Abstract
This phase II study evaluated the efficacy and safety of AMG 102 (rilotumumab), a fully human monoclonal antibody against hepatocyte growth factor/scatter factor (HGF/SF), in patients with recurrent glioblastoma (GBM). Patients with histologically confirmed, measurable recurrent GBM or gliosarcoma (World Health Organization grade 4) and ≤3 relapses or prior systemic therapies received AMG 102 (10 or 20 mg/kg) by infusion every 2 weeks. The primary endpoint was best confirmed objective response rate (central assessment) per Macdonald criteria. Of the 61 patients who enrolled, 60 received AMG 102. Twenty-nine patients (48%) had previously received bevacizumab. There were no objective responses per central assessment, but 1 patient had an objective response per investigator assessment. Median overall survival (95% CI) in the 10- and 20-mg/kg cohorts was 6.5 months (4.1–9.8) and 5.4 months (3.4–11.4), respectively, and progression-free survival (PFS) per central assessment was 4.1 weeks (4.0–4.1) and 4.3 weeks (4.1–8.1), respectively. PFS was similar among patients who had previously received bevacizumab compared with bevacizumab-naive patients. The most common adverse events were fatigue (38%), headache (33%), and peripheral edema (23%). AMG 102 serum concentrations increased approximately dose-proportionally with 2-fold accumulation at steady state. Plasma total HGF/SF and soluble c-Met concentrations increased 12.05- and 1.12-fold, respectively, from baseline during AMG 102 treatment. AMG 102 monotherapy at doses up to 20 mg/kg was not associated with significant antitumor activity in heavily pretreated patients with recurrent GBM.
Keywords: Rilotumumab, AMG 102, hepatocyte growth factor, c-Met, glioblastoma, phase II clinical trial
High-grade gliomas are associated with a poor prognosis.1–3 Surgical resection followed by radiotherapy and adjuvant temozolomide is the standard of care for patients with newly diagnosed glioblastoma (GBM), but median overall survival (OS) is 14.6 months, and the 2-year survival rate is approximately 27%.4 Because therapies for recurrent high-grade gliomas are limited, there has been increasing interest in investigational therapies.5,6 Bevacizumab recently received FDA approval as a salvage therapy for recurrent GBM and is currently the only available targeted therapy in recurrent GBM.7,8
Hepatocyte growth factor/scatter factor (HGF/SF) and its receptor c-Met have been implicated in the pathogenesis of GBM through autocrine and/or paracrine mechanisms, potentially affecting tumor cell growth, survival, invasion, migration, and angiogenesis.9–12 Intratumoral HGF/SF and c-Met expression and cerebrospinal fluid HGF/SF concentrations have been associated with poor prognosis in GBM.13–15 In preclinical studies, inhibition of the HGF/SF–c-Met axis resulted in regression of human GBM tumor xenografts.10,16,17
AMG 102 (rilotumumab) is a fully human monoclonal antibody that specifically targets HGF/SF and has shown antitumor activity in vitro and in U-87 MG tumor xenograft models as a single agent and in combination with temozolomide.18,19 In a phase I, first-in-humans study, AMG 102 was well tolerated and had linear pharmacokinetics (PK) up to the planned maximum dose of 20 mg/kg.22 The objective of this study was to evaluate the efficacy and safety of AMG 102 in patients with recurrent GBM.
Patients and Methods
Patients
Eligible patients (aged ≥18 years) had histologically confirmed GBM or gliosarcoma (World Health Organization grade 4), bidimensionally measurable and recurrent disease as assessed by MRI, Karnofsky performance score ≥60%, ≤3 prior relapses or systemic treatments, and archived tissue from initial diagnosis or upon transformation to glioblastoma. Key exclusion criteria were history of another neoplasm (except curatively treated); hemorrhagic stroke or intraocular bleeding within 6 months of enrollment; acute intracranial/intratumoral hemorrhage (except stable grade 1); severe or uncontrolled medical disease; inadequate cardiac, hepatic, hematologic, or renal function; prior treatment with c-Met- or HGF/SF-targeted therapy; surgical resection of brain tumor within 4 weeks of enrollment; major surgery or treatment with radiation therapy, immunotherapeutic agents, vaccines, monoclonal antibody therapy, or alkylating agents within 4 weeks of enrollment; or treatment with thalidomide, tamoxifen, or anticoagulation therapy (except low-molecular-weight heparins or warfarin) within 1 week of enrollment. Prior treatment with bevacizumab and other antiangiogenic agents was permitted. The study protocol was approved by an institutional review board at each participating site. All procedures were performed in accordance with the ethical standards of the Helsinki Declaration. All patients provided written informed consent.
Study Design
This multicenter, open-label, single-agent, 2-stage, phase II study of AMG 102 in patients with recurrent GBM was conducted at 6 centers. The primary endpoint was best confirmed objective response rate (ORR; complete response and partial response) per Macdonald criteria by independent radiologic assessment.23 Secondary endpoints included adverse events (AEs), OS, progression-free survival (PFS), duration of response, time to response, and AMG 102 PK. Exploratory endpoints included the pharmacodynamic tumor response of AMG 102 as assessed by HGF/SF–c-Met axis markers and variation in cancer and drug-target genes. Exploratory endpoints not reported in this manuscript due to space limitations included objective response predicted by changes in cerebral blood volume, functional diffusion maps, or proton magnetic resonance spectroscopic imaging.
