Abstract
Supratentorial ependymomas account for a minority of intracranial ependymomas, which still have uncertain prognostic markers. Among them, epidermal growth factor receptor (EGFR) overexpression correlates with a poor prognosis. In glioblastoma cells, EGFR function has been reported to be regulated by its migration from cell membrane infoldings called caveolae and by its colocalization with the caveolae-associated protein caveolin-1 (cav-1). Therefore, we decided to investigate cav-1 expression and coexpression with EGFR in a series of adult intracranial ependymomas. We analyzed 22 adult supratentorial ependymomas and compared tumor grades as determined by the WHO classification and patient survival rates with the expression of EGFR, cav-1, and p53 and the values of the proliferation marker Ki-67, all tested by immunohistochemistry; in addition, we investigated the mutational profile of cav-1. The results demonstrate that the tumor grade is directly correlated with EGFR, Ki-67, and cav-1 expression only, whereas (by univariate analysis) the expression of all the studied markers, as well as the tumor histological grade, significantly correlated with the patient's overall survival (OS). By multivariate analysis using the Cox proportional hazards model, among all variables considered, cav-1 was the only independent prognostic marker related to OS (relative risk = 13.92; P = .013). Among grade II ependymomas, only cav-1 correlated with poor OS (P = .011), distinguishing 2 distinct subgroups of tumors with different outcomes despite sharing identical grading. All the patients studied carried wild-type cav-1 sequences, demonstrating that cav-1 overexpression is not driven by activating mutations, as previously reported in other tumor types. Interestingly, after stratifying all cases into 4 distinct groups according to cav-1 and EGFR expression (cav-1+/EGFR+, cav-1−/EGFR−, cav-1+/EGFR−, and cav-1−/EGFR+), the coexpression of cav-1 and EGFR identified a subset of patients with definitively poor prognoses. Further studies are needed to support this evidence on a larger scale and to clarify how cav-1 and EGFR interaction can influence tumor aggressiveness.
Keywords: caveolin-1, EGFR, ependymomas, IHC, survival
Adult ependymal tumors can have either an intracranial or a spinal localization, and a different gene expression profile between intra- and extracranial neoplasms has been reported recently, definitively identifying 2 distinct neoplastic entities.1 Adult intracranial ependymomas account for 2% of all adult intracranial tumors,2 and this site is usually associated with a more aggressive clinical behavior.3 Except for the location, a definite relationship among morphological features, phenotypic and/or molecular markers, and patient outcomes remains controversial. The influence of the tumor grade seems to be relevant in predicting prognoses,2,4–9 despite conflicting results reported in the literature as a consequence of interobserver variability and World Health Organization (WHO) grading system criteria that are equivocal when distinguishing between classic (grade II) and anaplastic ependymomas (grade III).10,11 However, within grade II ependymomas, distinct groups of lesions with different prognoses can be envisaged. Several putative prognostic indicators, such as Ki-67 and p53, have been tentatively considered to aid in distinguishing prognostic categories for ependymomas, but a reliable marker is still lacking.6,9,12–20 Recently, Kuncova et al.10 performed a systematic review and meta-analysis of the published data on immunohistochemical prognostic markers in intracranial ependymomas, and they highlighted the role of MIB-1 as the main prognostic marker that is significantly related to poorer survival (with a cut-off value varying from 1% to 25% in different studies). Similarly, epidermal growth factor receptor (EGFR) immunohistochemical overexpression has been reported to be an independent prognostic marker in intracranial ependymomas: it is useful to stratify patients within Grade II lesions21 and is correlated with a reduced progression-free survival.12 As reported for gliomas,22 a genetic characterization of ependymomas may be informative in order to define distinct prognostic categories. This has been recognized among astrocyte-derived tumors based on the switch-on of specific genes involved in mesenchymal, proliferative, or proneural differentiation,22–24 and specifically, a mesenchymal phenotype has been identified as a predictor of a poorer prognosis.22 An alternative approach can be achieved at the protein level by tracing the immune-phenotypic profile using antibodies directed toward antigens expected to be overexpressed because of the known gene activation. Using such an approach, we discovered that a major component of cell membrane scaffolds, caveolin-1 (cav-1), which is highly (but not exclusively) expressed in mesenchymal/endothelial cells, bears diagnostic and prognostic significance in astrocytic tumors and oligodendrogliomas.25,26 The role of cav-1 in neoplastic cells of various origins is to date debated and controversial because it can act as a tumor promoter or inhibitor.27–29 Recently, a cav-1 gene mutation (P132L) has been reported to increase tumor aggressiveness and to promote cell migration and invasiveness through an interaction with new signaling protein partners that normally do not interact with cav-1 wild type (WT).30
In glioblastoma cells, in vitro experiments demonstrated that cav-1 is upregulated and colocalizes with EGFR.31 Such a pattern of expression may be significant to the functional status of the receptor and therefore could bear biological and therapeutic relevance, deserving further investigation in different glial tumors, such as ependymomas.
