Abstract
Due to its molecular heterogeneity and infiltrative nature, glioblastoma multiforme (GBM) is notoriously resistant to traditional and experimental therapeutics. To overcome these hurdles, targeted agents have been combined with conventional therapy. We evaluated the preclinical potential of a novel, orally bioavailable PI3K/mTOR dual inhibitor (XL765) in in vitro and in vivo studies. In vivo serially passaged human GBM xenografts that are more genetically stable than GBM cell lines in culture were used for all experiments. Biochemical downstream changes were evaluated by immunoblot and cytotoxicity by colorimetric ATP-based assay. For in vivo experiments, human xenograft GBM 39 grown intracranially in nude mice was altered to express luciferase to monitor tumor burden by optical imaging. XL765 resulted in concentration-dependent decreases in cell viability in vitro. Cytotoxic doses resulted in specific inhibition of PI3K signaling. Combining XL765 with temozolomide (TMZ) resulted in additive toxicity in 4 of 5 xenografts. In vivo, XL765 administered by oral gavage resulted in greater than 12-fold reduction in median tumor bioluminescence compared with control (Mann–Whitney test p = 0.001) and improvement in median survival (logrank p = 0.05). TMZ alone showed a 30-fold decrease in median bioluminescence, but the combination XL765 + TMZ yielded a 140-fold reduction in median bioluminescence (Mann-Whitney test p = 0.05) with a trend toward improvement in median survival (logrank p = 0.09) compared with TMZ alone. XL765 shows activity as monotherapy and in combination with conventional therapeutics in a range of genetically diverse GBM xenografts.
Keywords: PI3K/mTOR inhibitor, glioma, temozolomide, signaling inhibitor
The current standard of care for glioblastoma multiforme (GBM) is postoperative radiation and temozolomide (TMZ), producing a median survival of approximately 14 months.1 Despite extensive translational research and development of experimental therapeutics, there has been no significant improvement in overall survival for patients.2 A significant hurdle is the molecular heterogeneity of GBM,3,4 which impedes uniform application of specific molecularly targeted agents. One frequently dysregulated pathway is the receptor tyrosine kinase (RTK)/phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) molecular cascade, which is activated by various mechanisms in GBM.5 Analysis of 209 GBM clinical samples by the Cancer Genome Atlas group demonstrated that 86% had a genetic alteration (activating mutation or gene amplification) in the RTK/PI3K pathway.6 The majority of these alterations occurred in the various RTK signaling inputs including EGFR, ERBB2, PDGFRA, and MET, while alterations in genes encoding subunits of PI3K itself (PIK3R1 and PIK3CA) were the second most common event. In addition, 36% of GBM samples had mutations or homozygous deletions of phosphatase and tensin homolog (PTEN), a tumor suppressor protein that regulates PI3K activity. Clinically, retrospective data indicate that genetic alterations in low-grade gliomas resulting in PI3K pathway activation have a detrimental impact on patient survival.36
Attempts to inhibit the PI3K pathway with pan-PI3K inhibitors such as LY2940027,8 and wortmannin/PX-866,9,10 while successful preclinically, have not progressed to clinical use due to concerns over organ toxicity and poor bioavailability. Inhibition of the pathway distally using rapamycin resulted in paradoxical activation of Akt through loss of negative feedback in a subset of patients, which in turn was associated with shorter time-to-progression during postsurgical maintenance rapamycin therapy.11 Development of next-generation, class Iα–specific PI3K inhibitors with oral bioavailability has led to a resurgence in efforts to therapeutically modulate this pathway.12 Identification of one particularly effective PI3K inhibitor also led to the discovery of its ability to concomitantly inhibit mTOR.13 Since then, additional studies have focused on dual inhibition of both PI3K and mTOR in GBM.14–18 However, it remains unclear which subtypes of GBM are susceptible to this approach. To help answer this question, we obtained a panel of genetically characterized human GBM xenografts serially passaged in nude mice to maintain genetic stability.19,20 We used the PI3K/mTOR inhibitor XL765, which has demonstrated broad anticancer efficacy.21
In addition to evaluating effects of XL765 in vitro against a genetically diverse panel of GBM xenografts, we combined XL765 with TMZ to determine the combined cytotoxic efficacy of these agents. In an intracranial, orthotopic xenograft glioma model that allows tumor burden to be monitored noninvasively, we assessed the efficacy of XL765 in vivo as a single agent and in combination with TMZ. This study provides a basis for clinical investigation of XL765 combined with TMZ in phase Ib/II trials.
