Abstract
Long-term morbidity for children with low-grade glioma (LGG) requires exposure-specific characterization. Overall survival (OS) and progression-free survival (PFS) were estimated for 361 children diagnosed with LGG between 1985 and 2007 at a single institution. Five-year survivors (n = 240) received risk-based clinical assessment. Cumulative incidence of late effects 15 years from diagnosis were estimated. Risk factors for adverse health were identified using Fine and Gray's approach to Cox's proportional hazards model, accounting for death as a competing risk. OS at 15 years was 86% (95% confidence interval [CI] 82%–90%), and PFS was 55% (95% CI 51%–58%). Among the 240 5-year survivors, the 5-, 10-, and 15-year cumulative incidence of adverse outcomes included blindness: 10%, 13%, and 18%, respectively; hearing loss: 8%, 14%, and 22%; obesity/overweight: 18%, 35%, and 53%; hyperinsulinism: 1%, 5%, and 24%; growth hormone deficiency: 13%, 27%, and 29%;thyroid hormone deficiency: 16%, 28%, and 33%; and adrenocorticotropic hormone (ACTH) deficiency: 12%, 22%, and 26%. Multivariable models demonstrated radiation therapy to be a significant independent predictor of hearing loss, growth hormone deficiency, abnormal thyroid function, and ACTH deficiency. Diencephalic location was a statistically significant independent risk factor for blindness, growth hormone deficiency, abnormal thyroid function, and ACTH deficiency. Among the 182 5-year survivors assessed for intellectual function, 34% had an intelligence quotient (IQ) below average (<85), associated with younger age at diagnosis, epilepsy, and shunt placement. Survivors of childhood LGG experience substantial long-term adverse effects that continue to increase well beyond the 5-year survival time point.
Keywords: cancer, glioma, pediatric, survivor
Low-grade gliomas (LGGs) are a heterogeneous group of indolent tumors that comprise almost 40% of central nervous system tumors in childhood.1,2 These tumors typically involve midline structures such as the brainstem, hypothalamus, and optic pathway, in addition to their most common location, the cerebellum.3 Complete resection of these tumors provides excellent long-term survival; however, recurrence or progression frequently occurs and may necessitate adjuvant therapies, including radiation therapy (RT), chemotherapy, or further surgery.4–19 Thus, despite the low malignant potential of LGGs, patients harboring these tumors are recognized to be at risk for adverse long-term health outcomes.20 Comprehensive, exposure-specific risk assessment of late morbidity experienced by a large cohort of survivors of LGG has not occurred. Therefore, we report the survival experience of 361 children with LGG and long-term health outcomes of 240 5-year survivors.
Methods
Patient Population and Outcome Measures
Between 1985 and 2007, 361 children (≤21 years at diagnosis) were diagnosed and followed at St. Jude Children's Research Hospital (SJCRH) for LGG, defined as histopathologic confirmation of grade 1 astrocytoma (pilocytic) or grade 2 astrocytoma (including pilomyxoid fibrillary astrocytoma, oligoastrocytoma, oligodendroglioma, or low-grade astrocytic tumors not otherwise specified). Optic-pathway tumors presumed to be glioma by neuroimaging, but not biopsy proven, were included as “optic pathway glioma” (n = 27) and assumed to be grade 1 astrocytomas for analyses stratified by grade. Patients seen for a second opinion but not followed primarily at SJCRH were excluded. Details of tumor location, extent of resection, and treatment for this retrospective cohort were abstracted from the medical record. As the focal RT dose was relatively homogeneous across this population, RT exposure was analyzed as a dichotomous variable. Cumulative doses for all chemotherapy were abstracted from treatment records. Overall survival (OS) and progression-free survival (PFS) estimates were reported for the entire population (n = 361) utilizing vital status records updated annually by the SJCRH cancer registrar.
As of December 31, 2007, we identified 240 5-year survivors of LGG. Those with no evidence of recent tumor progression and at least 2 years after completion of antineoplastic therapy were followed in the After Completion of Therapy (ACT) clinic, which provides risk-based screening and counseling according to the Children's Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers until the age of 18 or 10 years from diagnosis, whichever is later.21 Health outcomes were prospectively identified by annual medical evaluations and diagnostic laboratory and imaging results, as well as the completion of a comprehensive health questionnaire. Health outcome definitions included: legal blindness, best corrected visual acuity ≥20/200; vision impaired, best corrected acuity ≥20 of 60 but <20 of 200; and visual field deficit, defined as bitemporal or one-sided homonymous hemianopsia, central scotoma, peripheral vision loss, or quadrant hemianopsia, all assessed by ophthalmologic examination. Hearing loss was defined as >25 db below the normal range at any frequency. Specific endocrine diagnoses were based on results of random or dynamic testing, including spontaneous overnight secretion of thyroid stimulating hormone, thyroid releasing hormone stimulation, overnight metyrapone testing, insulin-induced hypoglycemia, low-dose adrenocorticotropic hormone (ACTH) stimulation testing, stimulation of growth hormone secretion with arginine, l-dopa, clonidine, or hypoglycemia. Children who lacked clinical signs, symptoms, or laboratory values suggesting hormonal dysfunction were presumed to have a normal endocrine status.22 In survivors ≥18 years at the last evaluation, obesity was defined as body mass index (BMI) ≥30, and overweight as BMI <30 but ≥25. Survivors <18 years were described as overweight if their BMI was >95th percentile.23,24 Prevalence of focal neurologic deficits was reported from the most recent ACT clinic assessment by a single neurologist (E.B.M.). Assessment of health outcomes in the first 5 years from diagnosis occurred as part of a primary treatment protocol (n = 156, 43%) or as prescribed by the treating physician.
Among 5-year survivors, 182 (76%) participated in assessment of intellectual functioning (IQ) as part of routine clinical follow-up (n = 96) or protocol-based monitoring (n = 86). For individuals with multiple assessments, the most recent assessment was utilized to best characterize long-term cognitive outcomes. All IQ assessments used measures standardized on large representative normative samples that have demonstrated reliability and validity.25–37 Full scale IQ assessments were completed on 111 patients with estimated IQs available for an additional 71 patients. There was no statistical difference between estimated and full scale scores (t = −1.40, P = .16); therefore, these scores were combined for this analysis. This analysis was approved by the human investigations committee at SJCRH.