Patients received AMG 102 10 or 20 mg/kg by intravenous infusion (over 30–60 min) every 2 weeks. Based on assessments of serum concentrations of AMG 102 observed in the first-in-humans study22 and values of 90% inhibitory concentrations predicted with U-87 MG glioblastoma cell proliferation assays,18 10 mg/kg was selected as the starting dose of AMG 102. Initially, 20 patients were to receive the 10-mg/kg dose. If ≥1 minor response (>25% reduction from baseline tumor sum of longest diameters) was observed (per investigator assessment) at week 9, then an additional 20 patients were to be enrolled in the 10-mg/kg cohort. If no response was observed in the initial 20 patients or if <5 responses were observed in the total of 40 patients, then an additional 20 patients were to receive the 20-mg/kg dose. If either the 10- or 20-mg/kg dose continued to the second stage of accrual, then the primary evaluation of best ORR was determined by central assessment. Intrapatient AMG 102 dose modifications were not permitted. Doses of AMG 102 were withheld for grade 3 treatment-related toxicities or serious AEs or any grade 4 toxicity until resolved. Treatment was withdrawn in patients requiring ≥3 weeks to recover from grade 4 toxicities.
Tumor Assessments
For the primary analysis of efficacy, tumor response was assessed per Macdonald criteria23 by independent central radiologic assessment (MedQIA, Los Angeles, CA). Contrast- and non–contrast-enhanced MRI was performed at baseline (within 14 days before enrollment), weeks 5 and 9, and every 8 weeks thereafter or as clinically indicated.
Safety
All AEs occurring from enrollment until the end of study were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Clinical and laboratory assessments were performed at baseline, week 1, and every 2 weeks thereafter during treatment. Blood samples were collected predose; at weeks 1, 5, and 9; and every 8 weeks thereafter and analyzed for anti–AMG 102 antibodies using an electrochemiluminescent immunoassay.22
Pharmacokinetics
Blood samples for AMG 102 PK analysis were collected prior to the end of AMG 102 infusion at weeks 1, 5, and 9. The serum AMG 102 concentrations were measured as described previously.22 Descriptive statistics were used to summarize AMG 102 concentration data.
Biomarker Development
Plasma samples for the exploratory analysis of soluble c-Met and total HGF/SF were collected before and after AMG 102 dosing at week 1 and after dosing at weeks 5 and 9, every 8 weeks thereafter, and the end of treatment. As described previously,22 plasma total HGF/SF (pro-HGF/SF, mature HGF/SF, and AMG 102–bound HGF/SF) was measured by sandwich enzyme-linked immunosorbent assay, and soluble c-Met was measured by a Meso Scale Discovery assay. In an exploratory analysis, archival tumor samples were stained for cytoplasmic and membrane c-Met staining by immunohistochemistry as described previously.22 The c-Met assay was evaluated by a pathologist on a semiquantitative scale, and the percentage of cancer cells staining at each of the following 4 levels was recorded: 0 (unstained), 1+ (weak staining), 2+ (moderate staining), and 3+ (strong staining). The presence or absence of epidermal growth factor receptor (EGFR) mutant EGFRvIII RNA was determined by quantitative reverse transcription (qRT) PCR analysis.24
Changes in log total HGF/SF and soluble c-Met changes over time were analyzed using repeated-measures analysis of variance (ANOVA); their relationships with PFS and best ORR were assessed using proportional hazards and nominal logistics models and 2-way ANOVA. For c-Met immunohistochemistry, associations between categorical variables were assessed with a logistic model using a likelihood ratio test; a log-rank test was used to assess differences in PFS among categorical groups. Relationships between EGFRvIII mutations and PFS were assessed by log-rank and Wilcoxon tests.
Statistical Analysis
The study design had a power of 0.90 for a given cohort to distinguish between an active drug with a 20% true response rate and a drug with a response rate of ≤5% with an alpha level of 0.05. The best ORR (complete response and partial response) was summarized by dose by calculating the response rate with an exact 2-sided 95% confidence interval (CI) as described previously.25 For PFS and OS, Kaplan–Meier estimates and 2-sided 95% CIs for rates at 8-week intervals and for quartiles were calculated, and group differences were evaluated with the log-rank test. PFS was calculated as the time in weeks from the first dose of AMG 102 to disease progression (per Macdonald criteria) or death from any cause. The primary and secondary endpoint and safety analyses were conducted for all patients who received ≥ 1 dose of AMG 102 in each cohort. SAS software (version 9.1; SAS Institute, Cary, NC) was used for statistical analyses.