On the basis of this evidence, we investigated, in a series of adult intracranial ependymomas, (i) whether cav-1 expression varies according to tumor grade and Ki67, p53, and EGFR expression, (ii) whether cav-1 alone and/or in association with other markers may be useful in predicting patient survival and in distinguishing different outcome subgroups among tumors of an equivalent grade (II), and (iii) whether cav-1 overexpression is driven by an activating mutation.
Materials and Methods
Patients
A series of 22 adult intracranial ependymomas (operated on between December 2001 and December 2006 in the Neurosurgery Unit of the San Giovanni Battista-Molinette Hospital of Turin, Policlinico Le Scotte of Siena, Policlinico G. Rodolico of Catania, and Neurosurgery Unit of the Cannizzaro Hospital of Catania) were collected. The patients’ ages ranged from 18 to 63 years (mean age, 38.7 years); 6 were male and 16 were female. Biopsies and relapsed tumors were excluded from the study. All cases were reviewed by 2 independent pathologists, and the histological diagnoses were reconfirmed. The tumors were classified according to the WHO Classification of Brain Tumors (2007 edition). Fourteen of 22 (63.7%) cases were Grade II ependymomas, and 8 of 22 (36.3%) were diagnosed as Grade III ependymomas (anaplastic ependymomas).
Surgical treatment of patients consisted of gross total or incomplete resection followed by postoperative irradiation depending on the high grade or incomplete resection of the tumor through a standardized therapeutic approach.
Table 1 details the clinical and the anatomical features of the cases examined.
Table 1.
Clinical and histological features of 22 patients
| Characteristic | Number of patients |
|---|---|
| Age (y) | |
| Range | 18–63 |
| Mean | 38.7 |
| Sex | |
| Men | 6 |
| Women | 16 |
| Tumor location | |
| Ventricle (lateral or IIIth) | 13 |
| Parenchymal | 9 |
| Extent of surgery | |
| Gross total removal | 10 |
| Incomplete resection | 12 |
| WHO histology | |
| Grade II | 14 |
| Grade III | 8 |
| Follow-up (mo) | |
| Range | 13–109 |
| Median | 58.5 |
Follow-up
The follow-up data were retrieved from the neuro-oncologists’ clinical records. Overall survival (OS) was determined as the time from the date of initial diagnosis to the date of patient death.
The follow-up data were obtained for all patients. At the end point of follow-up, 13 of 22 (59%) patients were alive, whereas the remaining 9 of 22 patients (41%) had died within a period of 1–12 months after surgery. Among the living patients, follow-up ranged from 13 to 109 months with a mean of 58.5 months.
The study was performed according to the standards of the Institutional Ethical Committee and the Helsinki Declaration of 1975 as revised in 1983. The Institutional Review Board of our hospital approved the study. All tissue samples were anonymized by staff who were not involved in the study, according to the published procedures.32
Immunohistochemical Analysis
For each case, the surgical specimens were fixed in 4% buffered formaldehyde, routinely processed, and paraffin embedded. Three-micrometer-thick sections were prepared and routinely stained with hematoxylin and eosin (H&E); additional sections, collected on superfrost plus slides, were used for immunohistochemical analysis. Immunohistochemical reactions using antibodies anti-Ki-67 (monoclonal antibody, clone Mib1, diluted 1:100; Dako), anti-p53 (monoclonal antibody, clone D0-7, prediluted; Ventana-Diapath), anti-EGFR (monoclonal antibody, clone 31G7, diluted 1:50; Zymed), and anti-cav-1 (rabbit polyclonal antibody, diluted 1:350; Santa Cruz) were performed in an automated immunostainer (Ventana BenchMark Auto-Stainer, Ventana Medical Systems). A double cav-1/EGFR immunoreaction was also performed in the 8 cases that were positive for both markers to recognize their possible colocalization. Briefly, sections were stained for EGFR in the Ventana automated immunostainer, washed in phosphate buffered saline (PBS) solution, and treated with citrate solution for 10 min at 98°C. The slides were then incubated with polyclonal anti-cav-1 antibody (diluted 1:300, Santa Cruz) and subsequently with a biotinylated secondary antibody and alkaline phosphatase-conjugated streptavidin (Dako), both for 30 min at room temperature. Slides were washed in PBS solution and counterstained in alkaline phosphatase blue substrate. Ki-67 and p53 immunohistochemical variables were scored by evaluating the percentage of stained nuclei, counting positive nuclei of 10 separate fields at a ×400 magnification in the tumor areas with the highest density of positive nuclei. EGFR and cav-1 immunoreactivity was determined as a percentage of positive cells (0%–100%), applying a semiquantitative score determined on the evaluation of the whole histological section. Appropriate positive and negative controls for all of the studied antibodies were used. Pathologists carrying out these analyses were blinded to the clinical outcomes of patients.