Materials and Methods
Cell Culture
GBM 6, GBM 8, GBM 12, GBM 39, and GS-2 cell lines have been previously described.19 The identities of all cell sources used for these investigations were confirmed by short tandem repeat DNA fingerprinting using the Promega Powerplex 1.2 platform to determine the status of EGFR, PTEN, p53, MGMT, and p16. Xenografts were harvested from the flanks of nude mice, diced, filtered, and washed 3 times with fresh media. Cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 0.1 μg/mL penicillin, 100 units/mL streptomycin, nonessential amino acids, and 2 mM glutamine (UCSF Cell Culture facility). XL765 (Exelixis) stock was stored as a 10-mM solution in dimethyl sulfoxide (DMSO) at −20°C. Temozolomide (Schering-Plough) stock was stored as an 80-mM solution in DMSO at −20°C.
Cell Viability Assay
Glioblastoma cells were plated in 96-well black-walled plates at a density of 3000 cells per well. Cells were allowed to adhere for 16 hours. Medium was then removed and replaced with medium containing specified concentrations of drug(s). Viable cells were quantitated 72 hours after drug exposure using the CellTiter-Glo Assay (Promega) per manufacturer's instruction. Results are presented as the mean value ± standard error from three independent experiments.
Immunoblot
Effects of XL765 on activity of PI3K and mTOR pathways were analyzed by immunoblot. Cells in 10-cm plastic dishes were treated with specified concentrations of XL765. After 24 hours, the cells were lysed by incubation in lysis buffer (50 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) at pH 7.0, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 0.5% sodium deoxycholate, 1% NP-40, 1.5 mM MgCl2, and 10 mM EDTA) with proteinase inhibitor cocktail added (Roche Diagnostics Corporation) at 4°C. Protein lysate (20 µg) was loaded and separated on 4–20% Tris-Glycine gels (Invitrogen) and transferred to pre-wetted polyvinylidene fluoride membranes. Membranes were blocked with 4% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 hour at room temperature. Membranes were then incubated with primary antibody overnight at 4°C. Blots were washed with TBST three times for 15 minutes each and then incubated for 1 hour at room temperature with secondary antibody. Bands were visualized using enhanced chemiluminescence detection reagent (Amersham Pharmacia). Antibodies against pAKT, pS6, p4EBP1, pPRAS40, pJNK, pp38, total AKT, total S6, total 4EBP1, and total PRAS40 were obtained from Cell Signaling Technology; anti-β-actin antibody from Sigma-Aldrich; and anti-rabbit and anti-mouse antibodies from Amersham Pharmacia.
Animal Model
Athymic 4- to 6-week-old nude-Foxn1nu mice (Taconic Farms) were used for all in vivo experiments. All procedures were performed according to a protocol approved by the University of California −San Francisco (UCSF) Institutional Animal Care and Use Committee (IACUC). GBM 39 cells with luciferase gene modification were harvested from the flanks of nude mice. Cells were mechanically disaggregated, filtered, and washed 3 times with fresh media. Nude mice were manually injected with 3 ×105 cells intracranially in the right caudate-putamen using a 26G needle attached to a Hamilton syringe. Mice were randomized into groups of 10 animals per group based on bioluminescence on day 20 after injection, and treatments were started on day 21. Mice were treated as follows: (1) Control: 100 µl of Ora-Plus (Paddock Laboratories) by oral gavage once per day on days 21–25, 28–32, 49–53, and 56–60; (2) XL765: 100 µl (50 mg/kg dissolved in 10 mM HCl) by oral gavage twice per day (6 hours apart) on days 21–25, 28–32, 49–53, and 56–60; and (3) TMZ: 100 µl (5 mg/kg dissolved in Ora-Plus) once per day on days 21–25 and 49–53. The dose of XL765 (50 mg/kg twice a day) was chosen after higher doses (specifically 60 mg/kg twice a day and 100 mg once a day) resulted in progressive body weight loss and morbidity requiring euthanasia according to IACUC humane treatment guidelines. When XL765 and TMZ were given in combination, TMZ was given 30 minutes after XL765. Mouse weights were recorded daily. If an animal's weight dropped 10% below baseline, treatment was withheld until the weight recovered. Animals were sacrificed if they became symptomatic from intracranial tumor burden according to the IACUC protocol.
