Abstract
This phase II study was designed to assess the safety and efficacy of gefitinib given with and following radiation therapy in children newly diagnosed with a poor prognosis brainstem glioma. Eligible patients were those with a previously untreated nondisseminated diffuse intrinsic brainstem glioma. Histological confirmation was not required, provided patients had a characteristic clinical history and MRI findings. Treatment consisted of gefitinib, administered orally, 250 mg/m2/day, during standard external beam radiotherapy, continuing for up to 13 monthly courses in the absence of disease progression or unacceptable toxicity. Toxicities, particularly intratumoral hemorrhage, were monitored. Pharmacokinetics and investigational imaging studies were performed in consenting patients. Forty-three eligible patients were included in the study. Therapy was well tolerated; only 4 patients were withdrawn from the study for dose-limiting toxicity after receiving therapy for 6, 9, 17, and 24 weeks. The 12- and 24-month progression-free survival rates were 20.9 ±5.6 % and 9.3 ±4%, respectively. Overall survival rates were 56.4 ±7.6% and 19.6 ±5.9%, respectively, which appear nominally superior to other contemporaneous Pediatric Brain Tumor Consortium trials. Three patients remain progression-free survivors with ≥36 months follow-up. The observation that a subset of children with this generally fatal tumor experienced long-term progression-free survival, coupled with recent observations regarding the molecular features of brainstem gliomas, raises the possibility that prospective molecular characterization may allow enrichment of treatment responders and improvement in outcome results in future studies of biologically targeted agents.
Keywords: EGFR, gefitinib, radiation therapy, brainstem glioma, pontine glioma, outcome
Children with diffuse intrinsic brainstem gliomas (BSGs) have a poor prognosis, with 1-year progression-free survival (PFS) rates below 25% and 5-year overall survival (OS) rates below 5%.1 These lesions typically arise in the pons and have a characteristic appearance on MRI that generally obviates the need for biopsy to establish the diagnosis in children with an appropriate clinical history.2 Other than irradiation, which often provides transient symptomatic improvement, no therapy has favorably affected outcome.3−7 A paucity of data exists to document the histology and biology of these tumors, in most cases demonstrating high-grade or infiltrating glioma.8−11
Accordingly, there is a strong need to identify new therapeutic approaches that target molecular alterations that account for the dysregulated growth of these tumors. The aberrant activation of growth signaling pathways commonly observed in malignant gliomas12−15 constitutes a promising target for therapy. In this regard, epidermal growth factor receptor (EGFR) has been strongly implicated in the development of high-grade gliomas.13−16 EGFR amplification, often with mutation and constitutive activation of the gene product, is observed in 50% of adult primary glioblastomas.13−15 EGFR amplification is less common in pediatric high-grade gliomas,17−20 including in brainstem lesions,12,21 although overexpression of EGFR is detected in many pediatric malignant gliomas without gene amplification.16,17,19,21
Gefitinib (ZD1839, Iressa, AstraZeneca) is an oral inhibitor of the EGFR tyrosine kinase, which has demonstrated activity in preclinical studies against tumors with aberrantly activated EGFR signaling.22 Studies in patients with lung cancer have demonstrated a subset of patients with disease regression and long-term control, generally in tumors with EGFR amplification or, particularly, activating mutations.23−26 Although responses have also been reported to correlate with EGFR amplification or EGFRvIII-activating mutations in adults with malignant gliomas,27 rates of response to EGFR inhibitors have in other studies been comparatively low, with no clear association between response and EGFR pathway activation status.28−32 Convincing data are lacking to support the therapeutic relevance of EGFR overexpression, in the absence of EGFR gene alterations, as a predictor of response to EGFR-targeted small molecule inhibitors in these tumors.
