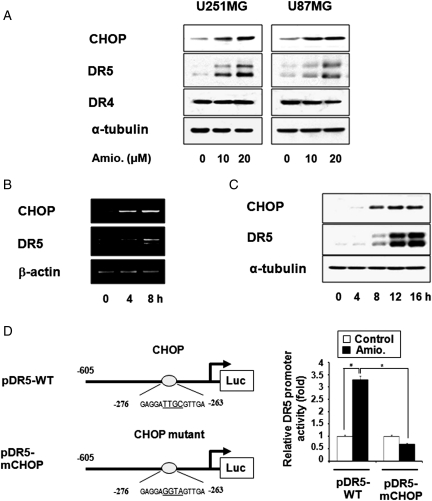
CHOP is known to be critically involved in the transcription of the DR5 gene.22 Thus, we next examined whether amiodarone-induced CHOP upregulation could modulate DR5 expression in glioma cells. We found that amiodarone treatment dose-dependently increased CHOP and DR5 protein levels, but not DR4 levels, in U251MG and U87MG cells (Fig. 3A). Amiodarone-induced upregulation of CHOP and DR5 was also observed in U343 and U251N cells (Supplementary Fig. 2). Furthermore, DR5 upregulation in amiodarone-treated U251MG cells was preceded by induction of CHOP, and this sequential enhancement could be observed at both the mRNA and protein levels for CHOP and DR5 (Fig. 3B and C). To confirm the direct involvement of CHOP in the amiodarone-mediated transcriptional activation of DR5, we employed two luciferase reporter plasmids: pDR5-WT, which contained the DR5 promoter sequence −605/+ 3,23 and pDR5-mCHOP, which contained the same promoter sequence with mutation of the potential CHOP-binding site (−281 to −261). U251MG cells were separately transfected with these plasmids and subjected to amiodarone treatment, and then we performed luciferase assay. Our results revealed that the transcriptional activity of pDR5-WT was significantly increased by 20 µM amiodarone treatment (Fig. 3D), but the promoter activity of pDR5-mCHOP was not enhanced by the same treatment, suggesting that CHOP directly mediates the amiodarone-induced upregulation of DR5.
Fig. 3.
CHOP-mediated DR5 upregulation is critical for amidarone-stimulated TRAIL-induced apoptosis. (A) Amiodarone increased the protein levels of CHOP and DR5. U251MG and U87MG cells were treated with amiodarone at the indicated concentrations for 24 h, and Western blotting of CHOP, DR5, DR4, and α-tubulin was performed. (B) Changes in the mRNA levels of CHOP and DR5 following amiodarone treatment. U251MG cells were treated with 20 µM amiodarone at the indicated time points, and RT-PCR was performed to detect the mRNA levels of CHOP, DR5, and β-actin. (C) Changes in the protein levels of CHOP and DR5 following amiodarone treatment. U251MG cells were treated with 20 µM amiodarone at the indicated time points, and Western blotting of CHOP and DR5 was performed. Western blotting of α-tubulin served as a loading control. (D) Mutation at CHOP binding site of the DR5 promoter abolishes amiodarone-mediated transcriptional activation of DR5. U251MG cells were transfected with pDR5-WT or CHOP-mutated pDR5-mCHOP and then treated with 20 µM amiodarone for 12 h, lysed, and assayed for luciferase activity. Columns indicate average of 3 individual experiments; bars represents ±SD; #P < 0.001, compared with untreated cells; *P < 0.001, compared with cell transfected with pDR5-WT and further treated with amiodarone.