Results
Patients
Between January 12, 2007, and February 20, 2008, 61 patients were enrolled in this study (10-mg/kg cohort, n = 41; 20-mg/kg cohort, n = 20) and received AMG 102, except for 1 patient (10-mg/kg cohort) who was not treated (Table 1). Approximately one half (n = 29, 48%) of patients had previously received bevacizumab (10-mg/kg cohort, n = 19 [48%]; 20-mg/kg cohort, n = 10 [50%]). Some patients received agents with potential anti-angiogenic activity, including vascular endothelial growth factor (VEGF) receptor 2 tyrosine kinase inhibitors (n = 5), aflibercept (n = 4), cilengitide (n = 2), thalidomide or lenalidomide (n = 2), and/or other non–anti-angiogenic targeted therapies (n = 15). Fifty-nine patients (98%) received prior radiotherapy (10-mg/kg cohort, n = 39 [98%]; 20-mg/kg cohort, n = 20 [100%]), including 6 (all in the 10-mg/kg cohort) who received treatment within 12 weeks of enrollment and thus may have exhibited pseudoprogression.26 The median (range) duration of treatment with AMG 102 was 4.1 weeks (2.1–167.7) and 4.1 weeks (1.9–25.0) in the 10- and 20-mg/kg cohorts, respectively. The median (range) follow-up times were 27.6 weeks (3.0–106.1) and 23.4 weeks (3.1–56.7) in the 10- and 20-mg/kg dose cohorts, respectively. The reasons for discontinuing the study were disease progression (n = 52 [87%]), consent withdrawn (n = 3 [5%]), AEs (n = 2 [3%]), protocol deviation (n = 1 [2%]), and death (n = 1 [2%]). As of May 2010, a 51-year-old white male patient (10-mg/kg cohort) diagnosed with stage IV GBM and 90% KPS was still receiving AMG 102 after 167.7 weeks.
Table 1.
Demographics and Key Baseline Characteristics
| Characteristics | AMG 102 10 mg/kg n = 40 | AMG 102 20 mg/kg n = 20 | All Enrolled Patients N = 60a |
|---|---|---|---|
| Sex, n (%) | |||
| Women | 15 (38) | 7 (35) | 22 (37) |
| Men | 25 (63) | 13 (65) | 38 (63) |
| Race, n (%) | |||
| White | 36 (90) | 18 (90) | 54 (90) |
| Black | 3 (8) | 0 (0) | 3 (5) |
| Hispanic | 1 (3) | 1 (5) | 2 (3) |
| Asian | 0 (0) | 1 (5) | 1 (2) |
| Age, median (range) | 54 (19–71) | 54 (26–71) | 54 (19–71) |
| Karnofsky performance status, n (%) | |||
| 100 | 1 (3) | 1 (5) | 2 (3) |
| 90 | 18 (45) | 7 (35) | 25 (42) |
| 80 | 14 (35) | 7 (35) | 21 (35) |
| 70 | 4 (10) | 3 (15) | 7 (12) |
| 60 | 3 (8) | 2 (10) | 5 (8) |
| Histologic type for primary tumor, n (%) | |||
| Glioblastoma multiforme | 38 (95) | 20 (100) | 58 (97) |
| Low-grade gliomab | 1 (3) | 0 (0) | 1 (2) |
| Other | 1 (3) | 0 (0) | 1 (2) |
| Months since initial diagnosis,c | |||
| median (range) | 16 (3.9–78) | 14 (1–40) | 16 (1–78) |
| Prior therapies,dn (%) | |||
| 0 | 0 (0) | 0 (0) | 0 (0) |
| 1 | 11 (28) | 3 (15) | 14 (23) |
| 2 | 13 (33) | 9 (45) | 22 (37) |
| ≥3 | 16 (40) | 8 (40) | 24 (40) |
| Prior radiotherapy, n (%) | |||
| 0 | 1 (3) | 0 (0) | 1 (2) |
| 1 | 33 (85) | 19 (95) | 52 (87) |
| 2 | 5 (13) | 1 (5) | 6 (10) |
| ≥3 | 1 (3) | 0 (0) | 1 (2) |
| Prior VEGF pathway inhibitors, n (%) | |||
| Bevacizumab | 19 (48) | 10 (50) | 29 (48) |
| Aflibercept | 3 (8) | 1 (5) | 4 (7) |
| Sorafenib | 0 (0) | 2 (10) | 2 (3) |
| Pazopanib | 1 (3) | 0 (0) | 1 (2) |
| Vandetanib | 1 (3) | 0 (0) | 1 (2) |
| Vatalanib | 1 (3) | 0 (0) | 1 (2) |
| Prior surgery,en (%) | 40 (100) | 20 (100) | 60 (100) |
aPatients who received ≥1 dose of AMG 102.
bEnrollment of the patient with low-grade disease was a protocol violation.
cDate of enrollment minus date of primary diagnosis.
dIncludes chemotherapy, immunotherapy, hormonal therapy, targeted biologics, small-molecule inhibitors, vaccines, and others.
ePrior surgery includes biopsy and resection procedures.