The phase II study reported here for children with diffuse BSG builds upon a prior Pediatric Brain Tumor Consortium (PBTC) phase I study of gefitinib with irradiation, in which this agent was generally well tolerated at a dosage of 250 mg/m2/d.33 Likewise, in adult glioma studies, gefitinib was well tolerated, with dose-limiting toxicity confined principally to the skin and gastrointestinal system.34 Given that radiotherapy is the one treatment modality that has proven, albeit limited, efficacy against BSGs, and that survival in affected patients is extremely poor,1–7 there was a strong rationale to administer irradiation immediately after diagnosis in conjunction with gefitinib. The theoretical benefit of this combination is supported by preclinical studies demonstrating radiosensitization by concurrent EGFR inhibition.35
The primary objective of this study was to assess safety and efficacy of gefitinib and irradiation in children with newly diagnosed poor-prognosis BSG. In addition, the relationship between pharmacogenetic polymorphisms and pharmacokinetics and pharmacodynamics for gefitinib was examined.
Patients and Methods
Patient Eligibility
Patients 3 to 21 years of age with newly diagnosed nondisseminated diffuse intrinsic BSG were eligible. A histopathological diagnosis was not required in the setting of characteristic MRI and clinical features.2 Other eligibility criteria included Karnofsky or Lansky performance score ≥50%, no prior chemotherapy (except corticosteroids) or radiotherapy, and adequate bone marrow, renal, and hepatic function. Patients could not be pregnant or have uncontrolled infection or a history of deep venous or arterial thrombosis within 6 weeks of study entry or be receiving enzyme-inducing anticonvulsant drugs. Patients with evidence of intratumoral hemorrhage (ITH) on pretreatment T1, T2, and gradient echo MR images were also ineligible.
The institutional review board of each participating PBTC institution approved the protocol before patient enrollment, and continuing approval was maintained throughout the study. Patients or their legal guardians gave written informed consent, and assent was obtained as appropriate at enrollment.
Studies Before and During Treatment
A complete history and physical examination, including neurological assessment, and laboratory studies were obtained before treatment and periodically thereafter. Pretreatment evaluation included: complete blood count, electrolytes, serum creatinine and blood urea nitrogen, liver function tests, fibrinogen, and β-human chorionic gonadotrophin in females of childbearing potential. MRI was obtained before therapy and at 8-week intervals during therapy to monitor for tumor size and ITH, and where feasible, MR spectroscopy, diffusion, and perfusion imaging were obtained at these time points. MRI was obtained at 3-month intervals for patients remaining on study after 13 courses of treatment. PET imaging was performed pretherapy and after 16 weeks of treatment.
Dosage, Drug Administration, and Treatment Plan
Gefitinib (250 mg/m2/d) was administered beginning at the start of irradiation, within 4 weeks of diagnosis. This dosage was selected based on the observation that 3 of 12 patients treated at the next highest dose (375 mg/m2/d) in the phase I study experienced ITH during treatment, whereas this complication was observed in only 1 of 10 patients (7 BSG and 3 nonbrainstem high-grade gliomas) treated at the 250 mg/m2/d dosage during the phase II planning period.33
Gefitinib was provided in tablets that could be dissolved in water. Patients received gefitinib once daily; dosing in relation to radiotherapy was not specified in the protocol, although consistent timing of drug administration each day was recommended. A course was defined as 4 weeks of therapy. In the absence of disease progression or dose-limiting toxicity, treatment continued for 13 courses (1 year). Patients who remained progression free at 1 year had the option to continue receiving gefitinib.
Patients received local irradiation using conventional or conformal techniques; imaging and treatment plan were centrally reviewed (Quality Assurance Review Center). A total dose of 5580 cGy was given in 180-cGy daily fractions.
Assessment of Toxicity and Dose Modifications
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 3.0. For patients experiencing grade 3 or 4 thrombocytopenia, grade 4 neutropenia, grade 3 nonhematologic toxicity, any grade 2 nonhematologic toxicity persisting for more than 7 days and considered sufficiently significant to warrant treatment interruption, gefitinib was withheld for at least 7 days and restarted at 100 mg/m2/d, provided the toxicity resolved to grade 0 or 1 within 14 days. Patients with recurrence of the toxicity and those with grade 4 nonhematologic toxicity, symptomatic ITH, or enlarging asymptomatic ITH on serial MRI scans were considered off treatment. For any toxicity requiring interruption of irradiation for more than 5 consecutive days or 10 days total, gefitinib was withheld for the remainder of irradiation but resumed thereafter.