CHOP-Mediated DR5 Upregulation Is Important for Amiodarone-Stimulated TRAIL-Induced Apoptosis
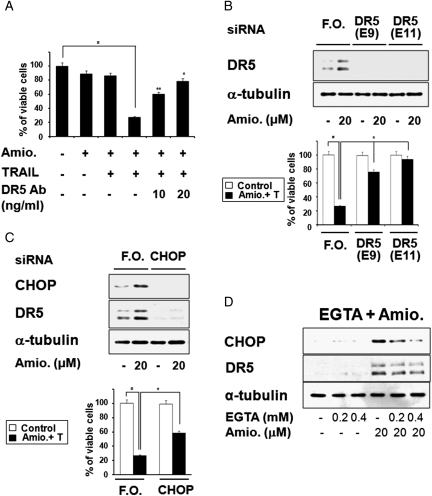
Next, to examine the functional significance of upregulation of DR5 in amiodarone/TRAIL-mediated apoptosis, we used DR5-specific blocking antibody and DR5-targeting siRNAs. We found that addition of DR5-specific blocking antibody dose-dependently inhibited amiodarone/TRAIL-induced apoptosis of U251MG cells (Fig. 4A). Furthermore, siRNA-mediated suppression of DR5 effectively blocked the cell death induced by amiodarone and TRAIL (Fig. 4B). Similarly, siRNA-mediated suppression of CHOP effectively inhibited amiodarone-induced DR5 upregulation and attenuated the cell death induced by amiodarone and TRAIL (Fig. 4C). These results clearly show that CHOP-mediated DR5 upregulation critically contributes to amiodarone/TRAIL-mediated apoptosis. Next, we investigated whether the amiodarone-induced influx of Ca2+ is actually involved in this process. We found that the EGTA-mediated removal of Ca2+ from the external medium dose-dependently decreased the amiodarone-induced upregulation of CHOP and DR5 (Fig. 4D). Taken together, these results suggest that the amiodarone-induced influx of Ca2+ is directly involved in sensitizing glioma cells to TRAIL-induced apoptosis via CHOP-mediated DR5 upregulation.
Fig. 4.
CHOP-mediated DR5 upregulation contributes to amiodarone-stimulated TRAIL-mediated apoptosis. (A) Effect of DR5-specific blocking chimera antibody on amiodarone/TRAIL-induced apoptosis. U251MG cells were pretreated with or without amiodarone for 30 min, followed by treatment with or without 100 ng/ml TRAIL for 24 h in the presence of indicated concentrations of DR5-specific blocking chimera antibody. Cellular viabilities were measured with calcein-AM and EthD-1 to detect live and dead cells, respectively. Similar results were obtained from 3 independent experiments; bars represent ±SD; #P < 0.001, compared with untreated cells; *P < 0.001, compared with amiodarone plus TRAIL–treated cells; **P < 0.01, compared with amiodarone plus TRAIL–treated cells. (B) Suppression of DR5 expression by siRNA blocks amiodarone-stimulated TRAIL-induced apoptosis in U251MG cells. U251MG cells were transfected with the control fluorescent oligonucleotide (F.O.) or two different siRNA duplexes against DR5 mRNA (E9 and E11). Twenty-four hours after transfection, cells were further treated with or without 20 µM amiodarone for 24 h. Western blotting of DR5 was done to confirm the downregulation of DR5 by siRNA transfection. α-Tubulin levels were assessed to show equal gel loading. To examine the effect of DR5 downregulation on amiodarone/TRAIL-induced apoptosis, U251MG cells were transfected with siRNAs, incubated for 24 h, and further treated with or without 20 µM amiodarone plus 100 ng/ml TRAIL for 24 h. Cellular viability was determined using calcein-AM and EthD-1. Columns indicate average of 3 individual experiments; bars represent ±SD; #P < 0.01, compared with untreated cells; *P < 0.01, compared with the cells transfected with the F.O. and further treated with amiodarone plus TRAIL. (C) Role of CHOP in amiodarone-induced DR5 upregulation and amiodarone-stimulated TRAIL-induced apoptosis. Suppression of CHOP expression by siRNA reduces amiodarone-induced DR5 upregulation and amiodarone-stimulated TRAIL-induced apoptosis in U251MG cells. U251MG cells were transfected with the control F.O. or siRNA duplexes against CHOP, incubated for 24 h, and further treated with 20 µM amiodarone alone for 24 h. First, Western blotting of CHOP was performed to confirm the downregulation of CHOP by siRNA transfection. Western blotting of DR5 was also performed to examine the knockdown effect of CHOP on amiodarone-induced DR5 upregulation. Equal loading of the protein samples was confirmed by Western blotting of α-tubulin. To examine the effect of CHOP downregulation on amiodarone-sensitized TRAIL-induced apoptosis, transfected cells with the control F.O. or CHOP siRNA were treated with 20 µM amiodarone plus 100 ng/ml TRAIL for 24 h. Cellular viability was determined using calcein-AM and EthD-1. Columns indicate average of 3 individual experiments; bars represent ±SD; #P < 0.01, compared with untreated cells; * P < 0.01, compared with the cells transfected with the F.O and further treated with amiodarone plus TRAIL. (D) Effect of EGTA on amiodarone-induced upregulation of CHOP and DR5. U251MG cells were pretreated with EGTA at the indicated concentrations and further treated with 20 µM amiodarone for 24 h. Western blotting of CHOP, DR5, and α-tubulin was performed.