Bioluminescence Imaging
In vivo bioluminescence images were obtained using the IVIS Imaging System 100 series (Xenogen Corporation). Beginning on day 11 post-injection, mice were injected with 150 mg/kg i.p. D-luciferin. Thirteen minutes after injection, mice were anesthetized with isoflurane and imaged using various exposure times (ranging from 1 s to 2 m) to optimize images. Whole brain bioluminescence was measured for each mouse as well as mean bioluminescence for each treatment group. All mice were imaged 3 times per week, on Monday, Wednesday, and Friday. Previously published data using our model have documented a good correlation between measured bioluminescence, tumor burden, and benefit or detriment in animal survival.40 For statistical analysis comparing treatment groups, bioluminescence values from the last day where all mice in both groups were alive were used.
Immunohistochemistry
Mice whose tumors were to be harvested for immunohistochemistry (IHC) were monitored with bioluminescence imaging until their relative radiance was between 5 ×105 and 1 ×106, at which point they were treated by the appropriate agents (control, XL765, TMZ, or XL765 + TMZ) for 2 consecutive days and then sacrificed. Brains were harvested and frozen in optimum cutting temperature compound (Tissue Tek) and placed in −80°C. Brains were then sectioned (UCSF tissue core facility), stained with primary antibody against pS6 (UCSF IHC and molecular pathology core facility), and photographed and interpreted by Dr. Joanna Phillips (UCSF Department of Pathology). After relative quantification of pS6 staining, representative slides were documented.
Statistical Analysis
All statistical analyses were done under the supervision of the biostatistics division of the UCSF Brain Tumor Research Center using the software program MedCalc. Data for cytotoxicity of XL765 at various concentrations were generated by regression analysis with fitting to a quadratic equation (y = a + bx + cx2) with XL765 concentration as the independent variable. To determine whether the combination of XL765 + TMZ significantly differed from single agent treatment alone in vitro, the most effective single agent (XL765 or TMZ) was compared with the combination using a Wilcoxon rank-sum test. In vivo, tumor bioluminescence between groups was statistically compared using the Mann–Whitney test for independent samples. Survival was analyzed using Kaplan–Meier survival curves.
Results
XL765 Inhibits Activation of PI3 Kinase and mTOR Pathways
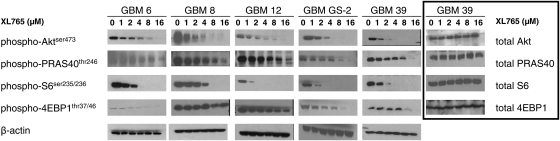
We sought to evaluate effects of XL765 treatment on the PI3K and mTOR pathways in vitro using a variety of human GBM xenografts (Table 1). These xenografts were harvested from the flanks of nude mice, grown in culture, and exposed to increasing concentrations of XL765 as a single agent. Immunoblots were done probing for phosphorylation of key molecules downstream of PI3K (pAkt and pPRAS40) and mTOR (pS6 and p4EBP1). In addition, phosphoproteins within other signaling cascades (pp38 and pJNK) were assessed to determine the specificity of XL765 and showed no changes in phosphorylation (data not shown). After exposure to XL765, cells were lysed and protein expression quantitated at various time points ranging from 1 to 36 hours, with 16 hours determined to be the optimal time point. At this time point, we were able to observe concentration-dependent inhibition of pAkt, pPRAS40, pS6, and p4EBP1 (Fig. 1), with β-actin used as a loading control. Levels of pAkt and pS6 were inhibited completely at concentrations of 2–8 µM, while pPRAS40 and p4EBP1 generally required 16 µM for similar levels of inhibition. Conversely, expression of nonphosphoprotein expression was not affected by XL765 in GBM 39, as shown in the last column of Figure 1.
Table 1.
