Abstract
We performed a phase II study to assess the efficacy and toxicity of tipifarnib, a farnesyltransferase inhibitor, administered with radiation therapy (RT) in children with newly diagnosed diffuse intrinsic pontine gliomas. Children 3-21 years old with pontine gliomas (BSGs) were treated with concurrent tipifarnib and RT, followed by adjuvant tipifarnib. Tipifarnib was taken orally twice daily (125 mg/m2/dose) during RT; after RT, it was taken at 200 mg/m2 twice daily for 21 days, in 28-day cycles. Initial and follow-up neuroimaging was centrally reviewed. Forty eligible patients (median age, 5.5 years; range, 3.3–16.5 years) had a median progression-free survival of 6.8 months (range, 0.2-18.6 months) and median overall survival of 8.3 months (range, 0.2-18.6 months). Kaplan–Meier estimates (± standard error) of 1-year progression-free and overall survival were 12.9% ±4.9% and 34.3% ±7.4%, respectively. A single patient remained on tipifarnib without progression at the completion of the study, two years after initiation of treatment. Seven patients were without disease progression for at least six months, three of whom remained controlled for more than a year. The most frequent toxicity was grade 3 lymphopenia. We documented a single instance of “pseudoprogression” by neuroimaging review. We found no discordance among 3 approaches to defining disease progression: as interpreted by treating institutions (based on clinical status and/or imaging) and by central review (using bi-dimensional tumor “area” versus volumetric measurements). For children with diffuse BSGs, tipifarnib administered with irradiation offered no clinical advantage over historical controls. Biopsies and molecular analyses of pediatric BSGs are vital for identification of new agents and for rational use of targeted agents.
Keywords: diffuse intrinsic pontine glioma, farnesyltransferase inhibitors, pediatric
Not a single clinical trial has shown unequivocal benefit from either chemotherapy or biological agents in children with diffuse intrinsic pontine gliomas (DIPGs), who have a notoriously poor prognosis, with a median survival of <1 year.1 Only local radiotherapy has shown benefit, alleviating neurological signs and symptoms for several months and increasing survival by ∼3 months.1,2 Despite numerous attempts to intensify treatment by increasing radiation doses, altering radiation fractionation schemes, or adding chemotherapeutic agents, long-term survival rates remain <10%.2 Thus, ongoing investigations are required to improve the outcome for pontine gliomas (BSGs), which comprise 10% of all pediatric brain tumors.2
A particularly provocative strategy for potentially improving outcome for BSGs is the administration of a molecularly targeted agent to enhance the cytotoxic effects of irradiation. Of all radiosensitizers, molecularly targeted agents hold particular appeal for their selective effects on tumor cells versus normal tissues. A class of promising radiosensitizers encompasses the farnesyltransferase inhibitors (FTIs) that block an essential step in the post-translational processing of Ras and related G-proteins.3,4 FTIs affect many cellular functions that are important for tumor formation, including survival, proliferation, differentiation, cytoarchitectural integrity, and membrane trafficking. Furthermore, FTIs may not only block Ras function directly but also interrupt the effects of tyrosine kinase receptors that signal through Ras receptors that are thought to be activated in pediatric BSGs.5
Tipifarnib (R115777; Zarnestra; Johnson & Johnson Pharmaceutical Research and Development), a potent and selective, orally available, non-peptidomimetic FTI, has been shown to have single agent anti-neoplastic activity in multiple pre-clinical models and to reverse radiation resistance of human glioma cells.6,7 Phase I and II studies in various tumor types, including gliomas, have shown single-agent anti-tumor activity.8–10 In a phase I Pediatric Brain Tumor Consortium (PBTC) study, the maximum-tolerated dose of tipifarnib with concurrent radiation therapy (RT) was established as 125 mg/m2 per dose, twice daily.11 We report here results of the subsequent phase II PBTC trial that evaluated the efficacy of tipifarnib administered concurrently with and after RT in children with newly diagnosed DIPGs.
Methods
Study Aims
The primary objective of the study was to estimate the distributions of progression-free survival (PFS) and overall survival (OS) of children with newly diagnosed nondisseminated DIPGs treated with tipifarnib administered concurrently with and following radiation therapy. Secondary aims were to describe toxicities associated with this regimen of tipifarnib and irradiation. An additional secondary objective was to characterize radiographic changes in DIPGs treated with irradiation and tipifarnib using MRI.