The Anti-arrhythmic Agents Bepridil and Cibenzoline Also Stimulate TRAIL-Induced Apoptosis via Upregulation of CHOP and DR5
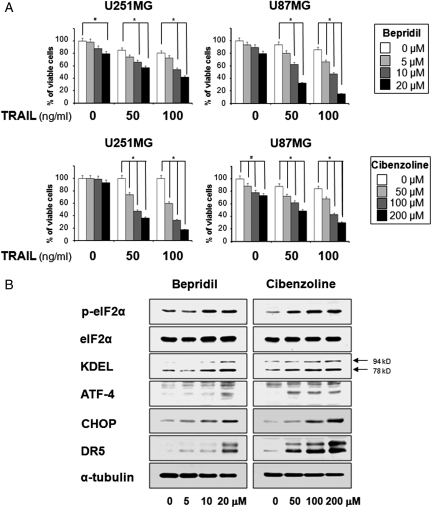
Since amiodarone is a representative anti-arrhythmic agent with NCX-inhibitor activity,17,24 we next tested whether two other anti-arrhythmic agents with NCX-inhibitor activity, bepridil and cibenzoline,17,24 could also sensitize glioma cells to TRAIL-mediated apoptosis. We found that treatment with bepridil alone did not induce marked cell death up to 20 µM, whereas cibenzoline alone was subtoxic up to 200 µM in U251MG and U87MG cells (Fig. 5A and B). In contrast, treatment with bepridil plus TRAIL or cibenzoline plus TRAIL effectively and dose-dependently induced cell death in these cells (Fig. 5A and B). These results suggest that bepridil and cibenzoline can also recover TRAIL sensitivity in TRAIL-resistant glioma cells. Furthermore, both bepridil and cibenzoline dose-dependently increased the levels of eIF2α phosphorylation, KDEL, and ATF-4 protein (Fig. 5C). Moreover, protein levels of CHOP and DR5 were increased by either bepridil or cibenzoline (Fig. 5C). Collectively, our results suggest that upregulation of CHOP and DR5 may be a common mechanism through which anti-arrhythmic agents with NCX-inhibitor activity can sensitize glioma cells to TRAIL-mediated apoptosis.
Fig. 5.
The anti-arrythmic drugs bepridil and cibenzoline also sensitize glioma cells to TRAIL-mediated apoptosis via CHOP-mediated DR5 upregulation. (A) Effect of bepridil plus TRAIL or cibenzoline plus TRAIL on the viability of various glioma cells. U251MG and U87MG cells were treated with bepridil or cibenzoline for 30 min and then further treated with TRAIL for 24 h at the indicated concentrations. Cellular viability was assessed using calcein-AM and EthD-1. Columns indicate average of 3 individual experiments; bars represent ± SD; #P < 0.001, compared with untreated cells; *P < 0.001, compared with TRAIL-treated cells. (B) Effect of bepridil or cibenzoline on the expression of ER stress–associated proteins and DR5. U251MG cells were treated with bepridil or cibenzoline for 24 h at the indicated concentrations. Western blotting to detect changes in the expression of indicated proteins was performed.