Statistical Analysis
Descriptive statistics were used to characterize LGG survivors. OS was defined as the time from diagnosis to the date of death, and all patients still surviving at the time of analyses were censored at the date of last contact. Similarly, PFS was defined as the time from diagnosis to the date of progression or date of death, and all patients surviving at the time of analyses were censored at the date of last contact. Kaplan–Meier plots and estimates were used to describe the survival distributions. For 5-year survivors, 15-year cumulative incidence rates of adverse long-term health outcomes were calculated, with death treated as a competing risk, according to the method described by Gray.38 For the visual field deficit, both death and bilateral blindness were treated as competing risks. Risk factors for adverse health (not including IQ) were identified using Fine and Gray's approach utilizing Cox's proportional hazards model, which accounts for competing risks.39 Date of last clinical assessment was used when onset of event date could not be clearly ascertained. Age at diagnosis, sex, race, presence of neurofibromatosis type 1 (NF-1), treatment era, tumor location, and history of RT, chemotherapy, and surgery were included as candidate independent variables. Results of Cox's multiple regression models within the proportional hazards framework were reported as hazard ratios with 95% intervals. SAS version 9.1 and R package cmprsk version 2.2-0 were used for all analyses.
All IQ scores were age standardized with a mean of 100 and standard deviation of 15. The proportion of the study sample with an IQ score <85 was calculated and compared with the normative sample. Univariate and multiple linear regression (using stepwise selection) models were developed to identify demographic and clinical factors predictive of lower IQ scores. Given that there was a strong statistical relationship between young age at diagnosis and treatment with chemotherapy (phi coefficient = −0.44), a well-established historical relationship between young age at diagnosis and poor cognitive outcomes,20,40,41 and significance of age at diagnosis in the univariate but not multiple regression model, the final model was constructed omitting chemotherapy.
Results
Among 361 children diagnosed with LGG, 54% were male and 78% non-Hispanic white (Table 1). Gliomas were most commonly pilocytic (63%) and located in the diencephalon (41%) or the cerebellum (26%) and less commonly located in the cerebral hemispheres (15%), brainstem (13%), or spinal cord (5%). Thirty-two percent received a gross total resection (GTR) at the time of diagnosis, with 40% ultimately achieving GTR with subsequent surgeries. Forty-six percent received surgery as their only treatment modality, while 47% had some combination of RT and chemotherapy. Thirty-six patients (10%) had NF-1. The 240 5-year survivors were last evaluated at a median age of 18.3 years (range 5.6–29.9) with a mean clinical follow-up of 10 years (range 5–21.5) from diagnosis.
Table 1.
Demographic and treatment characteristics
| Total cohort (N = 361) |
Five-year survivors (N = 240) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Age at diagnosis | ||||
| 0–4 | 122 | 33.8 | 82 | 34.2 |
| 5–9 | 118 | 32.7 | 80 | 33.3 |
| 10–20 | 121 | 33.5 | 78 | 32.5 |
| Gender | ||||
| Male | 196 | 54.3 | 137 | 57.1 |
| Female | 165 | 45.7 | 103 | 43.9 |
| Race/ethnicity | ||||
| Non-Hispanic, white | 281 | 77.8 | 194 | 80.8 |
| Non-Hispanic, black | 69 | 19.1 | 41 | 17.1 |
| Hispanic | 5 | 1.4 | 2 | 0.8 |
| Other | 6 | 1.7 | 3 | 1.3 |
| Tumor location | ||||
| Diencephalon | 147 | 40.7 | 97 | 40.4 |
| Hypothalamus/chiasmatic | 101 | 28.0 | 76 | 31.7 |
| Thalamic | 43 | 11.9 | 20 | 8.3 |
| Other | 3 | 0.8 | 1 | 0.4 |
| Cerebellum | 94 | 26.0 | 61 | 25.4 |
| Cerebral hemisphere | 56 | 15.5 | 38 | 15.8 |
| Frontal lobe | 15 | 4.2 | 10 | 4.1 |
| Temporal lobe | 28 | 7.8 | 20 | 8.3 |
| Parietal lobe | 7 | 1.9 | 4 | 1.7 |
| Occipital lobe | 2 | 0.6 | 2 | 0.8 |
| Other | 4 | 1.1 | 2 | 0.8 |
| Brainstem/spinal cord | 64 | 17.7 | 44 | 18.3 |
| Extent of resection of primary surgery | ||||
| <Gross total | 195 | 54.0 | 121 | 50.4 |
| Gross total | 117 | 32.4 | 81 | 33.8 |
| No surgery | 49 | 13.6 | 38 | 15.8 |
| Extent of resection of any surgery | ||||
| <Gross total | 190 | 52.6 | 114 | 47.5 |
| Gross total | 145 | 40.2 | 105 | 43.8 |
| No surgery | 26 | 7.2 | 21 | 8.8 |
| Primary treatment type | ||||
| Surgery only | 211 | 58.5 | 145 | 60.4 |
| Surgery + RT | 57 | 15.8 | 38 | 15.8 |
| Surgery + chemo | 34 | 9.4 | 14 | 5.8 |
| Surgery + RT + chemo | 10 | 2.8 | 5 | 2.1 |
| No Surgery | 49 | 13.6 | 38 | 15.8 |
| No surgery, chemo, or RT | 35 | 9.7 | 29 | 12.0 |
| Chemo only | 9 | 2.5 | 6 | 2.5 |
| RT only | 4 | 1.1 | 3 | 1.2 |
| Chemo + RT | 1 | 0.3 | 0 | 0.0 |
| Any treatment type | ||||
| Surgery only | 165 | 45.7 | 110 | 45.8 |
| Surgery + RT | 83 | 23.0 | 67 | 27.9 |
| Surgery + RT + chemo | 56 | 15.5 | 31 | 12.9 |
| Surgery + chemo | 31 | 8.6 | 11 | 4.6 |
| No surgery | 26 | 7.2 | 21 | 8.8 |
| No surgery, chemo, or RT | 5 | 1.4 | 4 | 1.7 |
| Chemo only | 8 | 2.2 | 4 | 1.7 |
| RT only | 6 | 1.7 | 6 | 2.5 |
| Chemo + RT | 7 | 1.9 | 7 | 2.9 |
| Histology | ||||
| Astrocytoma, pilocytic | 229 | 63.4 | 157 | 65.4 |
| Astrocytic tumor, NOS | 34 | 9.4 | 18 | 7.5 |
| Astrocytoma, fibrillary | 33 | 9.1 | 15 | 6.3 |
| Oligodendroglioma | 29 | 8.0 | 22 | 9.2 |
| Glioma, optic | 27 | 7.5 | 23 | 9.6 |
| Astrocytoma, pylomyxoid | 5 | 1.4 | 3 | 1.3 |
| Oligoastrocytoma | 4 | 1.1 | 2 | 0.8 |
| Treatment era | ||||
| 1985–1996 | 169 | 46.8 | 124 | 51.7 |
| 1997–2007 | 192 | 53.2 | 116 | 48.3 |
| Vital status | ||||
| Alive | 317 | 87.8 | 230 | 95.8 |
| Dead | 44 | 12.2 | 10 | 4.2 |
| Progression status | ||||
| Never progressed | 223 | 61.8 | 146 | 60.8 |
| Single progression (only) | 75 | 20.8 | 48 | 20.0 |
| Multiple progression | 63 | 17.5 | 46 | 19.2 |
| Neurofibromatosis type 1 | ||||
| No | 325 | 90.0 | 212 | 88.3 |
| Yes | 36 | 10.0 | 28 | 11.6 |
RT, radiation therapy ; NOS, not otherwise specified.