Patient Eligibility
Children aged 3 to 21 years with newly diagnosed nondisseminated DIPGs were eligible for this study. Other eligibility criteria included (1) a Lansky performance score ≥50, (2) adequate bone marrow function (absolute neutrophil count,≥1000 cells/mm3; hemoglobin concentration, ≥8 g/dL; and platelet count, ≥100,000 platelets/mm3, (3) adequate renal function (serum creatinine normal for age or a glomerular filtration rate >70 mL/min/1.73m2) and adequate hepatic function (serum bilirubin level, ≤1.5 mg/dL; and alanine and aspartate aminotransferase levels <2.5 times the upper limit of normal).
The institutional review boards of each PBTC institution approved the protocol before initial patient enrollment; continuing approval was maintained throughout the study. Patients, parents, or legal guardians gave written informed consent, and assent was obtained, as appropriate, in accordance with local institutional review board policies.
Studies Before and During Treatment
History and physical examinations, including detailed neurological examinations, were performed before treatment, at weekly intervals during the first 8 weeks of treatment, and monthly thereafter. Laboratory evaluations were performed before treatment and at 1–4-week intervals throughout the treatment. Pretreatment neuroimaging studies consisted of standard brain MRI (T1, T2, FLAIR, and post-gadolinium T1 images), gradient echo sequences, and perfusion/diffusion MRI. These studies were repeated at 8-week intervals.
Standard tumor response criteria were applied: complete response (complete resolution on MRI of all enhancing tumor and mass effect while clinically stable or improving on a level or decreasing dose of corticosteroids, maintained for ≥6 weeks); partial response (≥50% reduction in tumor size by bi-dimensional or volumetric measurement on MRI while clinically stable or improving on level or decreasing dose of corticosteroids, maintained for ≥6 weeks); stable disease (MR imaging meeting neither criteria for partial response nor those for progressive disease (see below) while clinically at least stable on level or decreasing corticosteroid dose); and progressive disease (progressive neurologic symptoms and signs or worsening neurologic status not explained by causes unrelated to tumor progression and/or >25% increase in the bi-dimensional or volumetric MRI measurement or the appearance of a new lesion).
Thirty-eight patients had baseline MRI measurements, of whom 36 had at least 1 on-treatment MRI scan. Thirty patients had at least 2 on-treatment MRI scans, and 22 had at least 3 on-treatment MRI scans. All MRIs for each of these patients were centrally reviewed by the PBTC Neuro-Imaging Center (Children's Hospital Boston) with bi-dimensional (AP and transverse) and volumetric measurements made using a perimeter technique with the Vitrea workstation (Vital Images) on FLAIR images. Documentation of response reported by the treating institution was based on clinical status and/or bi-dimensional or volumetric measurements comparing the current lesion to the smallest tumor measurements obtained at a prior time.
We defined “pseudoprogression” as previously described in publications relevant to glioblastoma: subacute imaging changes in gliomas after radiochemotherapy that unlike true tumor progression, recover or stabilize spontaneously.12,13 Thus, in this study, pseudoprogression was defined as tumors that met the 25% threshold for progressive disease either by MRI volume or area but improved spontaneously to a size of stable disease or smaller compared to initial imaging on subsequent scans.
RT and Drug Administration
Tipifarnib (125 mg/m2) was administered orally twice daily. Dosing was initiated 1–2 days before the start of RT and continued without interruption throughout the course of radiotherapy. After a 2-week break, at approximately week 9, patients resumed taking tipifarnib at 200 mg/m2 per dose orally twice daily in 28-day courses. Tipifarnib was administered on days 1–21 of each course followed by a 1-week break. In the absence of disease progression or unacceptable toxicity, patients could continue to receive tipifarnib for up to 2 years.
Patients received local irradiation using conventional or conformal, volume-based delivery techniques. The gross tumor volume was defined as the abnormal signal on MRI (usually T2-weighted, but a combination of T1 with contrast and T2 was acceptable). The clinical target volume for subclinical disease was defined as a 1.5-cm anatomic margin beyond the gross tumor volume, targeting at least the entire anatomic section of the brainstem within the clinical target volume. This margin was constrained by the anatomic limits of brain structures and skull. The planning target volume was an institution-specific margin to allow for daily patient set-up uncertainties (typically 3–5 mm). Treatment was administered once daily, 5 days per week, to a total dose of 55.8 Gy using conventional fractionation of 1.8 Gy per daily fraction.