Mutational Analysis
Formalin-fixed paraffin-embedded tumor sections were collected for all the cases enrolled in this study. Genomic DNA was extracted as described previously, and DNA quantification was checked using a spectrophotometer. The occurrence of cav-1 point mutations was assessed. The analysis included the full cav-1 coding sequence. Design and synthesis of exon-specific and sequencing primers and the PCRs were performed as described33, and amplicons were directly sequenced in an automatic genetic analyzer (CEQ 8800, Genetic Analyzer, Beckman-Coulter). The results of mutated samples were validated at least twice, starting from independent PCRs.
Statistical Analysis
All data were analyzed with Statistical Package for the Social Sciences version 17 (SPSS Inc.). Statistically significant P-values were defined as <.05.
Correlations between the immunohistochemical results for the various markers (with continuous readouts) and the ependymoma histological grades were evaluated using a Wilcoxon rank-sum test, whereas the associations between the different immunohistochemical markers were assessed by a Kendall correlation.
Univariate survival analysis was analyzed using a Mantel–Cox test34 and depicted using the Kaplan–Meier method.35 Multivariate survival analysis was performed by the Cox proportional hazards model with a backward stepwise selection procedure. To perform the survival analysis, since the standard cut-off values for p53, cav-1, and EGFR have not been set in the literature, we decided to use the median value as a cut-point for each variable, as follows: p53 = 8%, cav-1 = 30%, and EGFR = 20%. The Ki-67 value was set according to the literature (Ki-67 = 1%).36
Results
Immunohistochemical Results: Correlations Between Markers and Tumor Grade and Among Markers
Statistical analysis demonstrated a correlation between ependymoma histological grades and Ki-67 (P= .035), cav-1 (P= .001), and EGFR (P= .001). No correlation was observed between tumor grade and p53 immunoreactivity (P= .18).
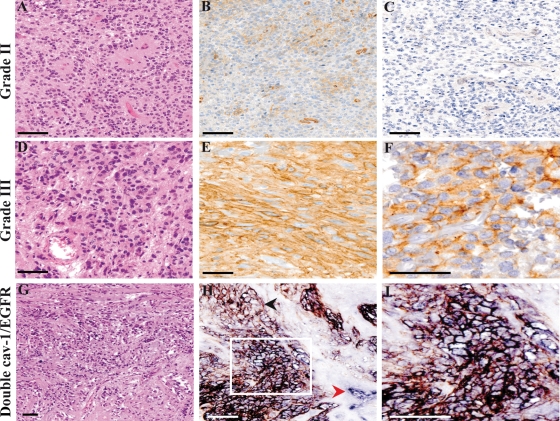
The cav-1 staining was localized both at the cell membrane and within the cell cytoplasm; however, in Grade III tumors, the membrane staining was prevalent and more intense. The patterns of cav-1 immunoreactivity are shown in Fig. 1.
Fig. 1.
Cav-1 and EGFR expression in ependymomas according to tumor grade. One case of Grade II ependymomas (A, H&E, 2×0) that proved to be negative for cav-1 staining (B, ×20). As for the majority of Grade II ependymomas studied, immunohistochemistry for EGFR was negative as well (C, ×20). One case of a Grade III ependymoma (D, H&E, ×20) from our series displaying an intense cav-1 membrane staining (E, ×20) and a diffuse EGFR positivity (F, ×40). In this case of a Grade III ependymoma (G, H&E, ×10), in the majority of positive cells, cav-1 (blue staining) and EGFR (brown staining) were colocalized on the cell membrane, resulting in a black staining (H, ×20; I, ×40). The red arrowhead indicates the internal positive control for cav-1 (blood vessel, in blue); the black arrowhead indicates a group of EGFR-positive/cav-1 negative cells (brown staining). An area of cav-1 and EGFR coexpression is shown in the white frame (bar = 100 μm).