Genetic Profiles of Human GBM Xenografts Used in This Study
| Xenograft | EGFR | PTEN | p53 | p16 | MGMT |
|---|---|---|---|---|---|
| GBM 6 | Amplified (vIII) | Null | wt | Null | Nonhypermethylated |
| GBM 8 | Amplified (wt) | Null | wt | Null | Hypermethylated |
| GBM 12 | Amplified (wt) | wt | Null | Null | Hypermethylated |
| GBM GS-2 | Nonamplified | Null | Mutant | Unknown | Unknown |
| GBM 39 | Amplified (vIII) | wt | wt | Null | Hypermethylated |
wt, wild type.
Fig. 1.
XL765 results in downregulation of phosphorylated proteins downstream of PI3K (pAkt and pPRAS40) and mTOR (pS6 and p4EBP1) in human xenografts cultured in vitro. Phosphoprotein levels with β-actin loading controls are shown for GBM 6, GBM 8, GBM 12, GBM GS-2, and GBM 39. The same corresponding non-phosphoproteins are shown for GBM 39. Each experiment was done in duplicate with representative examples shown.
Cytotoxic Activity of XL765 and TMZ in GBM Cells In Vitro
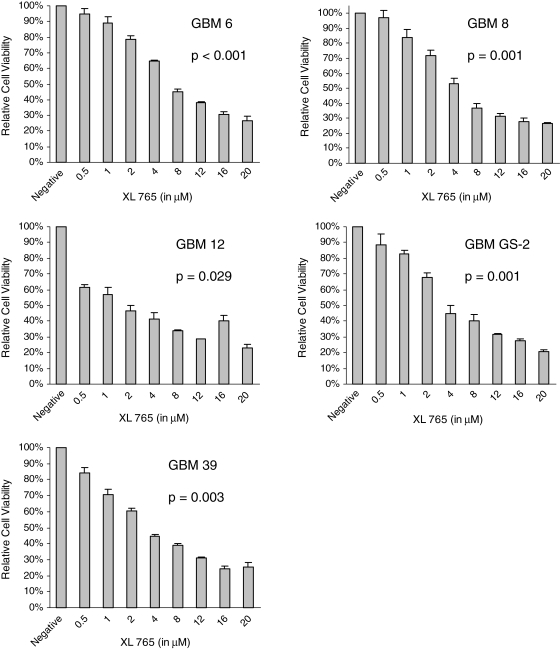
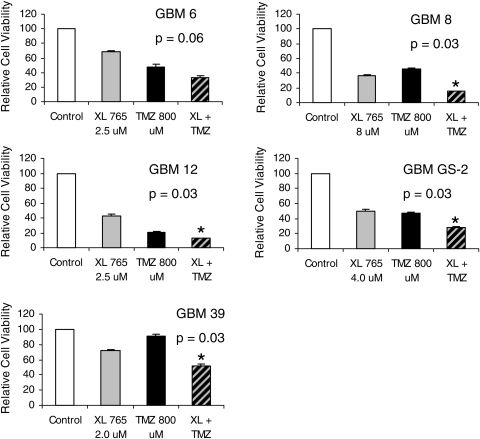
In order to determine the cytotoxicity of XL765 in GBM xenografts in vitro, we used a colormetric ATP-based assay. All 5 GBM xenografts demonstrated a concentration-dependent decrease in cell viability after exposure to XL765 that was statistically significant based on quadratic regression analysis (Fig. 2). Based on the regression model, values of the half maximal inhibitory concentration for each xenograft were determined to be 7.5 µM for GBM 6, 5.7 µM for GBM 8, 3.7 µM for GBM 12, 7.7 µM for GBM GS-2, and 5.0 µM for GBM 39. Addition of TMZ to XL765 resulted in a statistically significant improvement in cytotoxicity in 4 out of 5 GBM xenografts, compared with the most cytotoxic single agent (Fig. 3).
Fig. 2.
Inhibition of PI3K and mTOR results in a concentration-dependent increase of cytotoxicity in human GBM xenografts cultured in vitro. Results are presented as the mean ± standard error of experiments done in triplicate. p-Values were generated using quadratic regression analysis.
Fig. 3.
Combinations of XL765 and TMZ result in additive cytotoxicity in 4 out of 5 GBM xenografts cultured in vitro. p-Values were generated using a Wilcoxon rank-sum test comparing XL765 + TMZ with the most effective single agent (XL765 or TMZ) in each GBM xenograft. * denotes p<0.05.