Overall and Progression-Free Survival
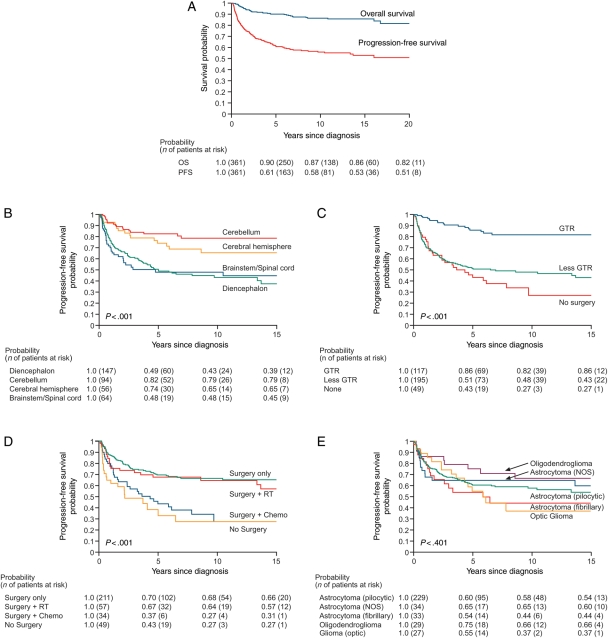
Of 361 cases, 44 (12%) were deceased, resulting in 10- and 20-year OS of 87% and 82%, respectively (Fig. 1A). Thirty-eight percent of the population experienced at least 1 progression of the primary tumor, while 18% had multiple tumor progression events. PFS at 10 years was 58% and stabilized by 15 years at 53%. PFS was associated with tumor location (P < .001; Fig. 1B), extent of primary surgical resection (P < .001; Fig. 1C), primary therapeutic modality (P < .001; Fig. 1D), and age at diagnosis (P < .001) but not by histology (P = .42; Fig. 1E), gender (P = .94), or race/ethnicity (P = .17).
Fig. 1.
Survival after diagnosis with low-grade glioma (n = 361). OS and PFS for the entire population (A), and PFS by tumor location (B), extent of primary resection: gross total vs less than gross total (C), by type of primary therapy (D), and histopathologic diagnosis (E).
Vision and Hearing
Overall, the cumulative incidence of monocular legal blindness among 5-year survivors at 5, 10, and 15 years from diagnosis was 10%, 13%, and 18%, respectively (95% confidence interval [CI] at 15 years 10.4%–24.6%, Table 2). 8.8% (95% CI 1.6%–16.0%) had bilateral blindness at 15 years. Lesions in the hypothalamic/chiasmatic region were responsible for most cases of vision loss (27 of 31, 87%), and among patients with progressive gliomas in this location the cumulative incidence of monocular legal blindness was 57% at 15 years from diagnosis. For all diencephalic tumors, while use of chemotherapy (hazard ratio [HR] = 2.1, 95% CI 1.0–4.3) and younger age at diagnosis (HR = 2.1, 95% CI 1.0–4.5) were associated with blindness on univariate analysis (Supplementary Material, Table S1), Cox's multiple regression model identified only hypothalamic/chiasmatic location (HR = 11.0, 95% CI 1.6–75.9) and earlier treatment era as significant independent risks for blindness (Table 3). Additionally, the cumulative incidence of a measured visual field deficit was 31.5% (95% CI 22.7%–40.3%) at 15 years, with a cumulative incidence of over 47% in hypothalamic/chiasmatic lesions, regardless of the progression status. Supratentorial tumor location was the only significant risk factor (HR = 9.3, 95% CI 3.7–23.7, Table 3).
Table 2.