Best Objective Response
Investigator assessment.—The rates of best-response stable disease, regardless of prior bevacizumab therapy, were generally consistent between central assessment and investigator assessment. However, a patient with GBM (10-mg/kg cohort) with stable disease per central assessment had a confirmed partial response per investigator assessment (Table 2).
Table 2.
Tumor Responsea
| Central Assessment |
Investigator Assessment |
|||
|---|---|---|---|---|
| Parameter | AMG 102 10 mg/kg (n = 40) | AMG 102 20 mg/kg (n = 20) | AMG 102 10 mg/kg (n = 40) | AMG 102 20 mg/kg (n = 20) |
| Tumor response | ||||
| Best confirmed response, n (%)b | ||||
| Complete response | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Partial response | 0 (0) | 0 (0) | 1 (3)c | 0 (0) |
| Stable disease | 4 (10) | 3 (15) | 4 (10) | 3 (15) |
| Progressive disease | 32 (80) | 13 (65) | 32 (80) | 15 (75) |
| Unable to evaluated | 2 (5) | 2 (10) | 1 (3) | 0 (0) |
| Not doned | 2 (5) | 2 (10) | 2 (5) | 2 (10) |
| Progression-free survivale | ||||
| Patients with events, n (%) | 38 (95) | 19 (95) | 37 (93) | 20 (100) |
| Disease progression | 33 (83) | 14 (70) | 34 (85) | 18 (90) |
| Death (any cause) | 5 (13) | 5 (25) | 3 (8) | 2 (10) |
| Kaplan-Meier median progression-free survival time, wk (95% CI) | 4.1 (4.0–4.1) | 4.3 (4.1–8.1) | 4.1 (4.0–4.3) | 4.1 (4.0–8.0) |
| Overall survivale | ||||
| Kaplan-Meier median overall survival time, mo (95% CI) | 6.5 (4.1–9.8) | 5.4 (3.4–11.4) | ||
aSafety analysis set. Tumor assessments taken after initiation of any non–AMG 102 antitumor therapy, tumor resection, or first tumor progression were excluded. Tumor assessments taken >70 d following the last dose of AMG 102 were also excluded.
bResponse based on the Macdonald criteria. Complete responses and partial responses were subsequently confirmed no less than 4 wk after the criteria for response were first met.
cOne patient with a partial response per investigator assessment subsequently had a complete response per investigator assessment following the cut-off date for analysis. The patient continues to receive treatment with AMG 102 (duration of therapy = 167.7 wk).
dPatients with complete responses, partial responses, or stable disease not confirmed after day 49 were classified as unevaluable. Patients without postbaseline assessments due to progressive disease or early death were classified as not done.
ePatients who did not progress or die were censored at the last evaluable radiograph.
Central Assessment
There were no complete or partial responses by central assessment (Table 2). Among patients who received prior bevacizumab therapy, no patients in the 10-mg/kg cohort and 1 patient (10%) in the 20-mg/kg cohort had a best response of stable disease. Among bevacizumab-naive patients, 4 (19%) in the 10-mg/kg cohort and 2 (20%) in the 20-mg/kg cohort had a best response of stable disease.
Progression-Free Survival
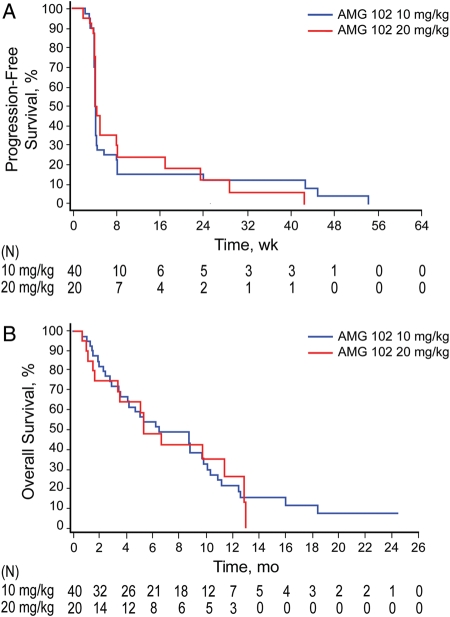
At the time of analysis, 38 patients (95%) in the 10-mg/kg cohort and 19 patients (95%) in the 20-mg/kg cohort had disease progression per central assessment or had died (Table 2). In a post hoc analysis, PFS, OS, and stable disease at 6 months were analyzed according to prior bevacizumab status (Table 3). Among patients who had received prior bevacizumab, estimated median (95% CI) PFS was 4.0 weeks (4.0–4.1) in the 10-mg/kg cohort and 4.1 weeks (3.9–8.1) in the 20-mg/kg cohort. Among patients who had received prior bevacizumab, the percentage (95% CI) of patients with stable disease at 6 months was 5.3% (0.4–21.4) in the 10-mg/kg cohort and 10.0% (0.6–35.8) in the 20-mg/kg cohort. In bevacizumab-naive patients, median PFS was 4.1 weeks (3.9–8.1) in the 10-mg/kg cohort and 4.7 weeks (4.1–17.0) in the 20-mg/kg cohort. The percentage (95% CI) of bevacizumab-naive patients with stable disease at 6 months was 17.9% (5.0–37.1) in the 10-mg/kg cohort and 15.0% (1.0–45.7) in the 20-mg/kg cohort. Overall median PFS is shown in Figure 1A. Median PFS in patients who completed radiotherapy/temozolomide <12 weeks from enrollment (6.0 weeks; n = 6) was not significantly different from that in patients who completed radiotherapy/temozolomide ≥12 weeks from enrollment (4.1 weeks; n = 34) (p = .38).