Synergistic Activity of XL765 and TMZ In Vivo
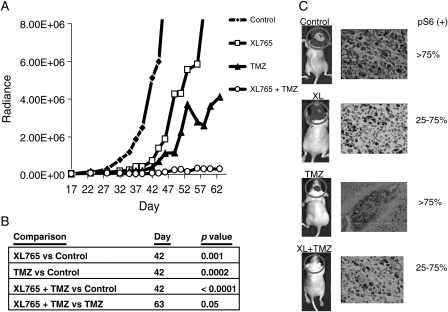
To evaluate the effects of XL765 on tumor growth in vivo, we established tumors as intracranial xenografts in nude mice. GBM 39 cells had been modified to express a luciferase reporter in order to track tumor growth in vivo noninvasively. Mice were implanted intracranially with 5 ×105 GBM 39-luc cells. Twenty-one days post-implantation, intracranial tumor volumes were quantitated and mice were randomized into treatment groups. On day 42, the last day that all animals were alive, median bioluminescence values were 4.0 ×106 for control, 3.2 ×105 for XL765 (30 mg/kg), 1.2 ×105 for TMZ (5 mg/kg), and 2.9 ×104 for the combined treatment (Fig. 4). Therefore, on the last day that all animals were alive, the tumor burden was decreased compared with control by 13-fold in the XL765 group (p = 0.001), 34-fold in the TMZ group (p = 0.0002), and 140-fold in the XL765 + TMZ group (p < 0.0001). Compared with TMZ alone, the combination of XL765 + TMZ showed a 16-fold decrease in tumor burden (p = 0.05) at day 63 (the final day on which all mice in both groups were alive). In addition, IHC staining of tumor sections from these intracranial xenografts showed reduced pS6 expression in groups treated with XL765 (Fig. 4B).
Fig. 4.
Tumor burden as measured by bioluminescence is decreased after oral administration of XL765, TMZ, or XL765 + TMZ in nude mice bearing intracranial GBM 39 xenografts. Two weeks after xenograft injection, mice were randomized into 1 of 4 treatment groups. (A) Average bioluminescent values in each treatment group measured 3 times a week are shown until the last day on which all mice in the group were alive. (B) p-Values represent statistical comparisons between treatment groups using the Mann–Whitney test between independent samples; XL765 and TMZ groups were compared with control at day 46; XL765 + TMZ was compared with control at day 46 and with TMZ at day 63. (C) Representative bioluminescence images in each treatment group on day 46. Representative tumor sections stained for phosphorylated S6 are shown with samples treated with control or TMZ alone demonstrating >75% positive staining and tumors treated with XL765 alone or XL765 + TMZ demonstrating <75% positive staining.
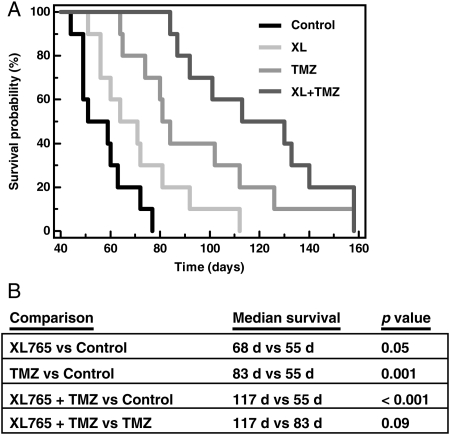
To analyze survival times of the treatment groups, we generated Kaplan–Meier survival curves (Fig. 5). Median survival times were 55 days for control, 68 days for XL765 (p = 0.05, compared with control), 83 days for TMZ (p = 0.001), and 117 days for XL765 + TMZ (p < 0.001). In comparison with TMZ monotherapy, XL765 + TMZ demonstrated a trend toward a survival advantage (p = 0.09). Clinically, there were no physical or behavioral differences noted between mice in the control group and those in the 3 treatment groups. Specifically, there were no statistically significant differences in constitutional signs, including animal weights in any of the treatment groups compared with control (data not shown), nor were there clinical signs of toxicity.
Fig. 5.
Survival of mice increases after oral administration of XL765, TMZ, or XL765 + TMZ in nude mice bearing intracranial GBM 39 xenografts. (A) Kaplan–Meier survival curves. (B) Statistical comparisons using log-rank tests. XL765 and TMZ were compared with the control group, while XL765 + TMZ was compared with the control group and TMZ alone.