A correlation between the studied markers indicated that cav-1 expression significantly correlated with Ki-67 (P= .004), EGFR (P< .001), and p53 (P= .001) immunoreactivity. In all of the 8 cases that were both EGFR and cav-1 positive, a double cav-1/EGFR immunoreaction was performed as well, which showed that cav-1 and EGFR immunoreactivity colocalized in approximately 60% of the tumor cells (Fig. 1G–I). In addition, Ki-67 expression significantly correlated with p53 positivity (P= .009). Aside from these, no other significant correlations between the remaining immunohistochemical markers studied were found.
Impact of Marker Expression on Survival
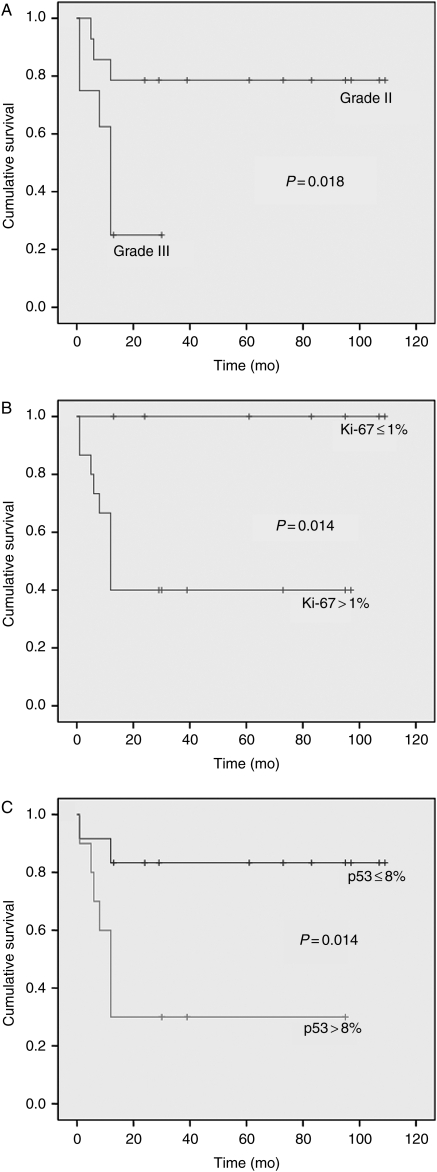
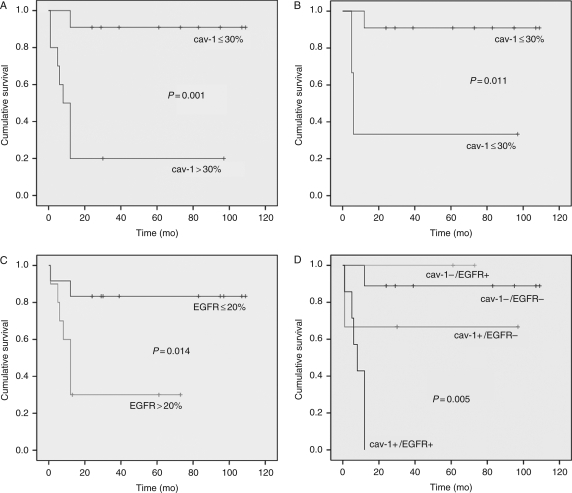
By univariate analysis, histological grade (P= .018), extent of surgery (P= .02), Ki-67 (P= .014), p53 (P= .014), cav-1 (P= .001), and EGFR (P= .014) expression significantly correlated with patient OS (Table 2; Figs 2 and 3).
Table 2.
Grade, Ki-67, p53, cav-1, and EGFR expression in censored patients by univariate analysis
| N | % Censored | P-value | |
|---|---|---|---|
| Grade | |||
| II | 14 | 78.6 | .018 |
| III | 8 | 25 | |
| Ki-67 | |||
| Ki-67 ≤ 1% | 7 | 100 | .014 |
| Ki-67 > 1% | 15 | 40 | |
| p53 | |||
| p53 ≤ 8% | 12 | 83.3 | .014 |
| p53 > 8% | 10 | 30 | |
| cav-1 | |||
| cav-1 ≤ 30% | 11 | 90.9 | .001 |
| cav-1 > 30% | 11 | 20 | |
| EGFR | |||
| EGFR ≤ 20% | 12 | 83.3 | .014 |
| EGFR > 20% | 10 | 30 | |
Fig. 2.