Pharmacokinetic Studies
Pharmacokinetic studies were performed in consenting patients. Serial blood plasma samples were collected on days 10, 11, or 12 of course 1 before gefitinib administration, and at 1, 2, 4, 6, 8, 12, and 24 hours after administration. Gefitinib concentrations were analyzed by isocratic reversed-phase high performance liquid chromatography with electrospray ionization mass spectrometric detection.36 For each patient, the maximum concentration (Cmax) and the time to maximum concentration (tmax) were the observed values. A one-compartment model was fitted to the gefitinib plasma concentrations using mixed effects nonlinear regression (NONMEM version VI) and the first-order conditional estimation method with interaction.37 Model parameters for each patient were used to simulate the plasma concentration-time profile, from which area under the curve (AUC) of plasma concentration-time was calculated using the log-linear trapezoidal method.
Pharmacogenetic Studies
In consenting patients, whole blood was collected prior to treatment for genomic DNA extraction. PCR restriction fragment length polymorphism (RFLP) techniques were used to genotype patients for CYP3A4*1 and CYP3A5*3, using established primer sequences.38,39 ABCB1 polymorphisms were genotyped as reported by Zheng et al.40 Amplification products (4 µl) were sequenced using the forward PCR primer. ABCG2 polymorphisms were genotyped as described by Zamber et al.41 with the following modifications: 10 ng of genomic DNA was used as template, and annealing for exons 2 and 5 PCR reactions was at 55°C. Amplification product (4 µl) was sequenced using the forward PCR primer.
Assessment of Response and Outcome
Complete response was defined as disappearance of enhancing tumor and mass effect, and partial response as ≥50% reduction in tumor cross-sectional area using maximal bidimensional measurements on MRI. Both response categories required stable or decreasing dose of corticosteroids, and stable or improving neurologic examination, maintained for at least 6 weeks. Stable disease was defined by a stable neurologic examination and corticosteroid dose, with MR imaging meeting neither criterion for response or progression. Progressive disease was defined as worsening neurologic status or increasing steroid requirement not explained by causes other than tumor progression, >25% increase in tumor cross-sectional area on MRI, or appearance of new lesions. Because gefitinib was considered to be a cytostatic agent, with a potential lag time between initiation of therapy and maximal antitumor effect, patients were allowed to continue on therapy until maximal cross-sectional area had increased 50% from baseline, provided the patient had no symptoms of tumor progression and the treating physician and patient/family elected to continue therapy. However, progression in this subset of patients was defined as the time at which 25% increase in cross-sectional area was observed, to permit comparisons with previous studies. Patients were “off therapy” for disease progression if tumor area increased at least 50% from baseline or patients exhibited clinical symptoms from tumor enlargement.
Statistical Design:
BSG patients treated at the 250 mg/m2/d dosage level on the phase I component of the study as well as patients enrolled on the phase II component were used to establish safety and efficacy of gefitinib given with and following irradiation. An early stopping rule was incorporated for inefficacy, using a version of the sequential probability ratio test42 that monitored the failure rate beginning with the seventh failure and would stop accrual if statistical confidence (α = 0.10) developed that the regimen did not meet efficacy expectations in the context of historical data with 1-year PFS ≥15%. The historical basis for choosing this threshold was CCG-9882 for children (N = 119), with diffuse BSGs treated with hyperfractionated irradiation (7200 and 7800 cGy), in which 1-year PFS was 18.8 ±3.5%.3 We were equally concerned with falsely continuing to investigate this regimen if it was not as effective as the historical treatment (α) as with falsely rejecting the regimen for further study (β) and thus developed the design with α = 0.10 and β = 0.10. The fixed sample size for testing that the 1-year PFS rate was <15% versus ≥30% required an accrual of 40 patients, although the primary objective was to estimate the distribution of PFS, not to test whether the 1-year PFS rate was greater than 30%.
Because ITH has been a concern in children enrolled in brain tumor studies involving molecularly targeted therapy, ITH monitoring constituted a second early stopping rule, such that accrual would be terminated if an excessive number of patients experienced symptomatic ITH or enlarging asymptomatic ITH on serial MRI scans, incorporating T1, T2, and echo gradient images. Based on data from other BSG trials, in which ITH rates have ranged from 3% to 19%,6,7,43,44 an “acceptable” rate of ITH during the 13 courses of treatment was defined as ≤10% (a bleed-free survival [BFS] rate ≥90%), whereas an ITH rate of ≥ 25% (BFS ≤75%) was felt to be unacceptable. The early stopping rule was designed with an error rate for falsely concluding that the BFS is ≤90% as α = 0.10 and 95% statistical power for correctly concluding that the BFS is ≤75%.