Cumulative incidence (%) and 95% CIs for long-term adverse health outcomes (cumulative incidence at 15 yrs from diagnosis, %): overall and by tumor location at presentation
| Overall (n = 240) | Posterior fossa, PA, GTR (n = 51) | Hypothalamic/chiasmatic, no progression (n = 35) | Hypothalamic/chiasmatic with progression (n = 41) | Cerebral hemisphere (n = 38) | Brainstem (n = 32) | |
|---|---|---|---|---|---|---|
| Visual acuity | ||||||
| Legal blindness (R or L) | 17.5 (10.4–24.6) | 0.0 | 27.1 (11.3–42.9) | 56.9 (33.3–100.0) | 5.3 (0.0–12.5) | 5.1 (0.0–15.0) |
| Field deficit (R or L) | 31.5 (22.7–40.3) | 5.9 (0.0–14.5) | NAR | 47.5 (24.1–100.0) | 58.6 (21.7–95.5) | 11.3 (0.0–24.0) |
| Endocrine | ||||||
| Obesity/overweight | 53.2 (42.8–63.6) | 25.7 (12.7–38.7) | NAR | 75.0 (57.6–100.0) | 41.6 (17.8–65.4) | 45.4 (24.3–66.5) |
| Growth hormone deficiency | 29.0 (22.2–35.8) | 0.0 | 57.9 (39.9–75.9) | 62.3 (45.4–100.0) | 6.6 (0.0–15.7) | 25.9 (8.0–43.7) |
| Hypothyroid | 32.7 (24.6–40.8) | 3.9 (0.0–11.5) | NAR | 67.9 (49.1–100.0) | 22.7 (7.0–38.4) | 16.0 (2.9–29.1) |
| ACTH deficiency | 25.7 (18.9–32.5) | 2.0 (0.0–5.8) | NAR | 55.6 (37.9–100.0) | 9.7 (0.0–20.6) | 18.8 (2.7–35.0) |
| Hyperinsulinism/insulin resistant | 24.0 (13.2–34.7) | 7.7 (0.0–18.3) | 56.8 (6.5–100.0) | 23.8 (5.5–69.9) | 6.8 (0.0–16.1) | 27.9 (0.0–59.6) |
| Hearing | ||||||
| Any abnormality (R or L) | 21.8 (13.2–30.3) | 24.2 (0.0–51.6) | 3.3 (0.0–9.8) | 20.3 (7.5–74.0) | 3.9 (0.0–11.5) | 38.2 (16.5–59.8) |
| Neurologic | ||||||
| Seizure | 38.0 (29.5–46.5) | 6.0 (0.0–12.7) | 26.5 (0.0–53.5) | 49.9 (30.6–100.0) | 76.3 (59.2–93.4) | 27.2 (10.6–43.9) |
| Chronic headache | 40.9 (31.7–50.1) | 55.4 (29.1–81.6) | 50.8 (27.2–74.5) | 23.2 (7.7–77.6) | 43.6 (19.4–67.8) | 37.3 (15.0–59.7) |
| Cranial nerve deficit | 23.7 (16.7–30.8) | 20.7 (7.8–33.5) | 24.2 (0.0–51.6) | 19.5 (3.6–56.7) | 10.5 (0.6–20.4) | 52.6 (32.9–72.3) |
PA, pilocytic astrocytoma; GTR, gross total resection; NAR, No patients remain at risk at 15 years from diagnosis; ACTH, adrenocorticotropic hormone.
Table 3.
Cumulative incidence (%) and 95% CIs for long-term adverse health outcomes (cumulative incidence at 15 yrs from diagnosis, %): overall and by tumor grade at presentation
| Overall (n = 240) | Grade 1 (n = 180) | Grade 2 (n = 42) | |
|---|---|---|---|
| Visual acuity | |||
| Legal blindness (R or L) | 17.5 (10.4–24.6) | 21.2 (12.2–30.2) | 2.4 (0.0–7.0) |
| Field deficit (R or L) | 31.5 (22.7–40.3) | 30.5 (19.2–41.7) | 45.4 (18.0–72.8) |
| Endocrine | |||
| Obesity/overweight | 53.2 (42.8–63.6) | 58.5 (46.4–70.6) | 33.8 (13.7–53.9) |
| Growth hormone deficiency | 29.0 (22.2–35.8) | 30.3 (22.6–38.1) | 11.0 (0.0–23.5) |
| Hypothyroid | 32.7 (24.6–40.8) | 31.5 (22.1–40.8) | 25.6 (10.3–40.9) |
| ACTH deficiency | 25.7 (18.9–32.5) | 28.1 (19.8–36.4) | 12.6 (0.1–25.1) |
| Hyperinsulinism/insulin resistant | 24.0 (13.2–34.7) | 27.2 (13.6–40.9) | 7.0 (0.0–16.4) |
| Hearing | |||
| Any abnormality (R or L) | 21.8 (13.2–30.3) | 25.1 (13.9–36.3) | 10.8 (0.4–21.1) |
| Neurologic | |||
| Seizure | 38.0 (29.5–46.5) | 25.6 (16.5–34.7) | 72.1 (55.6–88.5) |
| Epilepsy | 22.5 (14.4–30.6) | 14.7 (6.6–22.9) | 31.2 (14.8–47.6) |
| Chronic headache | 40.9 (31.7–50.1) | 38.6 (27.7–49.5) | 53.4 (30.1–76.8) |
| Cranial nerve | 23.7 (16.7–30.8) | 23.5 (15.5–31.6) | 17.1 (3.8–30.4) |
ACTH, adrenocorticotropic hormone.
The cumulative incidence of hearing loss was 8%, 14%, and 22% (95% CI at 15 years 13.2–30.3) at 5, 10, and 15 years, respectively, but a deficit severe enough to recommend a hearing aid was uncommon (6%) at 15 years. Hearing loss was associated with the use of RT (HR = 3.4, 95% CI 1.2–9.5) and tumor location in the brainstem (HR = 2.2, 95% CI 1.1–4.9, Table 4). Thirty-eight patients received platinum-based therapy (median carboplatin dose = 5351 mg/m2, range = 377–14068 mg/m2). No association between hearing loss and platinum-based chemotherapy (HR = 1.6, 95% CI 0.8–3.2) was identified.
Table 4.