The Kaplan–Meier plots of estimated OS time distribution in relation to tumor grade (A), Ki-67 (B), and p53 (C) expression (+, censored).
Fig. 3.
The Kaplan–Meier plots of estimated OS time distribution in relation to cav-1 and EGFR expression and cav-1/EGFR coexpression. Plots refer to all 22 of the cases studied (A, C, and D) or to the subgroup of Grade II ependymomas only (B) (+, censored).
In Grade II ependymomas, only cav-1 correlated with poor OS (P = .011), indicating the presence of 2 distinct subgroups of tumors with identical grading and different outcomes (Fig. 3).
By the Cox proportional hazards model, among all of the variables considered, cav-1 was the only independent prognostic factor (relative risk = 13.92; P= .013).
Finally, we decided to investigate whether cav-1 expression could impact the prognostic value of the major known prognostic indicator in ependymomas, EGFR. Therefore, we analyzed survival, stratifying all cases into 4 distinct groups according to cav-1 and EGFR expression (cav-1+/EGFR+, cav-1−/EGFR−, cav-1+/EGFR−, and cav-1−/EGFR+) independently of grade to define whether the coexpression of these 2 markers correlated with a significantly worse prognosis. Strikingly, all patients with cav-1 and EGFR-positive ependymomas (8 cases: 2 cases of Grade II and 6 of Grade III) died within 12 months of diagnosis (P< .005; Fig. 3).
Cav-1 Mutational Profiling in Supratentorial Ependymomas
The mutational profile of cav-1 was assessed in all of the studied cases of adult intracranial Grade II and Grade III ependymomas. All the samples analyzed carried WT cav-1 sequences.
Within the limits of the cohort in study, these results suggest that cav-1 overexpression in aggressive ependymomas is not driven by activating mutations.
Discussion
In the present paper, we provide evidence that the coexpression of EGFR and cav-1 in adult intracranial ependymomas significantly predicts a rapid unfavorable outcome and that this immune-phenotypic pattern identifies, among Grade II tumors, those with a poor prognosis.
Cav-1 is one of the main components of cell membrane unfolding called caveolae, which participate in various cell functions under both physiological and neoplastic conditions. To date, the role of cav-1 in tumors is still controversial: either promoting or inhibiting effects in tumor growth have been reported, consistently with cav-1 binding to heterogeneous “partners” involved in cell-signaling cascades.27–29
Among the molecular mechanisms previously investigated to explain the tumor-promoting role of cav-1, the presence of a cav-1 activating mutation (P132L) proved to be crucial in favoring invasiveness and aggressiveness of the mammary cell line Met-1; the cav-1 (P132L) mutation is supposed to favor the interaction of the resulting protein product with new signaling pro-oncogenic protein partners that do not normally interact with cav-1 WT.30 In contrast, cav-1 gene amplification does not seem to be a suitable molecular mechanism because no amplification of the 7q31 region, which harbors the cav-1 gene, was observed in a series of recurrent prostate carcinomas.37
For this reason, in order to investigate the possible molecular pathways involved in the observed overexpression of cav-1 associated with unfavorable outcomes in our series, we checked the full coding sequence of cav-1 and found no mutations.