Discussion
Despite the plethora of targeted inhibitors identified and studied preclinically in GBM, appreciable improvements in patient survival have not followed.2 One issue is the plasticity of GBM with its redundant signaling inputs22 and ability to bypass blockade of individual molecules by feedback loops.11,23 Paramount among these is the negative feedback loop by which inhibition of mTOR can trigger a negative feedback loop that results in PI3K/Akt activation. Therefore a combinatorial approach of PI3K inhibition, the common target for multiple RTKs, along with abrogation of mTOR to prevent feedback activation of Akt has been proposed and validated preclinically in multiple tumor types.24–29 XL765 is a novel, orally bioavailable PI3K/mTOR dual inhibitor that can be safely administered with no established maximally tolerated dose in phase I trials.21 In our study, we examined the effects of XL765 in a panel of genetically diverse GBM xenografts ± TMZ, the standard-of-care chemotherapeutic agent in postoperative GBM treatment.1 To our knowledge, this is the first study to evaluate a PI3K/mTOR inhibitor in GBM using multiple, distinct, and characterized xenografts. Given the genetic heterogeneity of GBM, this is an important consideration to identify susceptible genotypes.
Regardless of genetic background, XL765 demonstrated specific inhibition of the PI3K/mTOR pathway (Fig. 1). All 5 xenografts showed highly robust inhibition of pAkt (biomarker for PI3K) and pS6 (biomarker for mTOR), with >50% inhibition of phosphorylation within the 2- to 4-µM range. GBM 8 and GBM 12 (both of which are EGFR wt [amplified], p16 null, and MGMT hypermethylated) were relatively resistant to p4EBP1 modulation even at high concentrations of XL765. Expression of unrelated phosphoproteins pp38 and pJNK were also queried and showed no change after exposure to XL765 (data not shown). Similarly, there was no effect on non-phosphoproteins in the PI3K pathway.
Activation of the PI3K pathway has been shown to promote survival-related genes,30 invasion of surrounding brain parenchyma,31 and recruitment of neural stem cells that are pro-invasion.32 Given this central role of PI3K in GBM, it is not surprising that XL765 resulted in in vitro cytotoxicity. Since TMZ is an important component of multimodality GBM treatment, we combined it with XL765 and showed statistically significant chemosensitization in 4 of 5 xenografts. To determine the mechanism of XL765-induced cytotoxicity, we performed several additional in vitro assays, including staining with Annexin V–fluorescein isothiocyanate to quantify apoptosis, propidium iodide staining to measure cell cycle effects, and clonogenic assays to evaluate reproductive cell death (data not shown). These experiments demonstrated equivocal results when XL765-treated cells were compared with controls. This was likely due to the fact that our xenografts were removed from animals and cultured in vitro for short periods of time and not used to establish primary cell cultures, which are more amenable to prolonged in vitro manipulation.
To evaluate the effects of XL765 ± TMZ in vivo, we injected mice intracranially with luciferase-expressing GBM 39 xenografts. We began treatments on day 21 after tumor injection, and our XL765 dosing schedule of 30 mg/kg was given twice per day (Monday–Friday, 6 h apart) for a period of 2 consecutive weeks without evidence of clinical toxicity or significant weight loss. A dosing schedule of 30 mg/kg twice per day was chosen over 100 mg/kg every other day due to preliminary evidence of increased toxicity with the latter dose (data not shown). XL765 showed benefits in growth reduction (∼13 fold), survival, and in vivo reduction in pS6 expression by IHC compared with control, differences that were all statistically significant.
In GBMs that have MGMT hypermethylation, patients have improved outcomes particularly when TMZ is used.33 Thus we were cognizant of the fact that our GBM 39 xenograft model (with MGMT hypermethylation) would likely be sensitive to TMZ. Despite this, combination of XL765 with TMZ still demonstrated a greater than 10-fold decrease in average tumor bioluminescence compared to TMZ alone. As seen in Figure 4B, levels of bioluminescence in the combination group were essentially the same as background, indicating very robust tumor regression. While mice in the combination group did have a longer median survival than mice receiving TMZ alone, this did not quite reach the level of statistical significance (p = 0.09). This is likely due to the MGMT status of GBM 39, which already contributes to baseline TMZ sensitivity.