Toxicity Monitoring and Dose Modifications
Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) scale. Dose-modifying toxicities were defined as grade 4 neutropenia, grade 3 or 4 thrombocytopenia, any grade 3 or 4 nonhematologic toxicity except skin toxicity, grade 3 or 4 skin toxicity that persisted for >7 days despite withholding tipifarnib and treatment with topical agents and oral prednisone, grade 2 skin rash that progressed to grade 3 or greater despite treatment, symptomatic intra-tumoral hemorrhage or progressive asymptomatic hemorrhage, any toxicity that required interruption of radiation therapy for >5 consecutive days or 10 days total, or failure to recover sufficiently from toxicities to be eligible for resumption of tipifarnib within 2 weeks of receipt of the last dose of drug.
Statistical Considerations
OS was defined as the interval between initiation of treatment and death on study. PFS was defined as the interval between initiation of treatment and the earliest of either progressive disease or death during the study for patients whose treatment failed. Patients without failure for PFS or OS were censored at the date off-study or at the last date of follow-up. Distributions of PFS and OS were estimated using Kaplan–Meier method, and survival distributions were compared using log-rank test. Although the primary study objective was to estimate the distribution of PFS, the statistical design included a sequential probability ratio test for early stopping if there was statistical evidence that the true 1-year PFS rate was <0.15 (α = 0.10; 1-sided test). The design, which required 40 patients, had 90% statistical power to detect a 1-year PFS rate of 0.30. Patients who were treated at the maximum-tolerated dose of the phase I PBTC study were included in this phase II analysis. The eligibility, dose modification criteria, and response criteria were the same for both the phase I and II trials.
Results
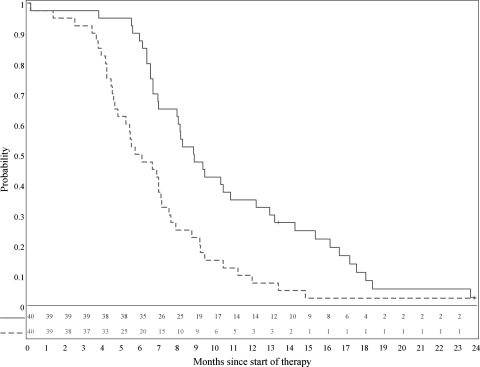
A total of 40 patients were included in the phase II analyses; 6 were accrued during the phase I portion of the study and were reported as part of the phase I manuscript11 and 34 were accrued in the phase II component. Of the 40 eligible patients, 25 (62.5%) were female and 15 (37.5%) were male; the median age was 5.5 years (range, 3.3–16.5 years), and the median Karnofsky Lansky Score was 70 (range, 50–100). The median PFS for the 40 eligible patients was 5.9 months (range, 0.2–23.9 months), and the median OS was 8.9 months (range, 0.2–23.9 months). One-year PFS and OS estimates (±standard error) were 7.5% ±3.6% and 35% ±7.3%, respectively (Figure 1). PFS and OS rates (±standard error) at 18 months were 2.5% ±1.8%) and 10% ±4.2%, respectively. The stopping criterion for inefficacy was not met during the accrual period.
Fig. 1.
Overall survival (solid line) and progression-free survival (dashed line) distributions.
The duration of presenting signs and symptoms varied among patients; onset occurred at a median of 6 days (range, 0-55 days) before the date of diagnosis. In decreasing order of frequency, these presenting signs and symptoms included cranial and motor neuropathies, ataxia, speech impairment, double vision, nystagmus, and mood alterations.
Tables 1 and 2 list toxicities that were at least grade 3 in severity and were considered possibly, probably, or definitely related to tipifarnib use. Those toxicities that occurred during course 1 and course 2 (the dose-limiting toxicity observation period for the phase I trial of PBTC-014) are shown in Table 1, whereas those toxicities that occurred later during therapy are shown in Table 2. The most frequent toxicity was lymphopenia: all cases but 1 were reported to be grade 3, and all patients but 1 received steroids prior to experiencing lymphopenia, a factor contributing to this toxicity.
Table 1.
