Abstract
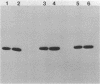
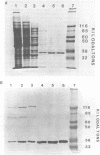
Two murine monoclonal antibodies to the proliferating cell nuclear antigen (PCNA), a rabbit anti-N-terminal peptide antibody and human auto-antibody to PCNA reacted with the auxiliary protein for DNA polymerase delta from fetal calf thymus following SDS-polyacrylamide gel electrophoresis, confirming the identity of PCNA and the auxiliary protein. Undenatured auxiliary protein was immunoprecipitated by the human autoantibody, but not by the monoclonal antibodies, which were raised to SDS-denatured PCNA, nor by the anti-N-terminal peptide antibody, suggesting that the epitopes recognized by both the monoclonal antibodies and the anti-peptide antibody are not exposed in the native protein. The human anti-PCNA autoantibody neutralized the activity of the auxiliary protein for DNA polymerase delta, but did not inhibit the activity of pol delta itself. The ability of pol delta to utilize template/primers containing long stretches of single-stranded template was inhibited by the anti-PCNA autoantibody, whereas the activity of pol alpha on such templates was not affected, confirming the specificity of the auxiliary protein for pol delta. The ability of PCNA, a cell cycle-regulated protein, to regulate the activity of pol delta suggests a central role for pol delta in cellular DNA replication.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P. Increased nuclear cyclin/PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986 Dec 15;209(2):277–283. doi: 10.1016/0014-5793(86)81127-9. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Gazin C., Rigolet M., Briand J. P., Van Regenmortel M. H., Galibert F. Immunochemical detection of proteins related to the human c-myc exon 1. EMBO J. 1986 Sep;5(9):2241–2250. doi: 10.1002/j.1460-2075.1986.tb04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U. DNA polymerases in prokaryotes and eukaryotes: mode of action and biological implications. Experientia. 1983 Jan 15;39(1):1–25. doi: 10.1007/BF01960616. [DOI] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Madsen P., Celis J. E. S-phase patterns of cyclin (PCNA) antigen staining resemble topographical patterns of DNA synthesis. A role for cyclin in DNA replication? FEBS Lett. 1985 Nov 25;193(1):5–11. doi: 10.1016/0014-5793(85)80068-5. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Ogata K., Ogata Y., Nakamura R. M., Tan E. M. Purification and N-terminal amino acid sequence of proliferating cell nuclear antigen (PCNA)/cyclin and development of ELISA for anti-PCNA antibodies. J Immunol. 1985 Oct;135(4):2623–2627. [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Sadaie M. R., Mathews M. B. Immunochemical and biochemical analysis of the proliferating cell nuclear antigen (PCNA) in HeLa cells. Exp Cell Res. 1986 Apr;163(2):423–433. doi: 10.1016/0014-4827(86)90073-x. [DOI] [PubMed] [Google Scholar]
- Takasaki Y., Fishwild D., Tan E. M. Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med. 1984 Apr 1;159(4):981–992. doi: 10.1084/jem.159.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Tan C. K., So M. J., Downey K. M., So A. G. Apparent stimulation of calf thymus DNA polymerase alpha by ATP. Nucleic Acids Res. 1987 Mar 11;15(5):2269–2278. doi: 10.1093/nar/15.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Witholt B. Assembly pathway of newly synthesized LamB protein an outer membrane protein of Escherichia coli K-12. J Mol Biol. 1984 Jun 5;175(4):511–528. doi: 10.1016/0022-2836(84)90182-7. [DOI] [PubMed] [Google Scholar]