PFS was defined as the period from diagnosis until earliest failure (disease progression or death) or last contact for patients who had not failed. Survival was measured from diagnosis until death or last contact for patients who had not failed. PFS and OS were estimated using the Kaplan–Meier method.
Results
Forty-four patients were enrolled in the study between February 2003 and November 2008; 43 were eligible and evaluable. Seven were enrolled at the 250 mg/m2/d dosage during the phase I dose-finding period, and the remainder to the phase II component of the study. Among the eligible cohort, ages ranged from 3.4 to 18.7 years at diagnosis, with a median of 7.0 years. There were 14 males and 29 females. In 37 patients, diagnosis was based solely on MRI; in 6 who underwent biopsy, diagnoses were anaplastic astrocytoma in 2, glioblastoma in 1, malignant glioma in 2, and astrocytoma not otherwise specified in 1.
Outcome
Six of 43 evaluable patients had partial response (PR) during treatment (14%, 95% CI: 5.3% −27.9%). In 4 of these patients, PR was observed immediately after radiation therapy, and these patients remained on gefitinib therapy for 6, 7, 10, and 10 courses, respectively, without progression. Three of these 4 children eventually progressed 32, 45, and 47 weeks after the start of protocol therapy, whereas 1 child remained progression free after 14 courses, at which point the patient withdrew from therapy; the patient ultimately died of tumor after 32 weeks from the last evaluation. The other 2 patients had PR during course 4 and course 7 and remained on therapy for 24 and 15 additional courses of therapy, respectively, without progression. The former child ultimately progressed during course 28, and the latter during course 22 after the therapy start date. Four patients had progressive disease during or immediately after initial therapy with radiation and gefitinib. The remaining 33 patients had stable disease during irradiation and initial gefitinib therapy.
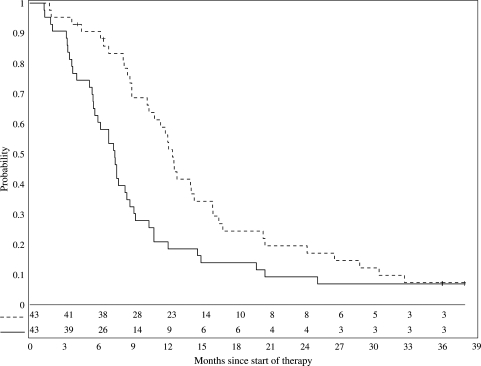
Failures and ITHs were monitored as per the statistical design and neither early stopping criteria for inefficacy nor safety were met. Kaplan–Meier estimates of the distribution of PFS is shown in Figure 1 with 1- and 2-year PFS rates of 20.9 ±5.6% and 9.3 ±4%, respectively. Median PFS was 7.4 months. OS rates were 56.4 ±7.6% and 19.6 ±5.9%, respectively (Fig. 1). Three patients, all with stable disease, remain progression-free survivors with ≥36 months follow-up. Because 2 of the 3 long-term survivors were in the subset of 10 patients who were less than 5 years of age at diagnosis, the effect of age on PFS was investigated using a Cox proportional hazards model with log transformation of age; however, no association between age and PFS was identified (p = 0.47). Dichotomizations of outcome as a function of age (<6 vs ≥6 years, and <8 vs ≥8 years) also showed no association between age and outcome (log-rank test p-values of 0.27 and 0.29, respectively).
Fig. 1.
Progression-free (solid line) and overall survival (dashed line) of 43 patients receiving gefitinib 250 mg/m2/d during and after irradiation.