Multivariable risk factors for poor health outcomes: adjusted hazard ratios and 95% CIs
| Outcome | Independent variable |
Hazard ratio | 95% CI | |
|---|---|---|---|---|
| Legal blindness (diencephalon tumors only, n = 97) | Age at diagnosis | <5 yrs | 1.0 | 0.4–3.0 |
| ≥5 yrs | 1.0 | |||
| Era | 1985–1996 | 3.3 | 1.3–8.5 | |
| 1997–2007 | 1.0 | |||
| Chemo | Yes | 1.2 | 0.4–3.3 | |
| No | 1.0 | |||
| Tumor location | Hypothalamic/chiasmatic | 11.0 | 1.6–75.9 | |
| Thalamic/other | 1.0 | |||
| Hearing abnormal | Age at diagnosis | <5 yrs | 1.7 | 0.9–3.5 |
| ≥5 yrs | 1.0 | |||
| Surgery | GTR | 0.7 | 0.3–1.7 | |
| <GTR | 1.0 | |||
| RT | Yes | 3.4 | 1.2–9.5 | |
| No | 1.0 | |||
| Tumor location | Brainstem | 2.2 | 1.1–4.9 | |
| Other | 1.0 | |||
| Field deficit | Chemo | Yes | 0.9 | 0.5–1.5 |
| No | 1.0 | |||
| RT | Yes | 1.3 | 0.8–2.3 | |
| No | 1.0 | |||
| Surgery | GTR | 0.8 | 0.4–1.6 | |
| <GTR | 1.0 | |||
| Tumor location | Supratentorial | 9.3 | 3.7–23.7 | |
| Infratentorial | 1.0 | |||
| Era | 1985–1996 | 0.7 | 0.4–1.2 | |
| 1997–2007 | 1.0 | |||
| NF-1 | Yes | 1.2 | 0.7–2.2 | |
| No | 1.0 | |||
| Growth hormone deficiency | Chemo | Yes | 0.8 | 0.4–1.4 |
| No | 1.0 | |||
| RT | Yes | 3.9 | 1.9–8.2 | |
| No | 1.0 | |||
| Surgery | GTR | 0.2 | 0.1–0.6 | |
| <GTR | 1.0 | |||
| Tumor location | Diencephalon | 3.5 | 1.6–7.7 | |
| Other | 1.0 | |||
| NF-1 | Yes | 1.1 | 0.5–2.1 | |
| No | 1.0 | |||
| Hypothyroid | Sex | Male | 1.4 | 0.8–2.5 |
| Female | 1.0 | |||
| Chemo | Yes | 1.1 | 0.6–1.9 | |
| No | 1.0 | |||
| RT | Yes | 2.8 | 1.4–5.4 | |
| No | 1.0 | |||
| Surgery | GTR | 0.5 | 0.2–1.1 | |
| <GTR | 1.0 | |||
| Tumor location | Diencephalon | 4.0 | 2.0–8.2 | |
| Other | 1.0 | |||
| NF-1 | Yes | 0.7 | 0.3–1.4 | |
| No | 1.0 | |||
| ACTH deficiency | Sex | Male | 1.6 | 0.9–3.0 |
| Female | 1.0 | |||
| Chemo | Yes | 0.8 | 0.4–1.5 | |
| No | 1.0 | |||
| RT | Yes | 4.6 | 2.1–10.0 | |
| No | 1.0 | |||
| Surgery | GTR | 0.4 | 0.2–1.2 | |
| <GTR | 1.0 | |||
| Tumor location | Diencephalon | 3.4 | 1.6–7.3 | |
| Other | 1.0 | |||
| Era | 1985–1996 | 0.5 | 0.3–0.9 | |
| 1997–2007 | 1.0 | |||
| Hyperinsulinism/insulin resistant | Race | White | 0.3 | 0.1–0.7 |
| Other | 1.0 | |||
| RT | Yes | 1.9 | 0.8–4.6 | |
| No | 1.0 | |||
| Surgery | GTR | 1.1 | 0.3–4.0 | |
| <GTR | 1.0 | |||
| Tumor location | Diencephalon | 2.0 | 0.6–6.1 | |
| Other | 1.0 | |||
| Era | 1985–1996 | 0.4 | 0.1–0.9 | |
| 1997–2007 | 1.0 | |||
| Obese/overweight | Race | White | 0.6 | 0.3–0.9 |
| Other | 1.0 | |||
| RT | Yes | 1.3 | 0.8–2.0 | |
| No | 1.0 | |||
| Chemo | Yes | 1.0 | 0.6–1.5 | |
| No | 1.0 | |||
| Surgery | GTR | 0.7 | 0.4–1.2 | |
| <GTR | 1.0 | |||
| Tumor location | Diencephalon | 1.8 | 1.1–2.8 | |
| Other | 1.0 | |||
| Era | 1985–1996 | 0.7 | 0.5–1.1 | |
| 1997–2007 | 1.0 | |||
| Epilepsy | Age at diagnosis | <5 yrs | 1.7 | 0.7–4.0 |
| ≥5 yrs | 1.0 | |||
| RT | Yes | 1.3 | 0.5–3.2 | |
| No | 1.0 | |||
| Chemo | Yes | 1.0 | 0.4–2.7 | |
| No | 1.0 | |||
| Surgery | GTR | 0.8 | 0.3–2.2 | |
| <GTR | 1.0 | |||
| Tumor location | Supratent | 4.8 | 1.7–14.0 | |
| Infratent | 1.0 | |||
| Seizure | Chemo | Yes | 1.0 | 0.6–1.6 |
| No | 1.0 | |||
| Tumor location | Supratent | 3.7 | 2.0–7.0 | |
| Infratent | 1.0 | |||
| Headache | Age at diagnosis | <5 yrs | 0.7 | 0.4–1.1 |
| ≥5 yrs | 1.0 | |||
| Sex | Male | 0.5 | 0.3–0.8 | |
| Female | 1.0 | |||
| RT | Yes | 0.7 | 0.4–1.0 | |
| No | 1.0 | |||
| Era | 1985–1996 | 0.5 | 0.3–0.8 | |
| 1997–2007 | 1.0 | |||
| Cranial nerve deficit | Race | White | 0.6 | 0.3–1.2 |
| Other | 1.0 | |||
| Surgery | GTR | 0.5 | 0.2–0.9 | |
| <GTR | 1.0 | |||
| Tumor location | Supratent | 0.4 | 0.2–0.8 | |
| Infratent | 1.0 | |||
GTR, gross total resection; ACTH, adrenocorticotropic hormone; NF-1, neurofibromatosis type 1.
Bold denotes covariates that are independantly statistically significant in the multivariate model.
Endocrine Outcomes
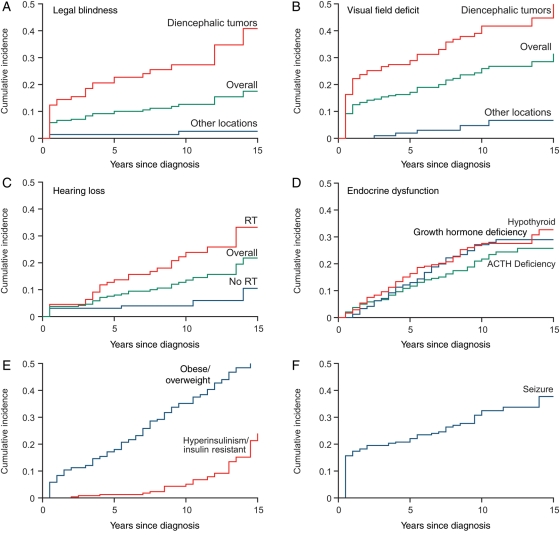
The cumulative incidence at 5, 10, and 15 years of hypothyroidism (16%, 28%, and 33%, respectively; 95% CI at 15 years 24.6%–40.8%), growth hormone (GH) deficiency (13%, 27%, and 29%; 95% CI at 15 years 22.2%–32.5%), and ACTH deficiency (12%, 22%, and 26%; 95% CI at 15 years 18.9%–32.5%) continued to increase, even at 15 years from diagnosis (Table 2, Fig. 2) and was accentuated among those with tumors in the hypothalamic/chiasmatic region. In Cox's multiple regression analysis, both location in the diencephalon and use of RT were statistically significant independent predictors of development of each of these outcomes (Table 4), while GTR was protective for development of GH deficiency (HR = 0.2, 95% CI 0.1–0.6). Notably, at 15 years from diagnosis, 53.2% (95% CI 42.8%–63.6%) of this population is overweight or obese, with the highest rates among those with progressive hypothalamic/chiasmatic lesions (75.0%; 95% CI 57.6%–100%), and the cumulative incidence of insulin resistance is 24.0% (95% CI 13.2%–34.7%). Obesity was associated with tumor location in the diencephalon (HR = 1.8, 95% CI 1.1–2.9) and was less common among non-Hispanic whites (HR = 0.6, 95% CI 0.3–0.9, Table 4).