In our study we have shown that a combined PI3K/mTOR inhibitor can be successfully utilized against GBM xenografts with a diverse genetic background. We hypothesize that XL765 would be most effective in patients with activating mutations in the PI3K pathway, specifically when EGFR is activated (through amplification of the wild-type form in GBMs 8, 12, and GS-2 or the vIII variant in GBMs 6 and 39) or PTEN is homozygously deleted (GBM 8 and GS-2). This hypothesis is based on two lines of reasoning: first, mTOR inhibitors are particularly effective against tumors with increased PI3K activity39; second, erlotinib, a small-molecule inhibitor of epithelial growth factor receptor (EGFR), is most valuable in tumors with permissive genetic backgrounds that include activating EGFR mutations.37,38
The utility of surrogate biomarkers in predicting responses to EGFR inhibitors in prospective clinical trials of GBMs is controversial. In a randomized trial by the European Organisation for Research and Treatment of Cancer comparing progression-free survival (PFS) using TMZ versus erlotinib in recurrent GBM, the activity of the latter could not be predicted by activating mutations in EGFR or expression of pAkt or PTEN.41 Similar conclusions were reached in 2 other multi-institutional single-arm prospective clinical trials that failed to identify biomarkers that predict response to erlotinib in recurrent GBMs.42,43 In studies in which erlotinib has been used up-front with chemoradiation as part of primary GBM treatment, attempts to identify biomarkers have also resulted in contradictory findings: while a single-arm prospective study by the North Central Cancer Treatment Group44 failed to find a relationship between biomarkers and tumor response, a similar study by UCSF45 found an improvement in overall survival in a subset of patients with MGMT hypermethylation and intact PTEN. Though it may be tempting to conclude that failure of biomarkers to predict erlotinib activity in some of these trials implies a lack of benefit of EGFR inhibition in GBMs, there are several non–mutually exclusive alternate hypotheses: (1) use of a single EGFR inhibitor simply forces cancer cells to adapt by coactivating nontargeted tyrosine kinases,22 (2) there exists an excess toxicity of erlotinib,46 (3) erlotinib exists in tumors in subtherapeutic concentration,42 and (4) insufficient patients were in surrogate biomarker groups to reach statistical significance.
The xenograft tested in our in vivo experiment had EGFR vIII amplification and wild-type PTEN and was indeed relatively sensitive to XL765. We would predict that a xenograft with EGFR vIII amplification and loss of PTEN would exhibit even greater sensitivity to XL765, and these experiments are ongoing. Furthermore, tumors without PI3K activation (granted, a minority of GBMs) may exhibit less in vivo sensitivity to XL765, and we are testing this hypothesis. Genetic background has bearing on a second issue—that of sensitization of tumors to TMZ. In vitro, inhibition of the PI3K pathway sensitized xenografts to TMZ in nearly all cases; and in vivo, XL765 showed marked sensitization to TMZ in a xenograft with MGMT hypermethylated. Whether such dramatic sensitization to TMZ will be evident in GBMs without MGMT hypermethylated is a topic for further studies. Lastly, combining erlotinib with XL765 is intriguing from mechanistic and efficacy viewpoints, particularly given recent clinical data using the former in GBM.34 Based in part on our preclinical data, a multi-institutional phase I trial studying the safety and tolerability of XL765 combined with TMZ is currently accruing.35
Acknowledgments
This research was supported in part by NIH-PO1 NS-42927-27A2 (D.H.-K., M.S.B.), NIH Brain Tumor SPORE grant P50 CA097257 (D.H.-K., C.D.J., M.-Y.P., M.S.B., M.D.P), UCSF Resident Research Fund (G.P., D.H.-K.), Nancy and Stephen Grand Philanthropic Fund (D.H.-K.), the V Foundation (D.H.-K.), and the Thrasher Foundation (D.H.-K.). Portions of this study were presented at the 2009 Society for Neuro-Oncology Annual Meeting as an oral presentation.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 4.Dahlback HS, Brandal P, Meling TR, Gorunova L, Scheie D, Heim S. Genomic aberrations in 80 cases of primary glioblastoma multiforme: pathogenetic heterogeneity and putative cytogenetic pathways. Genes Chromosomes Cancer. 2009;48:908–924. doi: 10.1002/gcc.20690. [DOI] [PubMed] [Google Scholar]
- 5.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3’-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin Cancer Res. 2000;6:880–886. [PubMed] [Google Scholar]
- 8.Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. [PubMed] [Google Scholar]