Table 3.
Patient Outcome According to Prior Treatment with Bevacizumaba
| Bevacizumab-Naive |
Prior Bevacizumab |
|||
|---|---|---|---|---|
| Parameter | AMG 102 10 mg/kg (n = 21) |
AMG 102 20 mg/kg (n = 10) |
AMG 102 10 mg/kg (n = 19) |
AMG 102 20 mg/kg (n = 10) |
| Median progression-free survival, wk (95% CI) | 4.1 (3.9–8.1) | 4.7 (4.1–17.0) | 4.0 (4.0–4.1) | 4.1 (3.9–8.1) |
| Patients with stable disease at 6 mo, % (95% CI) | 17.9 (5.0–37.1) | 15.0 (1.0–45.7) | 5.3 (0.4–21.4) | 10.0 (0.6–35.8) |
| Median overall survival, mo (95% CI) | 10.9 (8.7–16.0) | 11.4 (5.4–13.0) | 3.6 (1.9–6.2) | 3.4 (1.5–6.6) |
aPost hoc analysis of safety analysis set. Tumor assessments taken after initiation of any non–AMG 102 antitumor therapy, tumor resection, or first tumor progression were excluded. Patients who did not progress or die were censored at the last evaluable radiograph.
Fig. 1.
Progression-free survival (PFS) per central assessment (A) and overall survival (B). Tumor assessments that were taken after initiation of any non–AMG 102 antitumor therapy, tumor resection, or first tumor progression were excluded from PFS. All deaths were included. PFS was defined as the time in weeks from the first dose of AMG 102 to first progression by Macdonald criteria or death from any cause, whichever occurred first. Patients who did not progress or die were censored at the last evaluable radiograph. If a patient had no evaluable postbaseline radiograph and did not die, then the patient was censored at day 1.
Overall Survival
The Kaplan-Meier estimate of median (95% CI) OS was 6.5 months (4.1–9.8) for the 10-mg/kg cohort and 5.4 months (3.4–11.4) for the 20-mg/kg cohort (Fig. 1B). Among patients who received prior bevacizumab, estimated median (95% CI) OS was 3.6 months (1.9–6.2) in the 10-mg/kg cohort and 3.4 months (1.5–6.6) in the 20-mg/kg cohort (Table 3). Among bevacizumab-naive patients, estimated median (95% CI) OS was 10.9 months (8.7–16.0) in the 10-mg/kg cohort and 11.4 months (5.4–13.0) in the 20-mg/kg cohort.
Safety
The most common AEs (any grade) were fatigue (38%), headache (33%), and peripheral edema (23%). The incidence of AEs (any grade) considered by investigators to be related to treatment with AMG 102 was 52% (Table 4), the most common being fatigue (25%), nausea (10%), and peripheral edema (8%). The incidence of treatment-emergent edema of any grade (7%) was greater than that of treatment-related edema (3%). Eighteen patients (30%) had serious AEs, the most common (occurring in ≥2 patients) being convulsion (n = 4 [7%]), confusional state (n = 2 [3%]), and edema (n = 2 [3%]). Two patients (both in the 10-mg/kg cohort) discontinued AMG 102 as a result of treatment-related AEs. One patient had serious grade 3 edema considered related to treatment with AMG 102 (Table 4), and a second patient had grade 3 genital edema and peripheral edema; all resolved after treatment was discontinued. Deaths on study were attributed to disease progression (10-mg/kg cohort, n = 6 [15%]; 20-mg/kg cohort, n = 3 [15%]) and brain herniation (20-mg/kg cohort, n = 1 [5%]). Notable grade 3 or 4 laboratory findings included hypophosphatemia (n = 3 [5%]) and hypocalcemia (n = 1 [2%]). There were no notable changes from baseline in creatinine or albumin. No anti–AMG 102 antibodies were detected.
Table 4.