Combined Treatment with Amiodarone and TRAIL Does Not Induce Cell Death in Normal Astrocytes
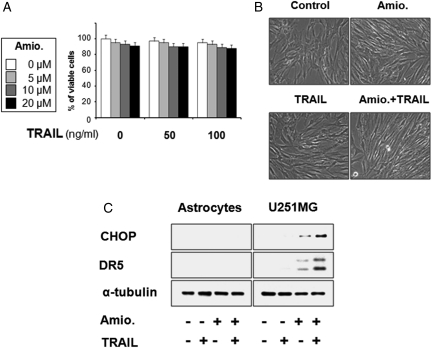
Next, we investigated whether the viability of normal astrocytes could be affected by amiodarone/TRAIL cotreatment. We found no induction of cell death in astrocytes treated with 20 µM amiodarone and/or 100 ng/ml TRAIL (Fig. 6A and B), suggesting that this combined regimen may be a safe and effective strategy for killing malignant glioma cells. Next, we examined the effect of amiodarone and/or TRAIL on the protein levels of CHOP and DR5 in normal astrocytes. We found that unlike our observations in U251MG glioma cells, amiodarone treatment did not upregulate CHOP or DR5 in astrocytes (Fig. 6C). Taken together, these results demonstrate that CHOP-mediated DR5 upregulation may explain the preferential cytotoxicity of amiodarone/TRAIL cotreatment in glioma cells, sparing normal astrocytes.
Fig. 6.
Effect of amiodarone and/or TRAIL on the viability of human normal astrocytes. (A) Human astrocytes were treated with amdiodarone and/or TRAIL at the indicated concentrations for 24 h. Cellular viability was assessed using calcein-AM and EthD-1. (B) Human astrocytes were treated with 20 µM amiodarone for 30 min and further treated with or without 100 ng/ml TRAIL for 24 h. Morphologies of human astrocytes treated with amiodarone and/or TRAIL are shown. (C) Effect of amiodarone/or TRAIL on the expression of CHOP and DR5 in astrocytes. Astrocytes and U251MG cells were treated with 20 µM amiodarone alone, 100 ng/ml TRAIL alone, or a combination of both for 24 h, and cell extracts were prepared for Western blotting of CHOP and DR5. Western blotting of α-tubulin served as a loading control of the sample.
Discussion
For effective cancer therapeutics, it is very important to induce cell death selectively in malignant tumor cells but not in normal cells. TRAIL has been shown to induce apoptosis in various tumor cells, while having minimal toxicity in normal cells.1,25 In this regard, TRAIL is an attractive candidate for cancer treatment. However, various cancer cells, including numerous glioma cells, have been reported to show resistance to the cytotoxic effects of TRAIL, even though these cells express the TRAIL receptor, DR5.5,26 Therefore, researchers are currently seeking to identify agents that may effectively increase the sensitivity of cancer cells to TRAIL-induced apoptosis. In this study, we show for the first time that amiodarone is a potent TRAIL sensitizer in malignant human glioma cells.