Grade 3–5 toxicities during dose-limiting toxicity observation period (courses 1 and 2) at least possibly attributable to tipifarnib
| Toxicity | No. of events (no. of patients) |
||
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | |
| CNS hemorrhage | 1 (1) | ||
| Seizure | 1 (1) | ||
| Encephalopathy | 1 (1) | ||
| Anorexia | 1 (1) | ||
| Transaminase elevations | 2 (2) | ||
| Infection with normal ANC or grade 1 or 2 neutropenia | 2 (2) | ||
| Ureteral obstruction | 1 (1) | ||
| Leukopenia | 1 (1) | ||
| Lymphopenia | 13 (12) | 1 (1) | |
| Hyponatremia | 1 (1) | ||
| Hypomagnesimia | 1 (1) | ||
| Rash/desquamation | 1 (1) | ||
Dosage was 125 mg/m2 per dose twice daily with concurrent radiation. ANC indicates absolute neutrophil count.
Table 2.
Grade 3–5 toxicities after dose-limiting toxicity observation period (courses 3 +) at least possibly attributable to tipifarnib
| Toxicity | No. of events (no. of patients) |
||
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | |
| Transaminase elevations | 1 (1) | ||
| Ataxia | 1 (1) | ||
| Low serum bicarbonate level | 1 (1) | ||
| Febrile neutropenia | 2 (2) | ||
| Infection with normal ANC or grade 1 or 2 neutropenia | 1 (1) | ||
| Neurology–Kluver-Bucy syndrome | 1 (1) | ||
| Leukopenia | 4 (2) | 1 (1) | |
| Lymphopenia | 11 (7) | ||
| Anemia | 2 (1) | ||
| Neutropenia | 3 (2) | 3 (2) | |
| Thrombocytopenia | 1 (1) | ||
| Hypokalemia | 2 (2) | ||
| Hyponatremia | 1 (1) | ||
Dosage was 125 mg/m2 per dose twice daily following radiation therapy. ANC indicates absolute neutrophil count.
Nonhematologic grade 4 toxicities included low serum bicarbonate, seizure, and encephalopathy (1 case each). The single grade 5 toxicity consisted of a central nervous system hemorrhage. This grade 5 hemorrhage occurred in a 4-year-old girl with BSG who had unusually rapid clinical deterioration and radiographic progression before enrollment on study. CNS hemorrhage resulted in death after 2 days of tipifarnib therapy, prior to commencement of RT. The patient had no thrombocytopenia, but tipifarnib could not be excluded as a contributing factor.
Seven patients experienced intratumoral hemorrhage, 1 case of which occurred before initiation of protocol treatment. Of the remaining 6 intratumoral hemorrhages, 4 occurred during the first 2 courses of treatment, and 2 occurred after completion of RT. Except for the case of grade 5 intratumoral hemorrhage described above, all cases were asymptomatic lesions noted on imaging.
Although the starting dose of tipifarnib was 125 mg/m2 per dose for all patients, 1 patient required a toxicity-related dose reduction due to rash. One patient continued to receive tipifarnib without evidence of progression or unacceptable toxicities at the completion of the study, 24.2 months (727 days) after initiation of treatment. At the first disease assessment, ∼8 weeks after initiation of treatment, 1 patient experienced disease progression. At this 8-week disease evaluation, 5 patients had documented partial responses, and 34 patients had stable disease by institutional call. At the second disease evaluation, ∼16 weeks after start of treatment, 2 additional patients had partial responses. Seven patients were without disease progression clinically or radiographically for at least 6 months, 3 of whom had disease controlled for >1 year.
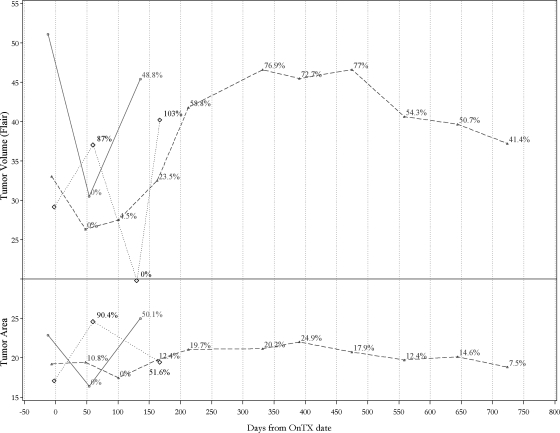
Central neuroimaging review revealed that 1 patient had a >25% increase in tumor size at the completion of irradiation at week 8, followed by 25% decrease on the next scheduled scan at week 16, thus meeting the criteria for “pseudo-progression” (Figure 2). On the basis of central review, 3 patients at week 8 showed progressive disease by MRI; for 2 of them, the treating site reported progressions as well, one concurrently and another 20 weeks later; the third patient withdrew from therapy without institutional documentation of progression at week 17. At the scheduled week 16 scan, central reviews of MRIs showed progression for 14 additional patients, of which 5 cases were synchronous with progression reports by the treating sites. One patient withdrew from therapy without institutional documentation of progression at week 20. For the remaining 8 patients, progression was reported by the treating site at a median of 16 weeks (range, 3.7–34 weeks) later than documented by central review.