Fig. 2.
Cumulative incidence of (A) legal blindness, (B) visual field deficit, (C) hearing loss, (D) endocrine dysfunction, (E) obesity and hyperinsulinism, and (F) seizure and epilepsy among 5-year survivors of low-grade glioma.
Neurologic Outcomes
The cumulative incidence of at least 1 seizure was 38.0% (95% CI 29.5%–46.5%) (Table 2). Both seizure and epilepsy were associated only with tumor location above the tentorium cerebri, but not with extent of resection. Cranial neuropathies were common (>50%) in survivors with brainstem lesions.
Time of Onset of Poor Health Outcomes
As expected, a large number of survivors sustain adverse health outcomes at the time of diagnosis and treatment, but over one-third of adverse events occurred beyond 5 years from diagnosis (Fig. 2). In particular, among those who ultimately developed a deficit, large percentages of patients had new onset of hearing loss (41%), field deficit (33%), GH deficiency (36%), hypothyroidism (37%), and epilepsy (34%) beyond 5 years from diagnosis.
Neurocognitive Outcomes
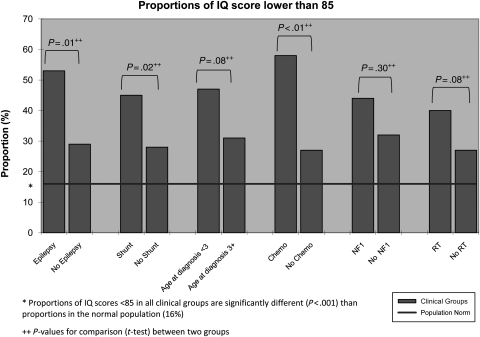
The 182 patients with IQ testing were not statistically different from the 58 patients who were not tested with respect to gender, age at diagnosis, tumor location, NF-1 status, number of surgeries, history of chemotherapy, or frequency of disease progressions. The group with IQ testing was more likely to have received RT (50% vs 34.5%). Assessments took place on average 6.3 ± 3.8 years following cancer diagnosis. The IQ for the sample as a whole fell in the low average range (92.5 ± 18.5). Sixty-one patients (34%) had an IQ score <85, compared with 16% in the normative sample (z = 0.18, P < .001, z-test for testing 1 sample proportion). Univariate linear regression revealed that younger age at diagnosis (P = .004), NF-1 diagnosis (P = .040), epilepsy (P = .010), shunt placement (P = .007), and chemotherapy (<.001) were each significant predictors of lower IQ. In the multiple regression model, younger age at diagnosis (P = .016), epilepsy (P = .048), and shunt placement (P = .029) were significantly predictive of lower IQ. Figure 3 depicts the relationship between significant univariate clinical predictors of IQ and risk for below average IQ scores.
Fig. 3.
Proportions (%) of survivors with IQ <85, by individual risk factor.
Outcomes by Tumor Grade
Acknowledging that grade 1 and grade 2 tumors are different with respect to clinical characteristics, treatments, and response to platinum-based chemotherapy, Table 3 demonstrates the 15-year cumulative incidence of long-term outcomes by tumor grade. In general, higher rates of morbidity are identified in the grade 1 population. Long-term outcomes for grade 1 tumors, stratified by location (Supplementary Material, Table S2) and multivariable assessment of risk factors for morbidity (Supplementary Material, Table S3) in grade 1 tumors is provided.
Discussion
While much attention has been focused on reporting OS and PFS after various treatment modalities, the fact remains that children with LGG have an excellent OS,7–10 yet are at high risk for long-term morbidity. To date, publications on late effects beyond 5 years from diagnosis have been limited in sample size (<100 patients) or have reported on only homogeneously treated populations.4,11–20 We report the survival experience of 361 children with LGG and the long-term health outcomes on 240 5-year survivors, including incidence rates stratified by both tumor location and tumor grade, and multivariable assessment of risk factors for a wide range of adverse health outcomes. Furthermore, we found that approximately one-third of adverse health outcomes occur beyond the 5-year time point, indicating that while many deficits occur at the time of initial presentation and surgery, ongoing risks attributable to subsequent tumor progression or exposure to RT or chemotherapy are not small. Studies that report “late effects” at the 5-year time point underestimate the true impact of LGG and its treatment. This population merits intensive long-term, risk-based follow-up.42
We found that 18% of patients have monocular blindness (>20/200 visual acuity) at 15 years from diagnosis. Tumor location in the hypothalamic/chiasmatic region has been previously documented in several small studies (rates of vision loss range: 7%–34%) as the primary threat to vision.11–15 However, among patients with tumors in the diencephalon that progress after initial diagnosis and treatment, we note that more than half (57%) will be rendered blind. With regard to hearing loss, while only 6% of survivors required an aid, 22% of survivors had measured loss that was associated with brainstem tumor location and the use of RT. Previous work at our institution has characterized the impact of RT on hearing.20,43 In addition, we now have found no association between the use of platinum-based chemotherapy and hearing loss. While the overall number of patients receiving platinum was relatively small, preventing dose-related assessment, this suggests that the use of platinum-based chemotherapy, with delay of RT, has resulted in no detectable harm in audiological function among these young children during crucial years of speech and language development. Future rates of hearing loss may be reduced with the use of conformal RT that allows a reduction of target radiation volumes.20
The risk of poly endocrinopathy in LGG survivors is well documented, with rates varying based on tumor location, exposure type, and length of follow-up.11–16,18–20,44 Fouladi et al.17 previously reported the St. Jude experience with LGG in the hypothalamic/chiasmatic region and documented that over two-thirds of patients (64%) developed at least 1 endocrinopathy. Utilizing multivariable analysis in this larger population with heterogeneity in tumor location, we add to these findings by quantifying the risk for tumor location in the diencephalon relative to other locations as well as quantifying the risk for RT exposure for endocrine deficits mediated through the hypothalamic–pituitary axis. Despite their high risk for sedentary lifestyle and deficits in physical function,45–47 the majority of this population demonstrates similar rates of excessive weight and obesity as the general age- and sex-matched population.24 However, an exception are the survivors with incompletely resected, progressive hypothalamic/chiasmatic tumors. Consistent with a known risk for hypothalamic injury-mediated obesity,48–51 75% of these patients are overweight or obese. Weight reduction interventions should be considered for all survivors who are overweight or obese.
