Abstract
Concomitant radiation therapy (RT) and temozolomide (TMZ) therapy after surgery is the standard treatment for glioblastoma multiforme (GBM). Radiation and chemotherapy can affect the immune system with implications on subsequent immune therapy. Therefore, we examined the phenotype and function of peripheral blood mononuclear cells in 25 patients with GBM prior to and 4 weeks after treatment with RT-TMZ using multicolor flow cytometry, as well as in vitro CD4+ regulatory T cell (Treg) suppressor and dendritic cell maturation assays. RT-TMZ induced significant lymphopenia, with a decrease in total CD4+ T cells, but did not significantly change monocyte counts. The proportion of functional Treg cells increased after treatment, whereas their absolute numbers remained stable. There was also a measurable decrease in the proportion of CD8+CD56+ and absolute number of CD3−CD56+ effector cells. Posttherapy monocytes retained the ability to mature into dendritic cells. Treatment with RT-TMZ is associated with changes in regulatory and effector peripheral blood mononuclear cells that tilt the balance towards an immune suppressive state. This shift can affect the outcome of immune therapy following RT-TMZ treatment and should be considered in the design of future combination therapy regimens.
Keywords: temozolomide, glioblastoma, immune modulation, radiation, regulatory T cells
Although the combination of surgery, radiation therapy (RT), and temozolomide (TMZ) treatment has improved the outcome of patients diagnosed with GBM,1 the prognosis remains dismal, with a 5-year survival rate of only 9%.2 Immune therapy, a potentially promising approach, has not yet translated into a consistent and significant clinical benefit for patients diagnosed with GBM.3 Limitations of immune therapy may be related to the tumor's weak immunogenicity, tumor-induced immune tolerance, and chemotherapy and radiation immune regulatory effects.4 On the other hand, there are some examples that suggest that the combination of immunotherapy and chemotherapy may benefit patients with GBM. Prolonged survival has been observed in patients with GBM who receive chemotherapy after dendritic cell (DC) vaccination, compared with patients who receive vaccination only.5,6 Preliminary data indicate that, after vaccination, glioma tumor cells are more sensitive to chemotherapy agents (including TMZ).7 Nevertheless, the influence of radiation and chemotherapy in the immune response triggered by adjuvant immune therapy remains uncertain.
Treatment with radiation and chemotherapy can have pronounced effects on the peripheral blood mononuclear cell (PBMC) compartment and, thus, on regulatory and effector mechanisms that modulate the immune destruction of cancer cells. One such mechanism is mediated through the activity of CD4+ regulatory T cells (Treg). Treg cells can function either by acquiring the ability to secrete immunosuppressive cytokines, such as transforming growth factor-β and interleukin (IL)–10, or are constitutively active and inhibit cytotoxic T lymphocyte function by cell-to-cell contact.8 This immune modulatory function is known to play a significant role in hampering antitumor immunity. On the other hand, natural killer (NK) cells and CD8+ T cells are effectors with the potential to lyse tumor target cells and induce pro-inflammatory cytokines that would enhance anti-tumor activity.9 Therapeutic modulation that effectively enhances the number and functionality of memory CD8+ T cells in cancer is being pursued.10 Prolonged exposure to low-dose TMZ in patients with melanoma significantly decreases the number of CD4+ T cells and, specifically, the CD4+CD25+ compartment, which includes the Treg population.11 Radiation and corticosteroids without chemotherapy for treatment of brain tumors also induces CD4 lymphopenia.12 Furthermore, the effect of RT-TMZ on the number of peripheral blood monocytes, as well as their ability to differentiate into DCs, is important in designing immune therapy regimens based on monocyte-derived DC vaccinations that follow RT-TMZ treatment. Ex vivo exposure of monocytes and monocyte-derived DCs to TMZ suggests that monocytes are more likely to be killed because they have a DNA base excision repair defect.13
It is unknown whether treatment with TMZ results in modulation from immune suppression to activation in patients with GBM. To evaluate how RT-TMZ may modulate regulatory and inflammatory immune pathways, we examined the treatment-related effects of RT-TMZ on the profile and recovery of peripheral blood lymphocytes and monocytes in patients with GBM.
Patients and Methods
Patients and study design
To participate in this trial, patients must have been aged ≥18 years and have undergone surgery for newly diagnosed GBM confirmed by neuropathology review. All patients had at least partial surgical resection of their tumor, as determined by postoperative MRI performed within 48 h after surgery. Patients with a Karnofsky Performance Scale index of ≥60 after surgery and who had the ability to provide informed consent were eligible. Patients who had a history of autoimmune disease or human immunodeficiency virus, hepatitis B virus, or hepatitis C virus infection or who had any other medical disorder that would otherwise affect their immune system were excluded. All patients completed 6 weeks of conformal external beam RT (5 days/week; total dose, 60-66Gy) with concomitant TMZ (75 mg/m2/day). All patients signed an informed consent that was approved by the Committee for the Protection of Human Subjects. Approximately 50 mL of peripheral venous blood was obtained after surgery but before starting RT/TMZ combined therapy (PRE) and ∼4 weeks after completing RT/TMZ therapy (POST). At the latter time point, after signing informed consent, a subset of 10 patients, who were going on to participate in a DC vaccination clinical trial, were leukapheresed, and the product was fractionated using elutriation.