In the United States, amiodarone is currently one of the most commonly prescribed drugs for ventricular and atrial arrhythmias, largely because of its minimal negative inotropic activity and very low rate of pro-arrhythmia.27 In addition, amiodarone has been shown to inhibit multidrug resistance in various cancer cells.9-12,28 For example, 20 µM amiodarone very effectively restored the drug sensitivity in doxorubicin-resistant rat glioblastoma cells,28 and the inclusion of amiodarone as a component of cocktails aimed at treating human cancer cells (e.g., prostate carcinoma, unresectable hepatocellular carcinoma) has shown promise.13,14 Here, we show that combined treatment with subtoxic doses of amiodarone and TRAIL can synergistically induce apoptosis in malignant glioma cells. Amiodarone is known to inhibit the activities of diverse ion channels, including the NCX6; this is a bidirectional transporter that removes a single Ca2+ ion from the cell while correspondingly importing three Na+ ions,17 and is considered one of the most important cellular mechanisms for removing Ca2+. 29 A previous study showed that amiodarone increased intracellular Ca2+ levels ([Ca2+]i) and that [Ca2+]i signaling depended primarily on extracellular Ca2+ in various cell types.18,19 Similar to the previous report, we found that amiodarone treatment of U251MG cells induced a persistent increase in [Ca2+]i (Fig. 2B). In addition, the EGTA-mediated removal of extracellular Ca2+ effectively blocked the cell death induced by amiodarone plus TRAIL (Fig. 2C), suggesting that the influx of Ca2+ may be critically involved in this cell death. Studies have shown that impairment of Ca2+ homeostasis induces defense mechanisms such as the unfolded protein response (UPR), but when it is severe and prolonged, the apoptotic pathway is activated.30,31 In our study, amiodarone-induced [Ca2+]i was accompanied by increases in eIF2α phosphorylation and the protein levels of KDEL, ATF-4, and CHOP, indicating that amiodarone induces the UPR in these cells. The CHOP gene shows extremely high induction during ER stress,21 and CHOP is known to critically modulate ER stress–induced cell death.21,31 In the present study, we found that amiodarone dose-dependently increased DR5 protein levels in various glioma cells, in parallel with the increase in CHOP protein levels. Given that TRAIL is known to trigger apoptosis through binding to its death receptors, DR4 and DR5,32 the expression levels of these death receptors may be critical to determining the intensity and/or duration of TRAIL-induced apoptotic signaling. Either pretreatment with the DR5-specific blocking antibody or siRNA-mediated suppression of DR5 expression efficiently blocked the cell death induced by the combined treatment, indicating that DR5 plays a critical role in amiodarone-sensitized TRAIL-induced apoptosis in glioma cells. Finally, we report three lines of evidence indicating that the CHOP transcription factor critically contributes to amiodarone-induced DR5 upregulation and amiodarone/TRAIL-induced apoptosis, as follows. (1) The amiodarone-induced increases in the mRNA and protein levels of CHOP preceded those of DR5 (Fig. 3B and C). (2) The amiodarone-induced activation of DR5 promoter activity was abrogated by mutation of the putative CHOP-binding site in the DR5 promoter. (3) Finally, siRNA-mediated CHOP knockdown inhibited amiodarone-induced DR5 upregulation and attenuated amiodarone/TRAIL-mediated cell death. In addition, EGTA treatment inhibited the amiodarone-induced upregulation of CHOP and DR5, thereby blocking the cell death induced by amiodarone plus TRAIL. These results suggest that the influx of Ca2+ acts upstream of the amiodarone-induced upregulation of CHOP and DR5. Consistent with our results, a previous study showed that an influx of Ca2+ and subsequent induction of CHOP were important for palmitate-induced apoptosis of MIN6N8a beta cell.33 Furthermore, Abdelrahim et al.34 reported that the release of ER-stored Ca2+ and subsequent CHOP-dependent DR5 induction were responsible for the apoptosis induced by 3,3′-diindolylmethane and its derivatives in pancreatic cancer cells.