Toxicity
Systemic toxicities were generally mild to moderate and reversible. Grade 3 lymphopenia was observed in 9 patients, neutropenia in 1, gastrointestinal toxicity in 4, infection in 2, and pulmonary, renal, and skin toxicity in 1 patient each. Grade 4 toxicities included 1 instance each of pulmonary, metabolic, infectious, and gastrointestinal toxicity. Grade 2 skin, gastrointestinal, and ocular toxicities were observed in 17, 13, and 10 patients, respectively. There were 3 instances of ITH classified as possibly, probably, or definitely attributable to gefitinib, 2 of which were symptomatic. Two were detected immediately after radiation therapy, and 1 at the end of course 6. The 1-year cumulative incidence of ITH ( ±SE) was 7.0% ±3.9%.
Pharmacokinetics
Serial samples for gefitinib pharmacokinetic studies were collected from 18 patients during week 2 of course 1. The median (range) gefitinib apparent oral clearance, apparent oral volume of distribution, and elimination half-life were 15.2 L/hr/m2 (9.2 to 24.9 L/hr/m2), 327 L/m2 (245 to 459 L/m2), and 15.1 hr (10.4 to 21.4 hr), respectively. The median (range) gefitinib AUC0–24, Cmax, and tmax were 16.4 μg/L*hr (10.0 to 27.1 μg/L*hr), 1.10 μg/ml (0.39 to 1.86 μg/ml), and 3.2 hr (2.0 to 6.5 hr), respectively. Pharmacokinetic parameters were available on only 1 of the long-term survivors and were close to the median values for the study: apparent oral clearance 19.4 L/hr/m2; half-life 13.3 hrs; AUC 12.9; Cmax 1.13 μg/ml.
Pharmacogenomics
Samples from 26 patients were available for pharmacogenomic studies. Six single nucleotide polymorphisms were analyzed in 4 genes of putative relevance for gefitinib absorption and disposition. For the C421A SNP in the ABCG2 gene, no homozygous variant was observed, consistent with its low frequency in Caucasians. For 18 patients in whom both genotype and gefitinib pharmacokinetic data were available, no relationship was apparent between CYP3A4*1B and CYP3A5*3 genotypes and gefitinib apparent oral clearance. Moreover, no relationship was evident between ABCG2 exon 2 (G34A), ABCG2 exon 5 (C421A), ABCB1 exon 21 (G2677T), or ABCB1 exon 26 (C3435T) genotypes and gefitinib absorption rate constant (ka) or long-term survival.
Neuroimaging Associations
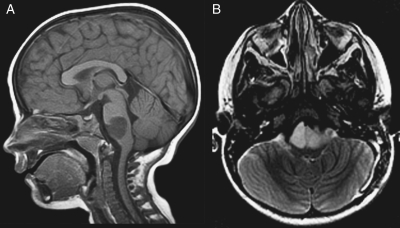
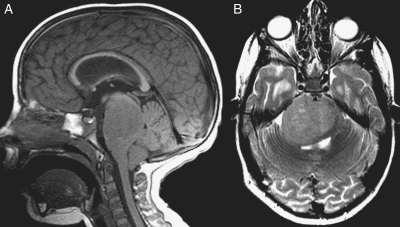
Neuroimaging data from this study are being combined with those from another PBTC phase II trial for patients with poor-prognosis BSGs (PBTC-014) and will be reported separately. However, because 3 of the patients in this series were long-term survivors, attention was directed at determining whether the tumors in these children had imaging features that differed from typical diffuse intrinsic BSGs. One tumor in a 4-year-old child had atypical features with an epicenter involving the lower pons extending into the medulla, which led to a biopsy and a resultant diagnosis of anaplastic astrocytoma (Fig. 2). The other two had characteristic imaging features for a diffuse intrinsic BSG (Fig. 3). Review of tumor size, diffusion, and perfusion values did not disclose any differences among these survivors and the remainder of the cohort (data not shown).
Fig. 2.
One long-term survivor had slightly atypical imaging features for a malignant brainstem glioma, with tumor involvement localized to the pontomedullary junction. Based upon tumor biopsy, the lesion was histologically demonstrated to be an anaplastic astrocytoma. Sagittal T1-weighted (A) and axial T2-weighted (B) MR images demonstrate a T1-hypointense and T2-hyperintense mass at the pontomedullary junction.
Fig. 3.