Isolation of PBMCs
Blood was collected in sodium heparin collection tubes (BD). PBMCs isolated via density-gradient centrifugation using Ficoll-Paque Plus (GE Healthcare) were washed, then frozen in stepwise fashion in 90% pooled human AB serum (Valley Biomedical) plus 10% DMSO (Sigma-Aldrich), and stored at −140°C until analyzed.
Antibody staining and flow cytometry (FCM)
Multicolor antibody staining was performed to examine multiple cell subsets of interest. PBMCs from the 2 collection time points for each patient were thawed, washed, and stained in one assay with combinations of the following fluorochrome-conjugated monoclonal antibodies for surface staining of the cells: Fluorescein isothiocyanate (FITC)–conjugated anti-CD4, CD8, CD3, CD19, CD45RO (Beckman Coulter), phycoerythrin (PE)–conjugated anti-CD4, CD56 (Beckman Coulter), CCR7 (BD/Pharmingen), PE-cyanin5 (PE-Cy5)–conjugated anti-CD3, CD4 (Beckman Coulter), CD25, CD45RA (BD/Pharmingen), allophycocyanin (APC)–conjugated anti-CD3, CD14 (Beckman Coulter), CD25 (BD/Pharmingen), CCR4 (R&D Systems), PE-cyanin 7 (PEGy-7) conjugated anti-CD8 (Beckman Coulter). Appropriate isotype controls were also used (Beckman Coulter). Intranuclear Fox-P3 staining was performed using the Biolegend Fox-P3-PE kit in accordance with the manufacturer's instructions.
All samples were acquired on a FACSCanto flow cytometer (BD) with acquired event number based on the frequency and limit of detection of the specific cell subset of interest for that sample. Flow cytometry files were analyzed using FlowJo software (Treestar). Gating strategies were determined for each subset of interest on the basis of selected parental population. The parental population was one of the following: lymphocytes, CD3+ cells, CD4+ cells, or CD8+ cells. Subsets of these populations were then further quantified. The subpopulations of CD4+ and CD8+ lymphocytes were further characterized according to their expression of the phenotypic markers CD45RA, CD45RO, and CCR7. In this study, we used CD45RO and CD45RA to distinguish between naive (CD45RA+) and memory (CD45RO+) T lymphocytes. The expression of CCR7 allowed further refining of the subpopulations CD45RA+CCR7+ (naïve), CD45RO+CCR7+ (central memory), CD45RO+CCR7− (effector memory), and CD45RA+CCR7− (terminally differentiated effector memory, TEMRA). We defined Treg cells as CD4+CD25+FOXP3+.14–17
Preparation of monocyte-derived DC
PBMCs were obtained from 10 patients via leukapheresis 4 weeks after completion of RT-TMZ treatment, and the cells fractionated by counter-flow elutriation. Isolated monocytes were cultured with IL-4 and granulocyte macrophage colony-stimulating factor, and the resulting monocyte-derived DCs were loaded with autologous tumor lysate on day 5 of culture, matured with overnight treatment of tumor necrosis factor–α and prostaglandin E2, and harvested on day 7. The methods for monocyte isolation and DC preparation have been previously described.18 DC maturation was assessed on the basis of comprehensive antibody staining for cell surface maturation and co-stimulatory molecules, including CD14, CD83, HLA-DR, CD80 (Beckman Coulter), CD86, and CD40 (Ancell).
Treg suppressor assay
We evaluated the suppressive function of the Treg cells in a CD4+ T-cell proliferation assay. In brief, CD4+ T cells were negatively isolated from leukapheresis product derived lymphocytes by magnetic labeling of non-CD4+ T cells with a biotin-antibody cocktail and anti-biotin MicroBeads using the AutoMacs Cell Separation system (Miltenyi). For the isolation of CD4+ CD25hi cells, negatively enriched CD4+ T cells were directly labeled with anti-CD25 Microbeads followed by separation into a CD25hi and a CD25low/− fraction (responder cells) using the Miltenyi Treg isolation kit. Purity of the isolated fractions was confirmed by FCM. CD4+CD25hi Treg and CD4+CD25low/− responder cells were cultured alone as controls, and they were co-cultured at a 1:1 ratio with Miltenyi activating beads (coated with anti-CD3, anti-CD28, and anti-CD2 antibodies) then incubated at 37°C for 6 days. On day 5, cultures were pulsed with 3H Thymidine for the last 18 h of culture, harvested on glass fiber filters, and incorporated radioactivity measured with a liquid scintillation counter. All treatment conditions were tested in triplicate.