Recently, our group and others reported that cav-1 expression in glial tumors varies according to histological grades and predicts a poor prognosis in brain tumors with oligodendroglial components.25,26 Also, a correlation between cav-1 and p53, but not EGFR, has been reported in glial tumors.38 However, Abulrob et al.31 showed that cav-1 and EGFR colocalize in glioblastoma cells, thus influencing the EGFR-phosphorylated status in vitro. The role and the biological significance of cav-1 interaction with EGFR have been recently discussed in various studies, mainly addressed to breast cancer.39 Among others, Agelaki et al. reported that breast carcinoma MCF7 cells expressing cav-1 exhibited higher EGFR phosphorylation and Akt activation levels. In addition, gefitinib treatment resulted in a more efficient inhibition of downstream Akt and mitogen-activated protein kinase in cav-1–positive neoplastic cells than in those that were cav-1 negative.39 These data suggest a crucial role for cav-1 in activating the EGFR cascade; alternatively, they also confirm previous studies that already demonstrated that following exposure to oxidative stress, EGFR undergoes src-1– and cav-1–dependent nuclear trafficking.40 Moreover, EGFR nuclear translocation, which can be radiation induced and occurs via caveolae, has been recently reported as crucial in the acquisition of radioresistance.41
On the basis of this evidence, we decided to investigate whether cav-1 could be expressed in ependymal tumors as we previously reported in astrocytic-derived gliomas and, if so, whether cav-1 and EGFR (which is to date among the few prognostic markers for ependymal tumors) can be coexpressed and this association bears a prognostic significance. The results obtained in this study demonstrate a correlation between cav-1 positivity and tumor grade and EGFR expression. In addition, both cav-1 and EGFR have prognostic relevance in distinguishing poor-prognosis patients, and their coexpression strengthens their possible impacts on the outcome. Mendrzyk et al.21 described previously, via univariate analysis, that EGFR overexpression correlates with a poor prognosis in intracranial ependymomas, and they suggested that other effectors could accompany EGFR expression. In our opinion, cav-1 could be among these partner molecules; in fact, recent data proved cav-1 to be a marker of sensitivity to the tyrosine kinase inhibitor dasatinib in solid tumor cell lines, which display a phenotype characterized by a mesenchymal transition.42 This result strengthens the evidence for a functional involvement of cav-1 in the src-family kinase pathway, which is required for EGFR-initiated mitogenesis and could thus bear biological and clinical relevance in EGFR-positive tumors of glial/ependymal origin.42,43
Additionally, concerns about heterogeneous outcomes among Grade II ependymomas had already necessitated a molecular approach aimed at identifying specific chromosomal abnormalities associated with different prognoses. This approach could account for a novel, more objective alternative/additional classification in addition to histological grades.21,44 We suggest that an immunohistochemical approach (such as has already proven useful with EGFR) to distinguish prognosis subgroups among grade II intracranial ependymomas21 using a marker such as cav-1, which is easily detectable and has a good internal positive control constituted by endothelial cells, alone or in combination with EGFR, might help in recognizing more aggressive lesions in a pool of relatively benign tumors and thus be useful as a first-step prognostic screening. In our series, multivariate analysis indicated that cav-1 is the only molecular marker significantly associated with patient outcomes. Owing to the cav-1 involvement in different signaling cascades,42 its expression could represent a preliminary signature for potential future adjuvant issue targeting.45
It should be stressed that although in the present study we focused on the interaction between cav-1 and EGFR, due to the previously reported unfavorable role of the latter in ependymomas,21 a large cohort of alternative molecules could interact with cav-1 within plasma membrane specializations, such as those described as “signalosomes” and involving tyrosine kinase receptors other than EGFR. Therefore, the findings described here could be extended to other proteins potentially associated with the cav-1–mediated modulation of pro-oncogenic signaling events.
Although the number of cases of this series is limited, the sample can be considered representative as a homogeneous group of supratentorial and adult ependymal tumors; therefore, due to the actual lack of reliable prognostic indicators in intracranial ependymomas, the potential impact of the results presented here is worth consideration as pivotal preliminary evidence to be confirmed in a larger series and to be investigated under a functional approach.