Fig. 2.
Examples of volumetric (above) and bi-dimensional or area (below) central imaging measures (provided in percentage compared with the smallest measurement in serial MRI scans during a patient's on-study time) and the duration of a patient's “on study” interval. The solid lines represent a patient who progressed early by both volumetric and area criteria (on the same scan) and was called “progressive disease” by the treating institution at the same time. The dashed lines represent a patient who progressed by volumetric measurements on day 210 but never met criteria for progressive disease by bi-dimensional measurements or clinical criteria; he remained in the study and receiving treatment for >103 weeks (725 days) and completed all therapy per protocol. The dotted lines represent the patient who had “pseudo-progression”.
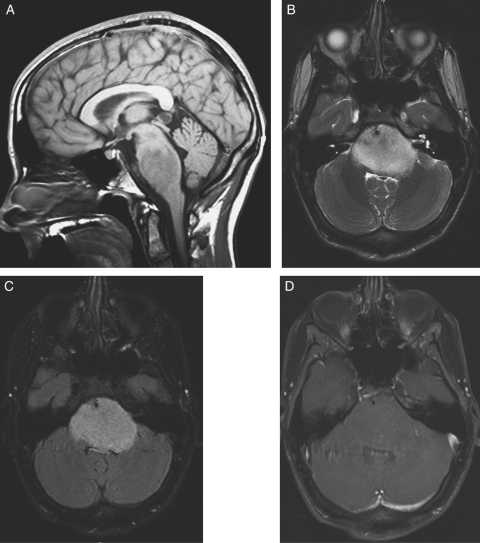
Overall, of 36 patients with at least 1 on-treatment MRI, 29 patients met the 25% threshold for progressive disease by central review, of which 11 cases (38%) were synchronous with progression reported by the treating sites. For 4 patients, the treating sites never reported progression; 3 of these 4 patients discontinued tipifarnib therapy without institutional documentation of progression and died 3, 6, and 6 months after discontinuation of therapy. The fourth patient for whom the treating site never reported progression completed tipifarnib therapy per protocol and is still alive 24.2 months after initiating therapy. This patient's MRI is shown in Figure 3. For the remaining patients, treating sites reported progression at a median of 14 weeks later (range, 3.7–40.4 weeks) documented by central review.
Fig. 3.
MRI for long-term survivor. (A) Sagittal T1 image demonstrates expansile T1 hypointense mass in pons with mass effect on fourth ventricle. (B) Axial T2 and (C) FLAIR images demonstrate expansile mass in the pons with mass effect on the fourth ventricle surrounding the basilar artery. (D) Axial T1 image with gadolinium demonstrates nonenhancing pontine mass.
Figure 2 shows an example of bi-dimensional and volumetric measurements for a patient whose condition progressed by both criteria on the same scan and who discontinued treatment at that time (Figure 2). Also shown is an example from a patient whose condition progressed at week 30 by volumetric but not by bi-dimensional measurements or clinical criteria. This patient continued to receive treatment, with clinical stability, for 104 weeks despite earlier progression by volumetric criteria; he completed all therapy per protocol, and remains alive as above (Figure 2).
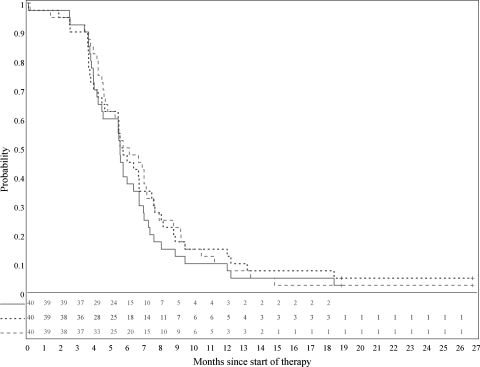
Four patients had progression noted by volumetric measurements but never had progression noted by bi-dimensional measurements, whereas 1 patient demonstrated progression by bi-dimensional measurements without reaching the threshold for progression by volumetric analysis. In 4 cases, disease progression was seen by bi-dimensional measurements earlier than by volumetric measurements, whereas in 6 cases, tumor volume indicated progressive disease earlier than tumor area; in each of the 10 cases, the opposite metric subsequently confirmed progression. In sum, Figure 4 demonstrates no differences in PFS regardless of whether progressive disease was defined by central documentation of either increased tumor volume or area or by the treating investigator's summary evaluation of clinical and imaging parameters.