One-third of long-term LGG survivors had below average IQ scores when assessed at a mean of 6 years following diagnosis. This is a significantly higher rate of deficit than found in healthy peers and is particularly concerning given the known association of cognitive lateeffects with academic difficulties, unemployment, and a reduced quality of life.52,53 While it may seem counterintuitive that RT was not a significant predictor of IQ, half (n = 45) of the patients in this study who received RT received conformal rather than craniospinal RT. We have previously demonstrated that IQ can be preserved years after treatment in children treated with conformal RT for a brain tumor.54 Additionally, the full effect of cognitive decline may not be evident in this cohort. Adults treated for LGG, while having minimal reduction in cognitive function at 6 years from RT, with further follow-up to 12 years from RT demonstrated significant cognitive decline associated with RT.55 NF-1 diagnosis has consistently been shown to confer a cognitive risk irrespective of treatment history.20,56 Shunt placement identifies a portion of the population with hydrocephalus, and considering the indolent nature of LGG, this group may have had a prolonged period of hydrocephalus prior to coming to medical attention, which can be associated with cognitive decline.22 Similarly, epilepsy is likely a marker of increased neurologic disruption associated with disease and/or treatment.57,58
A number of limitations to this analysis should be considered. The high rate of tumors in the diencephalon in this population suggests that a referral bias may exist, as a cerebellar location is established as the most common primary LGG site. It is likely that patients with a GTR of a cerebellar LGG may not be referred to our tertiary treatment institution at the same rates as patients with more complicated diencephalic tumors. Fortunately, our large sample size allowed for multivariable analysis adjusted for location, thus controlling for this potential confounder. Additionally, there is a risk for misclassification of the outcome. Patients were provided risk-based care; thus not all patients received the same screening and detection measures. However, for most outcomes, we expect that our comprehensive patient interview, examination, and comprehensive health questionnaire would identify new incident adverse outcomes resulting in appropriate diagnostic work up.
In conclusion, the 5- and 10-year OS and PFS in this study, as well as associations of PFS with tumor location and extent of resection, are generally consistent with previously published cohorts and population-based studies, affirming the generalizability of our findings. Notably, one exception is that no difference in PFS by histopathologic diagnosis was identified, which may be a consequence of a high percentage of mid-line hypothalamic and optic-pathway pilocytic tumors in the cohort. These data, taken collectively, provide the most comprehensive, exposure-specific assessment of long-term morbidity in survivors of LGG to date, and identify an ongoing increase in incidence of chronic medical conditions well beyond the 5-year time point and significant risk for cognitive decline. These risks for long-term morbidity, now being clearly defined, should be a component of patient education and therapeutic decision making at the time of diagnosis. In addition, because of their risk for late onset morbidity and tumor progression, survivors of LGG with residual tumor merit comprehensive, long-term follow-up under the guidance of neuro-oncology or late-effects expertise. Future studies should include prospective, longitudinal cognitive assessments that afford greater control of sample composition and allow more detailed predictive modeling. Direct measures of neurodevelopment, including neuroimaging would also provide a window into biological mechanisms underlying cognitive changes. In addition, modern applications of RT, such as conformal or proton-beam therapies, and chemotherapy (novel-targeted therapies) may improve the long-term outcomes and necessitate ongoing follow-up.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online.
Conflict of interest statement. None declared.
Funding
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC); National Institutes of Health Cancer Support (CORE, grant number P30 CA 21765); Musicians Against Childhood Cancer (MACC); an unrestricted grant from Research to Prevent Blindness and The Noyes Brain Tumor Foundation.
References
- 1.2008. C. Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Published by the Central Brain Tumor Registry of the United States. [Google Scholar]
- 2.Duffner PK, Cohen ME, Myers MH, Heise HW. Survival of children with brain tumors: SEER Program, 1973–1980. Neurology. 1986;36(5):597–601. doi: 10.1212/wnl.36.5.597. [DOI] [PubMed] [Google Scholar]
- 3.Rashidi M, DaSilva VR, Minagar A, Rutka JT. Nonmalignant pediatric brain tumors. Curr Neurol Neurosci Rep. 2003;3(3):200–205. doi: 10.1007/s11910-003-0079-9. doi:10.1007/s11910-003-0079-9. [DOI] [PubMed] [Google Scholar]
- 4.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. doi:10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 5.Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11(2):152–162. doi: 10.1053/srao.2001.21421. doi:10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]
- 6.Schmandt SM, Packer RJ. Treatment of low-grade pediatric gliomas. Curr Opin Oncol. 2000;12(3):194–198. doi: 10.1097/00001622-200005000-00002. doi:10.1097/00001622-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 8.Kandil A, Khafaga Y, ElHusseiny G, Allam A, Jamshed A, Schultz H. Low-grade astrocytoma—a retrospective analysis of 102 patients. Acta Oncol. 1999;38(8):1051–1056. doi: 10.1080/028418699432356. doi:10.1080/028418699432356. [DOI] [PubMed] [Google Scholar]
- 9.Yule SM, Hide TA, Cranney M, Simpson E, Barrett A. Low grade astrocytomas in the West of Scotland 1987–96: treatment, outcome, and cognitive functioning. Arch Dis Child. 2001;84(1):61–64. doi: 10.1136/adc.84.1.61. doi:10.1136/adc.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohrmann GJ, Farwell JR, Flannery JT. Astrocytomas in childhood: a population-based study. Surg Neurol. 1985;23(1):64–68. doi: 10.1016/0090-3019(85)90162-4. doi:10.1016/0090-3019(85)90162-4. [DOI] [PubMed] [Google Scholar]
- 11.Turner CD, Chordas CA, Liptak CC, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. doi: 10.1002/pbc.22081. doi:10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 12.Benesch M, Lackner H, Sovinz P, et al. Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neurooncol. 2006;78(2):199–205. doi: 10.1007/s11060-005-9091-z. doi:10.1007/s11060-005-9091-z. [DOI] [PubMed] [Google Scholar]