Patient Incidence of Treatment-Related Adverse Eventsa
| AEs | AMG 102 10 mg/kg n = 40 | AMG 102 20 mg/kg n = 20 | All Enrolled Patients N = 60 | |||
|---|---|---|---|---|---|---|
| Patients with any AE, n (%) | 23 (58) | 8 (40) | 31 (52) | |||
| Patients with grade ≥3 AEs,bn (%) | 4 (10) | 1 (5) | 5 (8) | |||
| AEs occurring in ≥2 patients, n (%) | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 |
| Fatigue | 9 (23) | 1 (3) | 6 (30) | 1 (5) | 15 (25) | 2 (3) |
| Nausea | 5 (13) | 0 (0) | 1 (5) | 0 (0) | 6 (10) | 0 (0) |
| Peripheral edemac | 4 (10) | 1 (3) | 1 (5) | 0 (0) | 5 (8) | 1 (2) |
| Diarrhea | 4 (10) | 0 (0) | 0 (0) | 0 (0) | 4 (7) | 0 (0) |
| Dry skin | 4 (10) | 0 (0) | 0 (0) | 0 (0) | 4 (7) | 0 (0) |
| Arthralgia | 3 (8) | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 0 (0) |
| Hypophosphatemia | 3 (8) | 3 (8) | 0 (0) | 0 (0) | 3 (5) | 3 (5) |
| Dysgeusia | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Hypoasthesia | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Edemab | 2 (5) | 1 (3) | 0 (0) | 0 (0) | 2 (3) | 1 (2) |
| Asthenia | 1 (3) | 0 (0) | 1 (5) | 1 (5) | 2 (3) | 1 (2) |
| Patients with any serious AE, n (%) | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
| Edema (grade 3) | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
AE, adverse event.
aAEs are reported for patients who received ≥1 dose of AMG 102 and include all AEs for ongoing patients and AEs with onset from the first dose of AMG 102 until 30 days after the last dose of AMG 102 for patients who discontinued treatment. Partial onset dates were imputed.
bGrade 5 adverse events included disease progression (n = 9) and brain herniation (n = 1). These events were not considered by investigators to be related to treatment with AMG 102.
cThe incidence of treatment-emergent peripheral edema of any grade was greater than the incidence of treatment-related peripheral edema (23% vs 8%), and the incidence of treatment-emergent edema of any grade was greater than the incidence of treatment-related edema (7% vs 3%).
Pharmacokinetics
Pre-infusion and end of infusion AMG 102 concentrations increased approximately dose-proportionally and accumulated by 2-fold at steady state. The end of infusion concentrations at the 10- and 20-mg/kg doses were 237 ± 64 µg/ml and 482 ± 92 µg/ml, respectively, at week 1 and 518 ± 77 µg/ml and 897 ± 437 µg/ml, respectively, at week 9. The pre-infusion concentration at the 10- and 20-mg/kg doses was 218 ± 37 µg/ml and 507 ± 168 µg/ml, respectively, at week 9.
Biomarker Development
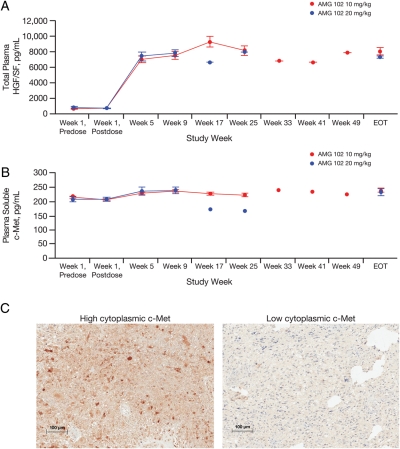
Plasma total HGF/SF and soluble c-Met were measured in 46 patients (n = 30, 10 mg/kg; n = 16, 20 mg/kg) and increased from baseline following AMG 102 administration (12.05-fold, p < .0001, and 1.12-fold, p = .012, respectively; Fig. 2). No significant associations between log baseline concentrations of either marker and PFS, best ORR, or change in target lesion dimensions were observed. Expression of cytoplasmic c-Met protein was detected in 30 of 50 (60%) evaluable samples, 9 (18%) of which had membrane staining. No significant association between c-Met expression and PFS or best ORR was observed. Analysis by qRT-PCR identified EGFRvIII in 26% of 53 evaluable samples, but its presence was not associated with PFS or best ORR.
Fig. 2.
Analysis of hepatocyte growth factor/scatter factor (HGF/SF) and c-Met as biomarkers. Plasma log total HGF/SF (A) and soluble c-Met (B) concentrations from week 1 until the end of treatment (EOT). (C) Representative images of high (maximum intensity scale of 3) versus low (maximum intensity scale of 1) cytoplasmic c-Met expression assessed by immunohistochemistry staining of patient archival tumor samples.
Discussion
AMG 102 did not have single-agent antitumor activity in patients with recurrent GBM, 48% of whom had received prior bevacizumab therapy. No responses were seen per central assessment, although 1 patient had an objective response per investigator assessment. AMG 102 was generally well tolerated at doses of 10 and 20 mg/kg once every 2 weeks.