Interestingly, we found that treatment with the anti-arrhythmic agents bepridil and cibenzoline also effectively sensitized glioma cells to TRAIL-induced apoptosis. Bepridil, a diarylaminopropylamine derivative, has been shown to have NCX-inhibitor activity, with an IC50 value of 8.1 µM,35 while cibenzoline, a diarylcyclopropylimidazoline derivative, was recently shown to inhibit NCX currents with an IC50 value of 95 µM.36 In addition to their NCX-inhibitor activities, bepridil and cibenzoline have inhibitory effects on various ionic channels, including those of Ca2+ and K+.17,24 Previously, bepridil has been shown to prolong increased intracellular Ca2+ levels by interfering with intracellular Ca2+ homeostasis, resulting in growth inhibition and potentiation of death among human brain tumor cells.37 Here, we found that both bepridil and cibenzoline upregulated eIF2α phosphorylation, and protein levels of KDEL and ATF-4 were increased (Fig. 5C), suggesting that they also induce ER stress in glioma cells. Furthermore, both bepridil and cibenzoline upregulated CHOP and DR5, thereby stimulating TRAIL-mediated apoptosis, in a manner similar to amiodarone. These results demonstrate that CHOP-mediated DR5 upregulation may be a common mechanism through which these anti-arrhythmic agents can sensitize glioma cells to TRAIL-mediated apoptosis.
One factor critical to the success of cancer therapy is the ability to selectively kill malignant cancer cells while sparing normal cells. The choice of an appropriate combination of anticancer drugs is also very important for successful chemotherapy. Fully understanding the differential signaling pathways between normal cells and cancer cells may help us maximize the synergistic death-inducing effects of combined drug treatments on cancer cells while minimizing their side effects in normal cells. Here, we found that normal astrocytes were very resistant to amiodarone/TRAIL cotreatment, whereas glioma cells were highly sensitive to this treatment. Furthermore, we uncovered a mechanistic basis for the difference by showing that the amiodarone-induced CHOP-mediated upregulation of DR5 did not occur in normal astrocytes. Thus, amiodarone/TRAIL cotreatment may offer an attractive and safe strategy for the treatment of malignant glioma cells. However, the presence of the blood–brain barrier (BBB), which prevents the delivery of potentially active therapeutic compounds, is particularly problematic for the treatment of infiltrating gliomas.38 In order to cross the BBB, the anticancer drugs should be small (<500 Da), highly lipid soluble, and able to pass the BBB by passive diffusion.38,39 Amoidarone is reportedly hydrophobic, but it does not cross the BBB efficiently.40 Radiation therapy is one of the main modalities in the treatment of glioblastoma,41 and opening of the BBB induced by irradiation with 20–40 Gy was shown to improve the efficacy of intracranial chemotherapy in glioblastoma patients.42,43 From this perspective, combined treatment with radiation and amiodarone/TRAIL may provide a more effective strategy for the treatment of malignant gliomas in the clinical setting. In addition, recent novel approaches for site-specific opening of the BBB, such as ultrasound, photodynamic therapy, and photochemical internalization,38 may be useful for the effective delivery of amiodarone and TRAIL to the tumor sites.
In summary, the present study demonstrates that apoptosis of malignant glioma cells, but not normal astrocytes, is effectively and selectively induced by combined treatment with amiodarone and TRAIL. Therefore, combined treatment with amiodarone and TRAIL seems to warrant additional study as a potential new strategy for treating TRAIL-resistant gliomas.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by a grant from the Nuclear Research & Development Program of the KOSEF grant funded by MEST (BAERI No. 2007-2001283) and a Mid-Career Researcher Program through NRF grant funded by MEST (No. 2008-0059745).