- 9.Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 10.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 11.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong D, Yamori T. Advances in development of phosphatidylinositol 3-kinase inhibitors. Curr Med Chem. 2009;16:2839–2854. doi: 10.2174/092986709788803222. [DOI] [PubMed] [Google Scholar]
- 13.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan QW, Cheng CK, Nicolaides TP, et al. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 16.Westhoff MA, Kandenwein JA, Karl S, et al. The pyridinylfuranopyrimidine inhibitor, PI-103, chemosensitizes glioblastoma cells for apoptosis by inhibiting DNA repair. Oncogene. 2009;28:3586–3596. doi: 10.1038/onc.2009.215. [DOI] [PubMed] [Google Scholar]
- 17.Crane C, Panner A, Pieper RO, Arbiser J, Parsa AT. Honokiol-mediated inhibition of PI3K/mTOR pathway: a potential strategy to overcome immunoresistance in glioma, breast, and prostate carcinoma without impacting T cell function. J Immunother. 2009;32:585–592. doi: 10.1097/CJI.0b013e3181a8efe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu TJ, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 20.Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. J Vis Exp. 2010;41 doi: 10.3791/1986. pii: 1986. doi: 10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LoRusso P, Markman B, Tabernero J, et al. A phase 1 dose-escalation study of the safety, pharmacokinetics and pharmacodynamics of XL765, a PI3K/TORC1/TORC2 inhibitor administered orally to patients with advanced solid tumors [abstract] J Clin Oncol. 2009;27 15s (suppl; abstr 3502) [Google Scholar]
- 22.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinnberg T, Lasithiotakis K, Niessner H, et al. Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol. 2009;129:1500–1155. doi: 10.1038/jid.2008.379. [DOI] [PubMed] [Google Scholar]
- 25.Presneau N, Shalaby A, Idowu B, et al. Potential therapeutic targets for chordoma: PI3K/AKT/TSC1/TSC2/mTOR pathway. Br J Cancer. 2009;100:1406–1414. doi: 10.1038/sj.bjc.6605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: A potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 27.Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH, Sun CF. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2010;48(4):291–296. doi: 10.1016/j.bjoms.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HY, Zhang PN, Sun H. Aberration of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol Reprod Biol. 2009;146:81–86. doi: 10.1016/j.ejogrb.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 29.McMillin DW, Ooi M, Delmore J, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–5842. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- 30.Ruano Y, Mollejo M, Camacho FI, et al. Identification of survival-related genes of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma multiforme. Cancer. 2008;112:1575–1584. doi: 10.1002/cncr.23338. [DOI] [PubMed] [Google Scholar]
- 31.Kleber S, Sancho-Martinez I, Wiestler B, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13:235–248. doi: 10.1016/j.ccr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Kendall SE, Najbauer J, Johnston HF, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26:1575–1586. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 34.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinicaltrials.gov. A study of XL765 in combination with temozolomide in adults with malignant gliomas. http://www.clinicaltrials.gov/ct2/show/NCT00704080 . Accessed February 4, 2010. [Google Scholar]
- 36.McBride SM, Perez DA, Polley MY, et al. Activation of PI3K/mTOR pathway occurs in most adult low-grade gliomas and predicts patient survival. J Neurooncol. 2010;97:33–40. doi: 10.1007/s11060-009-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 38.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 39.Brachmann S, Fritsch C, Maira SM, Garcia-Echeverria C. PI3K and mTOR inhibitors: a new generation of targeted anticancer agents. Curr Opin Cell Biol. 2009;21:194–198. doi: 10.1016/j.ceb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Dinca EB, Sarkaria JN, Schroeder MA, et al. Bioluminescence monitoring of intracranial glioblastoma xenograft: response to primary and salvage temozolomide therapy. J Neurosurg. 2007;107:610–617. doi: 10.3171/JNS-07/09/0610. [DOI] [PubMed] [Google Scholar]
- 41.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raizer JJ, Abrey LE, Lassman AB, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12:95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yung WK, Vredenburgh JJ, Cloughesy TF, et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a phase II open-label study. Neuro Oncol. 2010;12:1061–1070. doi: 10.1093/neuonc/noq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98:93–99. doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]