Although the reasons underlying the lack of activity by AMG 102 in this study are unknown, there are several plausible explanations. The patients were heavily pretreated, the majority having received at least 3 anticancer therapies and at least 1 bevacizumab-based regimen before enrollment. In studies of patients with recurrent disease who progressed following salvage bevacizumab, almost none appeared to benefit from treatment with subsequent bevacizumab-based regimens,7,27 and they had PFS times consistent with those reported in the current study. Similar results were observed in a study of patients with high-grade gliomas who progressed following treatment with VEGF receptor tyrosine kinase inhibitors, in which treatment with bevacizumab was not associated with durable tumor control in most patients.28 Therefore, it is possible that AMG 102 activity may have been more favorable if the study had enrolled only patients who had not previously received treatment with bevacizumab or other VEGF pathway inhibitors. Further, because patients treated with radiotherapy combined with temozolomide may exhibit early enhancement without evidence of recurrence within 3 months of treatment,29 it is possible that the study may have been limited by the inclusion of the 6 patients who received radiotherapy within 3 months of enrollment. However, PFS in these patients did not significantly differ from those patients who completed radiotherapy/temozolomide ≥3 months before enrollment. The blood-brain barrier may also have contributed to the lack of efficacy by AMG 102. Although it is unknown whether AMG 102 is capable of crossing the blood-brain barrier, the anti-HGF/SF monoclonal antibody L2G7 has been shown to accumulate in orthotopic human GBM xenografts in mice and cause tumor regression,20,21 suggesting that AMG 102 had a reasonable chance of crossing the blood-brain barrier. Further, multiple receptor tyrosine kinases are simultaneously activated in GBM tumors,30 potentially explaining the lack of single-agent AMG 102 activity in this study and suggesting that a combination treatment approach may be required. Inhibition of the HGF/SF–c-Met axis combined with radiation therapy or inhibition of EGFRvIII synergistically inhibited tumor growth in U-87 MG xenograft models.20,31 In a recent phase 2 study of patients with recurrent GBM, some of whom had received prior anti-angiogenic therapy including bevacizumab, treatment with XL184, an oral inhibitor of c-Met, Ret, Kit, and VEGF receptor 2, was associated with antitumor activity.32 However, because XL184 inhibits both the VEGF receptor 2 and c-Met, it is unclear to what extent the inhibition of c-Met contributed to the tumor reductions. Potentially, treatment with AMG 102 in combination with DNA-damaging agents and/or other targeted inhibitors may become a feasible therapeutic approach in recurrent glioma. In a phase Ib study, AMG 102 was well tolerated in combination with bevacizumab in patients with advanced solid tumors.33
The incidence and severity of treatment-emergent AEs were generally similar to or less than those observed in the AMG 102 first-in-human study.22 The incidence and severity of certain AEs, including fatigue, nausea, vomiting, and diarrhea, were similar to previous reports.34,35 Edema occurred in 23% of patients (compared with 30% in the first-in-human study22) and led to discontinuation of treatment in 2 patients. Peripheral edema was also reported in a phase I study of SCH 900105, a monoclonal antibody against HGF/SF, in patients with advanced solid tumors.36
The PK of AMG 102 maximum concentration was similar to that observed in the first-in-humans study.22 Serum AMG 102 trough concentrations at the studied doses exceeded the concentration that inhibits 50% of proliferation (15 µg/ml) and 90% of proliferation (75 µg/ml) estimated in HGF/SF-stimulated U-87 MG cells and human umbilical vein endothelial cells.18
The expression of HGF/SF and c-Met have been associated with poor survival in patients with GBM,13–15 indicating potential involvement of this pathway in disease progression. Plasma levels of total HGF and soluble c-Met and tumor expression of c-Met protein were assayed to investigate whether these analyte levels might predict response to AMG 102. Due to the lack of response to AMG 102, these hypotheses remain untested. However, consistent with results in the AMG 102 first-in-humans study, plasma total HGF/SF increased from baseline, likely as a result of HGF/SF half-life extension by AMG 102 and mobilization of HGF/SF from tissues.22 A slight increase in soluble c-Met was detected, which may indicate pathway inhibition. Based on the observation that HGF/SF inhibition lacked antitumor activity in PTEN-null/HGF+/c-Met+ mice harboring the constitutively active EGFR deletion mutant EGFRvIII,20 we hypothesized that patients expressing the EGFR deletion mutant EGFRvIII would not be responsive to AMG 102 monotherapy. Although the patient who exhibited a response per investigator assessment did not express EGFRvIII, this hypothesis remains untested because of the limited response observed in this study. Additionally, because the archival tumor samples were not collected immediately prior to initiation of AMG 102 treatment, they may not accurately represent the tumor state prior to treatment. Whether or not the presence of the EGFRvIII mutant is a prognostic factor or predictive of response in glioma is still under debate.37–40
In conclusion, these results suggest that AMG 102 monotherapy at doses up to 20 mg/kg is not associated with significant antitumor activity in heavily PYW has research support from Amgen pretreated patients with recurrent GBM, particularly patients who have received prior therapy with bevacizumab.
Conflict of interest: T.F.C. has served as a consultant/adviser for and has received research funding from Genentech/Roche and Excelixis and has provided expert testimony on behalf of Genentech/Roche. J.J.R. has received research funding from Genentech and Novartis and has served as a consultant/advisor/speaker for and received honoraria from Genentech and Merck. J.L. has served as a consultant for Amgen Inc. M.S., M.W., K.S.O., A.A., M.Z., and E.L. are employees of and shareholders in Amgen Inc. D.A.R. has served as a consultant/advisor for and has received honoraria from Schering-Plough/Merck, Genentech/Roche, and EMD Serono. P.Y.W. has received research support from Amgen Inc. D.S. declares no conflicts of interest.