- 13.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83(4):583–589. doi: 10.3171/jns.1995.83.4.0583. doi:10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 14.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75(4):1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. doi:10.1002/1097-0142(19950215)75:4<1051::AID-CNCR2820750423>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)—radiographic response, vision and late toxicity. Radiother Oncol. 2000;54(3):239–245. doi: 10.1016/s0167-8140(00)00149-3. doi:10.1016/S0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 16.Collet-Solberg PF, Sernyak H, Satin-Smith M, et al. Endocrine outcome in long-term survivors of low-grade hypothalamic/chiasmatic glioma. Clin Endocrinol (Oxf). 1997;47(1):79–85. doi: 10.1046/j.1365-2265.1997.2211032.x. doi:10.1046/j.1365-2265.1997.2211032.x. [DOI] [PubMed] [Google Scholar]
- 17.Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97(4):1084–1092. doi: 10.1002/cncr.11119. doi:10.1002/cncr.11119. [DOI] [PubMed] [Google Scholar]
- 18.Brauner R, Malandry F, Rappaport R, et al. Growth and endocrine disorders in optic glioma. Eur J Pediatr. 1990;149(12):825–828. doi: 10.1007/BF02072067. doi:10.1007/BF02072067. [DOI] [PubMed] [Google Scholar]
- 19.Due-Tonnessen BJ, Helseth E, Scheie D, Skullerud K, Aamodt G, Lundar T. Long-term outcome after resection of benign cerebellar astrocytomas in children and young adults (0–19 years): report of 110 consecutive cases. Pediatr Neurosurg. 2002;37(2):71–80. doi: 10.1159/000065108. doi:10.1159/000065108. [DOI] [PubMed] [Google Scholar]
- 20.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. doi:10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. doi:10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Morris EB, Shelso J, Smeltzer MP, et al. The use of bone age for bone mineral density interpretation in a cohort of pediatric brain tumor patients. Pediatr Radiol. 2008;38(12):1285–1292. doi: 10.1007/s00247-008-0991-x. doi:10.1007/s00247-008-0991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. J Am Med Assoc. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. doi:10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. doi:10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 25.Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 26.Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service, Inc.; 1990. [Google Scholar]
- 27.McCarthy D. Manual for the McCarthy Scales of Children's Abilities. New York: The Psychological Corporation; 1972. [Google Scholar]
- 28.Mullen E. Mullen Scales of Early Learning. Los Angeles: Western Psychological Services; 1995. [Google Scholar]
- 29.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. New York: The Psychological Corporation; 1949. [Google Scholar]
- 30.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1955. [Google Scholar]
- 31.Wechsler D. Manual for the Wechsler Intelligence Scale for Children - Revised. New York: The Psychological Corporation; 1974. [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale—Revised Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 33.Wechsler D. WPPSI-R, Manual: Wechsler Preschool and Primary Scale of Intelligence, Revised. San Antonio, TX: Harcourt Brace Jovanovich; 1989. [Google Scholar]
- 34.Wechsler D. Wechsler Intelligence Scale for Children—III. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 36.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 37.Wechsler D. WISC-IV Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 38.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi:10.1214/aos/1176350951. [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.2307/2670170. [Google Scholar]
- 40.Mulhern RK, Butler RW. Neurocognitive sequelae of childhood cancers and their treatment. Pediatr Rehabil. 2004;7(1):1–14. doi: 10.1080/13638490310001655528. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 41.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. doi:10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 42.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607. doi:10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hua C, Bass JK, Khan R, Kun LE, Merchant TE. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72(3):892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 44.Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005;23(28):7152–7160. doi: 10.1200/JCO.2005.01.214. doi:10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 45.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(9):1984–1994. doi: 10.1002/cncr.24209. doi:10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2382–2389. doi: 10.1200/JCO.2008.21.1482. doi:10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. doi: 10.1093/jnci/djp148. doi:10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lustig RH, Rose SR, Burghen GA, et al. Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J Pediatr. 1999;135(2 Pt 1):162–168. doi: 10.1016/s0022-3476(99)70017-x. [DOI] [PubMed] [Google Scholar]
- 49.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88(2):611–616. doi: 10.1210/jc.2002-021180. doi:10.1210/jc.2002-021180. [DOI] [PubMed] [Google Scholar]
- 50.Lustig RH. Obesity in childhood cancer survivors. Pediatr Endocrinol Rev. 2006;3(Suppl 2):306–311. [Google Scholar]
- 51.Lustig RH, Sen S, Soberman JE, Velasquez-Mieyer PA. Obesity, leptin resistance, and the effects of insulin reduction. Int J Obes Relat Metab Disord. 2004;28(10):1344–1348. doi: 10.1038/sj.ijo.0802753. doi:10.1038/sj.ijo.0802753. [DOI] [PubMed] [Google Scholar]
- 52.Crom DB, Lensing SY, Rai SN, Snider MA, Cash DK, Hudson MM. Marriage, employment, and health insurance in adult survivors of childhood cancer. J Cancer Surviv. 2007;1(3):237–245. doi: 10.1007/s11764-007-0026-x. doi:10.1007/s11764-007-0026-x. [DOI] [PubMed] [Google Scholar]
- 53.Mostow EN, Byrne J, Connelly RR, Mulvihill JJ. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J Clin Oncol. 1991;9(4):592–599. doi: 10.1200/JCO.1991.9.4.592. [DOI] [PubMed] [Google Scholar]
- 54.Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. doi: 10.1200/JCO.2007.15.9970. doi:10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. doi:10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 56.Levine TM, Materek A, Abel J, O'Donnell M, Cutting LE. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):8–20. doi: 10.1016/j.spen.2006.01.006. doi:10.1016/j.spen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Johnson AR, DeMatt E, Salorio CF. Predictors of outcome following acquired brain injury in children. Dev Disabil Res Rev. 2009;15(2):124–132. doi: 10.1002/ddrr.63. doi:10.1002/ddrr.63. [DOI] [PubMed] [Google Scholar]
- 58.Khan RB, Marshman KC, Mulhern RK. Atonic seizures in survivors of childhood cancer. J Child Neurol. 2003;18(6):397–400. doi: 10.1177/08830738030180060701. doi:10.1177/08830738030180060701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.