Conflict of interest statement. None declared.
Funding
This paper was supported by grants from Ricerca Sanitaria Finalizzata Regione Piemonte and MURST (ex 60%).
References
- 1.Palm T, Figarella-Branger D, Chapon F, et al. Expression profiling of ependymomas unravels localization and tumor grade-specific tumorigenesis. Cancer. 2009;115:3955–3968. doi: 10.1002/cncr.24476. doi:10.1002/cncr.24476. [DOI] [PubMed] [Google Scholar]
- 2.Metellus P, Figarella-Branger D, Guyotat J, et al. Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113:175–185. doi: 10.1002/cncr.23530. doi:10.1002/cncr.23530. [DOI] [PubMed] [Google Scholar]
- 3.McLendon RE, Wiestler OD, Kros JM, Korshunov A, Ng HK. Ependymal tumors. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyons: IARC Press; 2007. pp. 69–79. [Google Scholar]
- 4.Chiu JK, Woo SY, Ater J, et al. Intracranial ependymoma in children: analysis of prognostic factors. J Neurooncol. 1992;13:283–290. doi: 10.1007/BF00172482. [DOI] [PubMed] [Google Scholar]
- 5.Ernestus RI, Schroder R, Stutzer H, Klug N. The clinical and prognostic relevance of grading in intracranial ependymomas. Br J Neurosurg. 1997;11:421–428. doi: 10.1080/02688699745925. doi:10.1080/02688699745925. [DOI] [PubMed] [Google Scholar]
- 6.Figarella-Branger D, Civatte M, Bouvier-Labit C, et al. Prognostic factors in intracranial ependymomas in children. J Neurosurg. 2000;93:605–613. doi: 10.3171/jns.2000.93.4.0605. doi:10.3171/jns.2000.93.4.0605. [DOI] [PubMed] [Google Scholar]
- 7.Ho DM, Hsu CY, Wong TT, Chiang H. A clinicopathologic study of 81 patients with ependymomas and proposal of diagnostic criteria for anaplastic ependymoma. J Neurooncol. 2001;54:77–85. doi: 10.1023/a:1012590505236. doi:10.1023/A:1012590505236. [DOI] [PubMed] [Google Scholar]
- 8.Nazar GB, Hoffman HJ, Becker LE, Jenkin D, Humphreys RP, Hendrick EB. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72:408–417. doi: 10.3171/jns.1990.72.3.0408. doi:10.3171/jns.1990.72.3.0408. [DOI] [PubMed] [Google Scholar]
- 9.Zamecnik J, Snuderl M, Eckschlager T. Pediatric intracranial ependymomas: prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod Pathol. 2003;6:980–991. doi: 10.1097/01.MP.0000087420.34166.B6. doi:10.1097/01.MP.0000087420.34166.B6. [DOI] [PubMed] [Google Scholar]
- 10.Kuncova K, Janda A, Kasal P, Zamecnik J. Immunohistochemical prognostic markers in intracranial ependymomas: systematic review and meta-analysis [published online ahead of print] Pathol Oncol Res. 2009;15:604–615. doi: 10.1007/s12253-009-9160-2. [DOI] [PubMed] [Google Scholar]
- 11.Massimino M, Buttarelli FR, Antonelli M, Gandola L, Modena P, Giangaspero F. Intracranial ependymoma: factors affecting outcome. Future Oncol. 2009;5:207–216. doi: 10.2217/14796694.5.2.207. doi:10.2217/14796694.5.2.207. [DOI] [PubMed] [Google Scholar]
- 12.Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58:255–270. doi: 10.1023/a:1016222202230. doi:10.1023/A:1016222202230. [DOI] [PubMed] [Google Scholar]
- 13.Kurt E, Zheng PP, Hop WC, et al. Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer. 2006;106:388–395. doi: 10.1002/cncr.21608. doi:10.1002/cncr.21608. [DOI] [PubMed] [Google Scholar]
- 14.Prayson RA. Clinicopathologic study of 61 patients with ependymoma including MIB-1 immunohistochemistry. Ann Diagn Pathol. 1999;3:11–18. doi: 10.1016/s1092-9134(99)80004-5. doi:10.1016/S1092-9134(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 15.Rickert CH, Paulus W. Prognosis-related histomorphological and immunohistochemical markers in central nervous system tumors of childhood and adolescence. Acta Neuropathol (Berl). 2005;109:69–92. doi: 10.1007/s00401-004-0959-3. doi:10.1007/s00401-004-0959-3. [DOI] [PubMed] [Google Scholar]
- 16.Ritter AM, Hess KR, McLendon RE, Langford LA. Ependymomas: MIB-1 proliferation index and survival. J Neurooncol. 1998;40:51–57. doi: 10.1023/a:1006082622699. doi:10.1023/A:1006082622699. [DOI] [PubMed] [Google Scholar]
- 17.Rushing EJ, Brown DF, Hladik CL, Risser RC, Mickey BE, White CL. Correlation of bcl-2, p53, and MIB-1 expression with ependymoma grade and subtype. Mod Pathol. 1998;11:464–470. [PubMed] [Google Scholar]
- 18.Shuangshoti S, Rushing EJ, Mena H, Olsen C, Sandberg GD. Supratentorial extraventricular ependymal neoplasms: a clinicopathologic study of 32 patients. Cancer. 2005;103:2598–2605. doi: 10.1002/cncr.21111. doi:10.1002/cncr.21111. [DOI] [PubMed] [Google Scholar]
- 19.Suri VS, Tatke M, Singh D, Sharma A. Histological spectrum of ependymomas and correlation of p53 and Ki-67 expression with ependymoma grade and subtype. Indian J Cancer. 2004;41:66–71. [PubMed] [Google Scholar]
- 20.Suzuki S, Oka H, Kawano N, Tanaka S, Utsuki S, Fujii K. Prognostic value of Ki-67 (MIB-1) and p53 in ependymomas. Brain Tumor Pathol. 2001;18:151–154. doi: 10.1007/BF02479429. doi:10.1007/BF02479429. [DOI] [PubMed] [Google Scholar]