MR imaging of one of the 2 long-term survivors with typical imaging features of a diffuse intrinsic brainstem glioma. Sagittal T1-weighted (A) and axial T2-weighted (B) MR images demonstrate a T1-hypointense and T2-hyperintense mass in the pons surrounding the basilar artery with posterior mass effect on the fourth ventricle, moderate hydrocephalus, and tonsillar herniation.
Discussion
Gefitinib is one of the first molecularly targeted agents to be evaluated in the treatment of newly diagnosed brain tumors of childhood. Because the PBTC previously reported that a subset of high-grade intrinsic BSG overexpresses EGFR in the context of gene amplification,21 there was a strong rationale to examine the safety and efficacy of an EGFR inhibitor such as gefitinib in these tumors.
The current study demonstrated that administration of gefitinib with irradiation in children with BSGs was generally well tolerated, with an acceptable incidence of ITH, which has been a concern with other growth factor receptor inhibitors.44 The rate of ITH attributed to the study drug (7%) compares favorably with other studies involving radiation plus conventional43 or molecularly targeted chemotherapy.44 Other toxicities were generally mild and reversible. The pharmacokinetics of gefitinib are similar to those in the phase I component of this study,33 paralleling results for children with solid tumors.45 Wide interpatient variation was noted in gefitinib apparent oral clearance, maximal plasma concentrations, and area under the concentration–time curve. No relationship was apparent between ABCG2 single nucleotide polymorphisms and gefitinib absorption rate. Maximal serum concentrations were within the range at which inhibition of EGFR tyrosine kinase activity has been noted in in vitro studies.46 Although a previous study questioned whether EGFR inhibitory concentrations could be achieved in adult gliomas with oral gefitinib therapy,28 another report suggested that gefitinib undergoes preferential distribution from the blood into brain tumor tissue.47 However, a recent study demonstrated that transport of gefitinib across the blood-brain barrier may be significantly limited by active efflux proteins.48 Given the potentially distinctive features of brainstem gliomas and the impracticality of sampling to obtain tissue drug levels, it remains unknown whether effective drug concentrations were achieved in the brainstem tumor site.
Although PFS and OS data from this study are consistent with the generally disappointing therapeutic results observed for these tumors, they appear nominally more favorable than some other recent reports, albeit within the range of outcomes observed. In particular, the 2-year survival rate of 19.6 ±5.9% compares with a rate of 3% in a recent trial of topotecan plus irradiation,6 3 ±2% in a study involving radiation, etoposide, and vincristine,7 6.8 ±3.8% in a trial of imatinib plus irradiation,44 5.5 ±3.1% in a trial of zarnestra plus irradiation (unpublished data), 9.2 ±2.7% in a pooled analysis of the HIT (Hirntumor-Studie)-GBM database,49 14 ±5% in a large institutional review,50 and 14 ±5.4% in a previous study of hyperfractionated irradiation administered to a dose of 7200 cGy.3 In most of these studies, longer-term survival rates have been less than 5%. Although 3 of 43 patients (7.0%) in the current study remain progression free with 36 months of follow-up, one of these cases had atypical features, and 2 of these patients were younger than 5 years of age, of potential relevance in light of observations from some prior reports that younger children with BSGs appeared to have a more favorable outcome.49,51,52 Moreover, it is known that stringency of entry criteria in terms of duration and type of symptoms and imaging definitions required for protocol eligibility can also influence outcome interpretations,1 and it is thus important to emphasize that inferences cannot be made regarding efficacy of the regimen employed in this study versus those in other reports that may have used somewhat different criteria. Finally, since data were not systematically collected on second-line therapy among patients who had progressive disease, the impact of such interventions on the OS data cannot be assessed.
In the absence of molecular data in these tumors, it is impossible to know whether the long-term survivors had lesions that were within the small subset of BSGs that exhibit EGFR amplification.21 Studies in other tumor types indicate that tumors with activating mutations or amplification of EGFR are more likely to have favorable responses to EGFR inhibitors than tumors without EGFR alterations, particularly in the absence of other molecular features, such as PTEN deletions, which counteract sensitivity to EGFR blockade.23–26 In view of the intriguing, albeit small, percentage of patients with long-term disease control in the current study, there is some rationale to consider strategies to combine targeted therapy with molecular characterization of BSGs in future trials, to define molecular features that correlate with therapeutic efficacy. Because EGFR kinase domain mutations, which are associated with the greatest sensitivity to EGFR inhibitors,53 are uncommon in malignant gliomas,28 including those arising in children,54 it remains to be determined whether other molecular alterations involving EGFR signaling pathways may occur selectively in treatment responders.