Fig. 4.
Progression-free survival estimates based on central review tumor volume (solid line), central review tumor area (dashed line), and evaluations by treating sites (dotted line).
Discussion
Tipifarnib has been studied for the treatment of adult gliomas by the North American Brain Tumor Consortium (NABTC), yielding promising evidence of activity.10,14 PFS was 9 weeks among 41 patients who were not receiving enzyme-inducing anti-epileptic drugs and 6 weeks among 23 patients who were receiving enzyme-inducing anti-epileptic drugs. Such modest activity against recurrent adult GBM prompted study of this targeted agent in pediatric gliomas. Despite initially encouraging results of tipifarnib for adult recurrent malignant gliomas, the data for adults, as for the results presented herein, proved lackluster as the subjects matured.
Key to the rationale of this study is the ability of FTIs in general and tipifarnib in particular to sensitize human cancer cells to irradiation, specifically if they harbor Ras mutations or exhibit elevated Ras activity due to constitutive activation of upstream signals. Upstream signaling molecules are indeed activated in pediatric BSGs as evidenced by grade-dependent amplification and over-expression of ERBB1.5
Despite a compelling scientific rationale, this phase II study failed to show a clinical benefit for tipifarnib administered with and after radiation therapy in pediatric BSGs. One-year PFS and OS rates of 12.9% and 34.3%, respectively, are not very different from historical control data. A recent Children's Oncology Group (COG) clinical trial for children with diffuse BSGs treated with oral VP-16 plus vincristine along with standard radiotherapy reported 1-year OS of 27% ± 7% and a 2-year OS of 3% ± 2%, which are rates similar to those observed in this Phase II trial.15
This disappointing outcome parallels the most recent published study of tipifarnib in adult gliomas. Lustig et al.16 reported results of a study in which 28 patients with newly diagnosed GBM and residual enhancing disease after surgery received tipifarnib prior to radiation. This study was stopped early due to disease progression in nearly one-half of the patients and no evidence of measurable responses or improvement in survival.
We evaluated measurements of DIPGs in this trial by central review using 2D and 3D volumetric techniques and correlated these with the time that progressive disease was declared by the physician clinical investigators in their respective institutions. We used FLAIR images for tumor measurement, because earlier studies suggested that FLAIR sequences are most likely to demarcate tumor boundaries.17 A computer-assisted perimeter method was used for tumor volume calculations because of its high reproducibility for determining brain tumor volume.18 When comparing central imaging and institutional assessments of tumor progression, whether by bi-dimensional or volumetric measures, we found no differences in PFS defined by tumor volume, tumor area, or determination of progressive disease by the treating institutions. Sorensen et al.19 reviewed response criteria in adult malignant gliomas and concluded that volumetric measures were more reflective of true “response” than bi-dimensional measurements; they recommended the inclusion of clinical criteria (eg, changes in neurologic status and steroid dosage) in addition to neuroimaging in determining response in adult malignant gliomas. The interaction of imaging metrics and clinical factors, included in defining response in the current protocol, may explain differences in timing of disease progression by imaging and the determination of progression by the treating institutions. This differs from earlier data in related studies of tumor measurement suggesting that volumetric analyses may be superior for measuring lesion size, compared with simple cross-sectional area, because of irregular tumor shapes.19,20
As has been determined for adults with brain tumors, updated response assessment criteria for children with BSGs are needed to develop new standardized response criteria for clinical trials of these patients.21 Correlations among clinical and imaging factors when determining response and progression in children with BSGs are undergoing additional analysis from this and other PBTC studies and warrant further study.