Funding
This trial was funded by Amgen Inc.
Acknowledgments
The authors would like to acknowledge Benjamin Scott, PhD, whose work was funded by Amgen Inc., for assistance in the preparation of this manuscript.
The results of this study have not been previously published or submitted for publication elsewhere. The results were presented in part at the 44th American Society of Clinical Oncology annual meeting, Chicago, 2008.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Radhakrishnan K, Mokri B, Parisi JE, O'Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(1):67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 3.Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro Oncol. 2009;11(2):183–191. doi: 10.1215/15228517-2008-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist. 2009;14(6):621–636. doi: 10.1634/theoncologist.2008-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen PY, Brandes AA. Treatment of recurrent high-grade gliomas. Curr Opin Neurol. 2009;22(6):664–675. doi: 10.1097/WCO.0b013e32833229e3. [DOI] [PubMed] [Google Scholar]
- 7.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 9.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57(23):5391–5398. [PubMed] [Google Scholar]
- 10.Abounader R, Lal B, Luddy C, et al. In vivo targeting of SF/HGF and c-Met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002;16(1):108–110. doi: 10.1096/fj.01-0421fje. [DOI] [PubMed] [Google Scholar]
- 11.Moriyama T, Kataoka H, Seguchi K, Tsubouchi H, Koono M. Effects of hepatocyte growth factor (HGF) on human glioma cells in vitro: HGF acts as a motility factor in glioma cells. Int J Cancer. 1996;66(5):678–685. doi: 10.1002/(SICI)1097-0215(19960529)66:5<678::AID-IJC16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Bowers DC, Fan S, Walter KA, et al. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 2000;60(15):4277–4283. [PubMed] [Google Scholar]
- 13.Arrieta O, Garcia E, Guevara P, et al. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94(12):3210–3218. doi: 10.1002/cncr.10594. [DOI] [PubMed] [Google Scholar]
- 14.Kong DS, Song SY, Kim DH, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115(1):140–148. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Navarrete R, Garcia E, Arrieta O, Sotelo J. Hepatocyte growth factor in cerebrospinal fluid is associated with mortality and recurrence of glioblastoma, and could be of prognostic value. J Neurooncol. 2010;97(3):347–351. doi: 10.1007/s11060-009-0037-8. [DOI] [PubMed] [Google Scholar]
- 16.Cao B, Su Y, Oskarsson M, et al. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci USA. 2001;98(13):7443–7448. doi: 10.1073/pnas.131200498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12(20 Pt 1):6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 18.Burgess T, Coxon A, Meyer S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66(3):1721–1729. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 19.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13(22 Pt 1):6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 20.Lal B, Goodwin CR, Sang Y, et al. EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther. 2009;8(7):1751–1760. doi: 10.1158/1535-7163.MCT-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KJ, Wang L, Su YC, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12(4):1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MS, Sweeney CJ, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16(2):699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto K, Dang J, Zhu S, et al. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14(2):488–493. doi: 10.1158/1078-0432.CCR-07-1966. [DOI] [PubMed] [Google Scholar]
- 25.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 26.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 27.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott BJ, Quant EC, McNamara MB, Ryg PA, Batchelor TT, Wen PY. Bevacizumab salvage therapy following progression in high-grade glioma patients treated with VEGF receptor tyrosine kinase inhibitors. Neuro Oncol. 2010;12(6):603–607. doi: 10.1093/neuonc/nop073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignantgliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 30.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 31.Lal B, Xia S, Abounader R, Laterra J. Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clin Cancer Res. 2005;11(12):4479–4486. doi: 10.1158/1078-0432.CCR-05-0166. [DOI] [PubMed] [Google Scholar]
- 32.Wen PY M., Prados M, Schiff D, et al. Phase 2 study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2 and RET in patients with progressive glioblastoma. J Clin Oncol. 2010;28:153. [Google Scholar]
- 33.Rosen PJ, Sweeney CJ, Park DJ, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. 2010;16(9):2677–2687. doi: 10.1158/1078-0432.CCR-09-2862. [DOI] [PubMed] [Google Scholar]
- 34.Yap TA, Harris D, Barriuso J, et al. Phase I trial to determine the dose range for the c-Met inhibitor ARQ 197 that inhibits c-Met and FAK phosphorylation, when administered by an oral twice-a-day schedule [abstract] J Clin Oncol. 2008;26:3584. [Google Scholar]
- 35.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16(13):3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan RK, Payumo FC, Papadopoulos KP, et al. A phase 1, first in human, study of SCH 900105, an antihepatocyte growth factor (HGF) monoclonal antibody, in subjects with advanced solid tumors [abstract] Mol Cancer Ther. 2009;8 A100. [Google Scholar]
- 37.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viana-Pereira M, Lopes JM, Little S, et al. Analysis of EGFR overexpression, EGFR gene amplification and the EGFRvIII mutation in Portuguese high-grade gliomas. Anticancer Res. 2008;28(2A):913–920. [PubMed] [Google Scholar]
- 39.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 40.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]