Funding
This work was supported in part by National Institutes of Health grant U01 CA81457 for the Pediatric Brain Tumor Consortium, AstraZeneca, and the American Lebanese Syrian Associated Charities.
Acknowledgment
The authors and the PBTC acknowledge Malcolm A. Smith, MD, PhD for reviewing and providing suggestions on the manuscript, the administrative and statistical support of Ms. Dana Wallace and the clinical research assistant support of Ms. Stacye Richardson.
References
- 1.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 2.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children's Cancer Group. Neurosurgery. 1993;33:1026–1030. doi: 10.1227/00006123-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiotherapy (7200 cGy) for children with brain stem gliomas: a Children's Cancer Group phase I/II trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Jennings MT, Sposto R, Boyett JM, et al. Pre-radiation chemotherapy in primary high risk brain stem tumors: CCG-9941, a phase II study of the Children's Cancer Group. J Clin Oncol. 2002;20:3431–3437. doi: 10.1200/JCO.2002.04.109. [DOI] [PubMed] [Google Scholar]
- 5.Lefkowitz IB, Packer RJ, Sutton LN, et al. Results of the treatment of children with recurrent gliomas with lomustine and vincristine. Cancer. 1988;61:896–902. doi: 10.1002/1097-0142(19880301)61:5<896::aid-cncr2820610507>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Bernier-Chastagner V, Grill J, Doz F, et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas. Results of French Society of Paediatric Oncology phase II study. Cancer. 2005;104:2792–2797. doi: 10.1002/cncr.21534. [DOI] [PubMed] [Google Scholar]
- 7.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: A Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 8.Mantravadi RV, Phatak R, Bellur S, Liebner EJ, Haas R. Brain stem gliomas: An autopsy study of 25 cases. Cancer. 1982;49:1294–1296. doi: 10.1002/1097-0142(19820315)49:6<1294::aid-cncr2820490636>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Cartmill M, Punt J. Diffuse brain stem glioma. A review of stereotactic biopsies. Childs Nerv Syst. 1999;15:235–237. doi: 10.1007/s003810050379. [DOI] [PubMed] [Google Scholar]
- 10.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107:1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 11.Pirotte BJ, Lubansu A, Massager N, Wikler D, Goldman S, Levivier M. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107:392–399. doi: 10.3171/PED-07/11/392. [DOI] [PubMed] [Google Scholar]
- 12.Zarghooni M., Bartels U., Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 13.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey PA, Gangarosa LM, Wong AJ, et al. Deletion-mutant epidermal growth factor receptor in human gliomas: effects of type II mutation on receptor function. Biochem Biophys Res Commun. 1991;178:1413–1420. doi: 10.1016/0006-291x(91)91051-d. [DOI] [PubMed] [Google Scholar]
- 16.Khatua S, Peterson KM, Brown KM, et al. Overexpression of the EGFR/FKBP12/HIF-2α pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63:1865–1870. [PubMed] [Google Scholar]
- 17.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 18.Cheng Y, Ng HK, Zhang SF, et al. Genetic alterations in pediatric high-grade astrocytomas. Human Pathology. 1999;30:1284–1290. doi: 10.1016/s0046-8177(99)90057-6. [DOI] [PubMed] [Google Scholar]
- 19.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg: Pediatr. 2006;105:3431–3437. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 20.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.7252. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 22.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 23.Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 24.Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23:2493–2501. doi: 10.1200/JCO.2005.01.388. [DOI] [PubMed] [Google Scholar]