References
- 1.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11(2):255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins CJ. TRAIL and malignant glioma. Vitam Horm. 2004;67:427–452. doi: 10.1016/S0083-6729(04)67022-1. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Cavenee WK. World Health Organization Classification of Tumors. Pathology and Genetics: Tumors of the Nervous System. 2nd. Albany, NY: WHO Publication Center USA; 2000. [Google Scholar]
- 4.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 5.Knight MJ, Riggkin CD, Muscat AM, et al. Analysis of FasL and TRAIL induced apoptosis pathways in glioma cells. Oncogene. 2001;20(41):5789–5798. doi: 10.1038/sj.onc.1204810. [DOI] [PubMed] [Google Scholar]
- 6.Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84(9A):20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 7.Halici Z, Dengiz GO, Odabasoglu F, et al. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol. 2007;566(1–3):215–221. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Dengiz GO, Odabasoglu F, Halici Z, et al. Gastroprotective and antioxidant effects of amiodarone on indumethacin-induced gastric ulcers in rats. Arch Pharm Res. 2007;30(11):1426–1434. doi: 10.1007/BF02977367. [DOI] [PubMed] [Google Scholar]
- 9.Chauffert B, Martin M, Hammann A, et al. Amiodarone-induced enhancement of doxorubicin and 4′-deoxydoxorubicin cytotoxicity to rat colon cancer cells in vitro and in vivo. Cancer Res. 1986;46(2):825–830. [PubMed] [Google Scholar]
- 10.van der Graaf WT, de Vries EG, Timmer-Bosscha H, et al. Effects of amiodarone, cyclosporine A, and PSC 833 on the cytotoxicity of mitoxantrone, doxorubicin, and vincristine in non-P-glycoprotein human small cell lung cancer cell lines. Cancer Res. 1994;54(20):5368–5373. [PubMed] [Google Scholar]
- 11.Wigler PW. Cellular drug efflux and reversal therapy of cancer. J Bioenerg Biomembr. 1996;28(3):279–284. doi: 10.1007/BF02110701. [DOI] [PubMed] [Google Scholar]
- 12.Abdul M, Santo A, Hoosein N. Activity of potassium channel-blockers in breast cancer. Anticancer Res. 2003;23(4):3347–3351. [PubMed] [Google Scholar]
- 13.Theodossiou TA, Galanou MC, Paleos CM. Novel amiodarone-doxorubicin cocktail liposomes enhance doxorubicin retention and cytotoxicity in DU145 human prostate carcinoma cells. J Med Chem. 2008;51(19):6067–6074. doi: 10.1021/jm800493j. [DOI] [PubMed] [Google Scholar]
- 14.Guiu B, Colin C, Cercueil JP, et al. Pilot study of transarterial chemoembolization with pirarubicin and amiodarone for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2009;32(3):238–244. doi: 10.1097/COC.0b013e3181845529. [DOI] [PubMed] [Google Scholar]
- 15.Kim SU, Moretto G, Lee V, et al. Neuroimmunology of gangliosides in human neurons and glial cells in culture. J Neurosci Res. 1986;15(3):303–321. doi: 10.1002/jnr.490150303. [DOI] [PubMed] [Google Scholar]
- 16.Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Koide Y, Kimura J. Topics on the Na+/Ca2+ exchanger: pharmacological characterization of Na+/Ca2+ exchanger inhibitors. J Pharmacol Sci. 2006;102(1):7–16. doi: 10.1254/jphs.fmj06002x2. [DOI] [PubMed] [Google Scholar]
- 18.Powis G, Olsen R, Standing JE, et al. Amiodarone-mediated increase in intracellular free Ca2+ associated with cellular injury to human pulmonary artery endothelial cells. Toxicol Appl Pharmaol. 1990;103(1):156–164. doi: 10.1016/0041-008x(90)90271-u. [DOI] [PubMed] [Google Scholar]
- 19.Kodavanti PR, Pentyala SN, Yallapragada PR, et al. Amiodarone and desethylamiodarone increase intrasynaptomal free calcium through receptor mediated channel. Naunyn Schmiedebergs Arch Pharmacol. 1992;345(2):213–221. doi: 10.1007/BF00165739. [DOI] [PubMed] [Google Scholar]
- 20.Nicolay JP, Bentzen PJ, Ghashghaeinia M, et al. Stimulation of erythrocyte cell membrane scrambling by amiodarone. Cell Physiol Biochem. 2007;20(6):1043–1050. doi: 10.1159/000110713. [DOI] [PubMed] [Google Scholar]