- 21.Mendrzyk F, Korshunov A, Benner A, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12:2070–2079. doi: 10.1158/1078-0432.CCR-05-2363. doi:10.1158/1078-0432.CCR-05-2363. [DOI] [PubMed] [Google Scholar]
- 22.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. doi:10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–6625. : [PubMed] [Google Scholar]
- 24.Nigro JM, Misra A, Zhang L, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65:1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. doi:10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 25.Cassoni P, Senetta R, Castellano I, et al. Caveolin-1 expression is variably displayed in astroglial-derived tumors and absent in oligodendrogliomas: concrete premises for a new reliable diagnostic marker in gliomas. Am J Surg Pathol. 2007;31:760–769. doi: 10.1097/01.pas.0000213433.14740.5d. doi:10.1097/01.pas.0000213433.14740.5d. [DOI] [PubMed] [Google Scholar]
- 26.Senetta R, Trevisan E, Rudà R, et al. Caveolin 1 expression independently predicts shorter survival in oligodendrogliomas. J Neuropathol Exp Neurol. 2009;68:425–431. doi: 10.1097/NEN.0b013e31819ed0b7. doi:10.1097/NEN.0b013e31819ed0b7. [DOI] [PubMed] [Google Scholar]
- 27.Burgermeister E, Liscovitch M, Röcken C, Schmid RM, Ebert MP. Caveats of caveolin-1 in cancer progression. Cancer Lett. 2008;268:187–201. doi: 10.1016/j.canlet.2008.03.055. doi:10.1016/j.canlet.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. doi:10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 29.Williams TM, Sotgia F, Lee H, et al. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. Am J Pathol. 2006;169:1784–1801. doi: 10.2353/ajpath.2006.060590. doi:10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonuccelli G, Casimiro MC, Sotgia F, et al. Caveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. Am J Pathol. 2009;174:1650–1662. doi: 10.2353/ajpath.2009.080648. doi:10.2353/ajpath.2009.080648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abulrob A, Giuseppin S, Andrade MF, McDermid A, Moreno M, Stanimirovic D. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene. 2004;23:6967–6979. doi: 10.1038/sj.onc.1207911. doi:10.1038/sj.onc.1207911. [DOI] [PubMed] [Google Scholar]
- 32.Merz JF, Sankar P, Taube SE, LiVolsi VA. Use of human tissues in research: clarifying clinician and researcher roles and information flows. J Invest Med. 1997;45:252–257. [PubMed] [Google Scholar]
- 33.Li T, Sotgia F, Vuolo MA, et al. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168:1998–201. doi: 10.2353/ajpath.2006.051089. doi:10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox D. Regressions models and life table. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 35.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi:10.2307/2281868. [Google Scholar]
- 36.Verstegen MJ, Leenstra DT, Ijlst-Keizers H, Bosch DA. Proliferation- and apoptosis-related proteins in intracranial ependymomas: an immunohistochemical analysis. J Neurooncol. 2002;56:21–28. doi: 10.1023/a:1014471714058. doi:10.1023/A:1014471714058. [DOI] [PubMed] [Google Scholar]
- 37.Nupponen NN, Kakkola L, Koivisto P, Visakorpi T. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153:141–148. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barresi V, Buttarelli FR, Vitarelli EE, Arcella A, Antonelli M, Giangaspero F. Caveolin-1 expression in diffuse gliomas: correlation with the proliferation index, epidermal growth factor receptor, p53, and 1p/19q status. Hum Pathol. 2009;40:1738–1746. doi: 10.1016/j.humpath.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Agelaki S, Spiliotaki M, Markomanolaki H, et al. Caveolin-1 regulates EGFR signalling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol Ther. 2009;8:1470–1477. doi: 10.4161/cbt.8.15.8939. [DOI] [PubMed] [Google Scholar]
- 40.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. doi:10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 41.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol Cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. doi:10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. doi:10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 43.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. doi:10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 44.Hirose Y, Aldape K, Bollen A, James CD, Brat D, Lamborn K, Berger M, Feuerstein BG. Chromosomal abnormalities subdivide ependymal tumors into clinically relevant groups. Am J Pathol. 2001;158:1137–1143. doi: 10.1016/S0002-9440(10)64061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefranc F, Rynkowski M, DeWitte O, Kiss R. Present and potential future adjuvant issues in high-grade astrocytic glioma treatment. Adv Tech Stand Neurosurg. 2009;34:3–35. doi: 10.1007/978-3-211-78741-0_1. doi:10.1007/978-3-211-78741-0_1. [DOI] [PubMed] [Google Scholar]