- 25.Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 26.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 27.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. New Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 28.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 29.Raizer JJ, Abrey LE, Lassman AB, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-Oncol. 2010;12:95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radation in patients with newly diagnosed glioblastoma multiforme. J Neuro-Oncol. 2010;98:93–99. doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 33.Geyer JR, Stewart CF, Kocak M, et al. A phase I and biology study of gefitinib and radiation in children with newly diagnosed brainstem gliomas or supratentorial malignant gliomas. Eur. J. Cancer. 2010 doi: 10.1016/j.ejca.2010.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prados MD, Yung WK, Wen PY, et al. Phase-1 trial of gefitinib and temozolomide in patients with malignant glioma: a North American brain tumor consortium study. Cancer Chemother Pharmacol. 2008;61:1059–1067. doi: 10.1007/s00280-007-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Rourke DM, Kao GD, Singh N, et al. Conversion of a radioresistant phenotype to a more sensitive one by disabling erbB receptor signaling in human cancer cells. Proc Natl Acad Sci U S A. 1988;95:10842–10847. doi: 10.1073/pnas.95.18.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai F, Iacono LC, Johnston B, Stewart CF. Determination of gefitinib in plasma by liquid chromatography with a C 12 column and electrospray tandem mass spectrometry detection. J Liquid Chromatography. 2004;27:2743–2758. [Google Scholar]
- 37.Beal SL, Sheiner LB. NONMEM Users' Guide Part I-VIII. 1998 NONMEM Project Group, University of California at San Francisco, CA. [Google Scholar]
- 38.Kadlubar FF, Berkowitz GS, Delongchamp RR, et al. The CYP3A4*1B variant is related to the onset of puberty, a known risk factor for the development of breast cancer. Cancer Epidemiol Biomarkers Prevention. 2003;12:327–331. [PubMed] [Google Scholar]
- 39.Lee SJ, Goldstein JA. Functionally defective or altered CYP3A4 and CYP3A5 single nucleotide polymorphisms and their detection with genotyping tests. Pharmacogenomics. 2005;6:357–371. doi: 10.1517/14622416.6.4.357. [DOI] [PubMed] [Google Scholar]
- 40.Zheng H, Webber S, Zeevi A, et al. The MDR1 polymorphisms at exons 21 and 26 predict steroid weaning in pediatric heart transplant patients. Human Immunology. 2002;63:765–770. doi: 10.1016/s0198-8859(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 41.Zamber CP, Lamba JK, Yasuda K, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Piantadosi S. Clinical Trials: A Methodologic Perspective. New York: John Wiley & Sons; 1997. pp. 238–239. Wiley Series in Probability and Statistics. [Google Scholar]
- 43.Broniscer A, Laningham FH, Kocak M, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer. 2006;106:1364–1371. doi: 10.1002/cncr.21749. [DOI] [PubMed] [Google Scholar]
- 44.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: A Pediatric Brain Tumor Consortium report. Neuro-Oncology. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daw NC, Furman WL, Stewart CF, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- 46.Premkumar DR, Arnold B, Pollack IF. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with 17-AAG on glioma cell growth. Molec Carcinogenesis. 2006;45:288–301. doi: 10.1002/mc.20141. [DOI] [PubMed] [Google Scholar]
- 47.Hofer S, Frei K. Gefitinib concentrations in human glioblastoma tissue. J Neurooncol. 2007;82:175–176. doi: 10.1007/s11060-006-9257-3. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of defitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)–mediated active efflux. J Pharmacol Exp Ther. 2010;334:147–155. doi: 10.1124/jpet.110.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner S, Warmuth-Metz M, Emser A, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79:281–287. doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 50.Massimino M, Spreafico F, Biassoni V, et al. Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neurooncol. 2008;87:355–361. doi: 10.1007/s11060-008-9525-5. [DOI] [PubMed] [Google Scholar]
- 51.Broniscer A, Laningham FH, Sanders RP, et al. Young age may predict a better outcome for children with diffuse pontine glioma. Cancer. 2008;113:566–572. doi: 10.1002/cncr.23584. [DOI] [PubMed] [Google Scholar]
- 52.Wolff JE, Classen CF, Wagner S, et al. Subpopulations of malignant gliomas in pediatric patients: Analysis of the HIT-GBM database. J Neurooncol. 2008;87:155–164. doi: 10.1007/s11060-007-9495-z. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen MW, Pedersen N, Ottesen LH, Poulsen HS. Differential response to gefitinib of cells expressing normal EGFR and the mutant EGFRvIII. Br J Cancer. 2005;93:915–923. doi: 10.1038/sj.bjc.6602793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bax DA, Gaspar N, Little SE, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]