- 21.Oyadomari S, Mori M. Role of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T, Shiraishi T, Nakata S, et al. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005;65(13):5662–5667. doi: 10.1158/0008-5472.CAN-05-0693. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Maeda A, Tani N, et al. Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett. 2001;507(3):381–385. doi: 10.1016/s0014-5793(01)02947-7. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto T, Watanabe Y, Kita S, et al. Na+/Ca2+ exchange inhibitors: a new class of calcium regulators. Cardiovasc Hematol Disord Drug Targets. 2007;7(3):188–198. doi: 10.2174/187152907781745288. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277(5327):818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 26.Hao C, Beguinot F, Condorelli G, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 2001;61(3):1162–1170. [PubMed] [Google Scholar]
- 27.Punnam SR, Goyal SK, Kotaru VP, et al. Amiodarone—a ‘broad spectrum’ antiarrhythmic drug. Cardiovasc Hematol Disord Drug Targets. 2010;10(1):73–81. doi: 10.2174/187152910790780032. [DOI] [PubMed] [Google Scholar]
- 28.Huet S, Chapey C, Robert J. Reversal of multidrug resistance by a new lipophilic cationic molecule, S9788. Comparison with 11 other MDR-modulating agents in a model of doxorubicin-resistant rat glioblastoma cells. Eur J Cancer. 1993;29A(10):1377–1383. doi: 10.1016/0959-8049(93)90005-z. [DOI] [PubMed] [Google Scholar]
- 29.Zheng YM, Wang YX. Sodium-calcium exchanger in pulmonary artery smooth muscle cells. Ann N Y Acad Sci. 2007:1099, 427–425. doi: 10.1196/annals.1387.017. [DOI] [PubMed] [Google Scholar]
- 30.Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29(1):1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 31.Rao RV, Elleby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11(4):372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 32.Pan G, O'Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276(5309):111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 33.Choi SE, Kim HE, Shin HC, et al. Involvement of Ca2+-mediated apoptotic signals in palmitate-induced MIN6N8a beta cell death. Mol Cell Endocrinol. 2007;(1–2):272, 50–62. doi: 10.1016/j.mce.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Abdelrahim M, Newman K, Vanderlaag K, et al. 3,3′-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27(4):717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Kimura J. Blocking effect of bepridil on Na+/Ca2+ exchange current in guinea pig cardiac ventricular myocyte. Jpn J Pharmacol. 2001;85(4):370–375. doi: 10.1254/jjp.85.370. [DOI] [PubMed] [Google Scholar]
- 36.Kimura J, Watanabe Y, Li L, et al. Pharmacology of Na+/Ca2+ exchanger. Ann N Y Acad Sci. 2002;976:513–519. doi: 10.1111/j.1749-6632.2002.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee YS, Sayeed MM, Wurster RD. Intracellular Ca2+ mediates the cytotoxicity induced by bepridil and benzamil in human brain tumor cells. Cancer Lett. 1995;88(1):87–91. doi: 10.1016/0304-3835(94)03619-t. [DOI] [PubMed] [Google Scholar]
- 38.Madsen SJ, Hirschberg H. Site-specific opening of the blood-brain barrier. J Biophotonics. 2010;3(5–6):356–367. doi: 10.1002/jbio.200900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;5(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer H, Gottschlich R, Seelig A. Blood-brain permeation: molecular parameters governing passive diffusion. J Membr Biol. 1998;165(3):201–211. doi: 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- 41.Norden AD, Wen PY. Glioma therapy in adults. Neurologist. 2006;12(6):179–192. doi: 10.1097/01.nrl.0000250928.26044.47. [DOI] [PubMed] [Google Scholar]
- 42.Qin D, Ma J, Xiao J, et al. Effect of brain irradiation on blood-CSF barrier permeability of chemotherapeutic agents. Am J Clin Oncol. 1997;20(3):263–265. doi: 10.1097/00000421-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Qin D, Ou G, Mo H, et al. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier: clinical results. Int J Radiat Oncol Biol Phys. 2001;51(4):959–962. doi: 10.1016/s0360-3016(01)01735-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.