The lack of clinical benefit observed in this trial highlights the pressing need for comprehensive biological studies of pediatric BSGs. Pediatric and adult gliomas are known to differ in their molecular pathogenesis and determinants of response to therapy.22,23 For example, microsatellite instability, a molecular marker for defective DNA mismatch repair genes, occurs more commonly in pediatric gliomas than in adult tumors.24 This may explain why temozolomide, which requires intact DNA mismatch repair function to exert cytotoxicity, prolongs survival in adult patients but shows little benefit in pediatric gliomas.25 Importing novel agents from adult trials into pediatric practice has been an excellent starting point and has propelled clinical and scientific investigations of pediatric brain tumors. However, pediatric BSGs clearly represent a unique genetic entity, and therefore biopsies and molecular analyses of pediatric BSGs are vital to the intelligent and rational utilization of targeted agents.
Acknowledgments
We and the PBTC acknowledge the study management and coordination of Ms Stacey Richardson.
Conflict of interest statement. None declared.
Funding
National Institutes of Health (U01 CA81457 for the Pediatric Brain Tumor Consortium), Brain Tumor SPORE grant (P50 CA097257 to D.H.K.), National Center for Research Resources (M01 RR00188), American Lebanese Syrian Associated Charities, and The Nancy and Stephen Grand Philanthropic Fund (to D.H.K.).
References
- 1.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40:265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 2.Fangusaro J. Pediatric high-grade gliomas and diffuse intrinsic pontine gliomas. J Child Neurol. 2009;24(11):1409–1417. doi: 10.1177/0883073809338960. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast GC. Farnesyltransferase inhibitors: antineoplastic mechanism and clinical prospects. Curr Opin Cell Biol. 2000;12:166–173. doi: 10.1016/s0955-0674(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 4.Prendergast GC, Oliff A. Farnesyltransferase inhibitors: antineoplastic properties, mechanisms of action, and clinical prospects. Semin Cancer Biol. 2000;10:443–452. doi: 10.1006/scbi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 6.Delmas C, Heliez C, Cohen-Jonathan E, et al. Farnesyltransferase inhibitor, R115777, reverses the resistance of human glioma cell lines to ionizing radiation. Int J Cancer. 2002;100:43–84. doi: 10.1002/ijc.10439. [DOI] [PubMed] [Google Scholar]
- 7.Jones HA, Hahn SM, Bernhard E, et al. Ras inhibitors and radiation therapy. Semin Radiat Oncol. 2001;11:328–37. doi: 10.1053/srao.2001.26020. [DOI] [PubMed] [Google Scholar]
- 8.Moyal EC, Laprie A, Delannes M, et al. Phase I trial of tipifarnib (R115777) concurrent with radiotherapy in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;68:1396–1401. doi: 10.1016/j.ijrobp.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer. 2007;110:2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 10.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 11.Haas-Kogan DA, Banerjee A, Kocak M, et al. Phase I trial of tipifarnib in children with newly diagnosed intrinsic diffuse brainstem glioma. Neuro Oncol. 2008;10:341–347. doi: 10.1215/15228517-2008-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 13.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for Differentiation of Pseudoprogression from True Tumor Progression with Dynamic Susceptibility-weighted Contrast-enhanced Magnetic Resonance Imaging using Ferumoxytol vs. Gadoteridol: A Pilot Study. Int J Radiat Oncol Biol Phys. 2011;79:514–523. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Kuhn J, Robins HI, et al. Phase I trial of tipifarnib in patients with recurrent malignant glioma taking enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2005;23:6647–6656. doi: 10.1200/JCO.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 15.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 16.Lustig R, Mikkelsen T, Lesser G, et al. Phase II preradiation R115777 (tipifarnib) in newly diagnosed GBM with residual enhancing disease. Neuro Oncol. 2008;10:1004–1009. doi: 10.1215/15228517-2008-070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayward RM, Patronas N, Baker EH, et al. Inter-observer variability in the measurement of diffuse intrinsic pontine gliomas. J Neurooncol. 2008;90:57–61. doi: 10.1007/s11060-008-9631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19:551–557. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29:419–424. doi: 10.3174/ajnr.A0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 22.Okada H, Low KL, Kohanbash G, et al. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88:245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 24.Szybka M, Bartkowiak J, Zakrzewski K, et al. Microsatellite instability and expression of DNA mismatch repair genes in malignant astrocytic tumors from adult and pediatric patients. Clin Neuropathol. 2003;22:180–186. [PubMed] [Google Scholar]
- 25.Burzynski SR. Treatments for astrocytic tumors in children: current and emerging strategies. Paediatr Drugs. 2006;8:167–178. doi: 10.2165/00148581-200608030-00003. [DOI] [PubMed] [Google Scholar]