Statistical analysis
The nonparametric Wilcoxon matched pairs test was used to compare the peripheral blood immune profile shift from PRE to POST RT-TMZ treatment time points. This method eliminates patient-to-patient heterogeneity, compared with unpaired testing. Statistical significance was established at the 5% significance level (P < .05). Statistical analysis was performed using the Graph Pad Prism software program. Mean values are shown with standard deviations.
Results
Patient characteristics
During the period from July 2005 through November 2007, there were 25 patients with the pathologic diagnosis of GBM who were included in this study (Table 1). One patient had a history of melanoma treated 4 years before the diagnosis of brain tumor and without evidence of recurrence. There was no history of cancer in the other 24 patients. Eighteen patients had received dexamethasone (median dose, 2 mg/day; range, 1–12 mg/day) ≤1 week prior to the PRE blood draw, but only 1 had received steroids ≤2 weeks before the POST blood draw. Of the 25 enrolled patients, 12 were subsequently included in therapeutic clinical trials and received a different maintenance therapy that started after the POST blood draw. All patients were observed until death or for a minimum of 34 months. The median duration of survival for the 25 patients was 19 months (range, 11 to >60 months). As of September 2010, 4 patients were alive, 2 without evidence of progression.
Table 1.
Patient characteristics and survival durations
| Steroidsa |
|||||
|---|---|---|---|---|---|
| Patient | Age, years | Sex | PRE | POST | Survival duration, months |
| 1 | 61 | M | Y | N | 12 |
| 2 | 67 | F | N | N | 19 |
| 3 | 73 | F | N | N | 11 |
| 4 | 52 | M | Y | N | 61+ |
| 5 | 71 | M | N | N | 24 |
| 6 | 73 | M | N | N | 19 |
| 7 | 69 | M | Y | N | 17 |
| 8 | 66 | F | Y | N | 31 |
| 9 | 66 | F | Y | N | 17 |
| 10 | 69 | F | Y | N | 9 |
| 11 | 59 | F | Y | N | 52+ |
| 12 | 66 | M | Y | N | 20 |
| 13 | 78 | M | N | N | 26 |
| 14 | 54 | F | Y | N | 15 |
| 15 | 60 | F | Y | N | 17 |
| 16 | 57 | M | Y | N | 30 |
| 17 | 47 | M | N | N | 35+ |
| 18 | 59 | M | Y | N | 34+ |
| 19 | 55 | M | Y | Y | 20 |
| 20 | 55 | M | Y | N | 15 |
| 21 | 64 | M | Y | N | 12 |
| 22 | 23 | M | Y | N | 33 |
| 23 | 75 | M | N | N | 14 |
| 24 | 59 | M | Y | N | 18 |
| 25 | 65 | M | Y | N | 12 |
| Median | 64 | 19 | |||
| Range | 23–78 | 11–60+ | |||
F indicates female, and M indicates male. Y indicates yes, and N indicates no. + indicates that the patient is alive.
aPatient receiving steroids at the PRE and POST time points.
Mononuclear cells
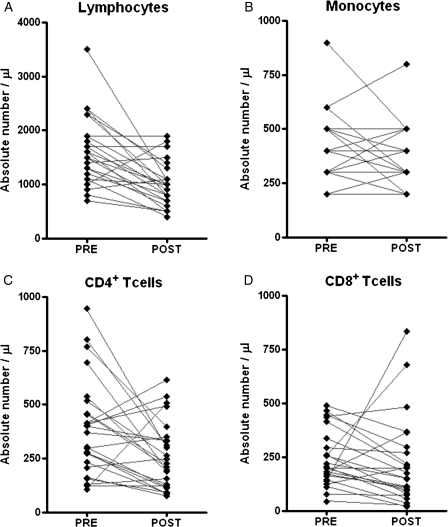
The mean total white blood cell count was 9424 ± 3830 cells/μL at the PRE and 5376 ± 1915 cells/μL at the POST time points (P = .006). RT-TMZ resulted in a significant decrease in the mean lymphocyte count 4 weeks after treatment, compared with baseline (1628 ± 624 vs 1000 ± 411 cells/μL; P = .032) (Figure 1A). The mean absolute monocyte count had no significant change with the treatment (400 ± 168 vs 348 ± 142 cells/μL) (Figure 1B). The combination of RT-TMZ significantly reduced the mean absolute number of CD3+ (768 ± 486 vs 259 ± 156 cells/μL) and CD4+ (390 ± 224 vs 259 ± 156 cells/μL) (Figure 1C), but not CD8+ cells (318 ± 401 vs 233 ± 209 cells/μL) (Figure 1D), although there was no statistically significant difference in the percentages of the CD3+, CD4+, or CD8+ compartments (data not shown).
Fig. 1.
Effect of concomitant radiation therapy and temozolomide therapy on mononuclear cell count (n = 25). The Wilcoxon matched pairs test P values were as follows: (A) lymphocytes, P = .003; (B) monocytes, P = .1427; (C) CD4+ T cells, P = .0105; and (D) CD8+ T cells, P = .0649.
CD4+ Treg cells
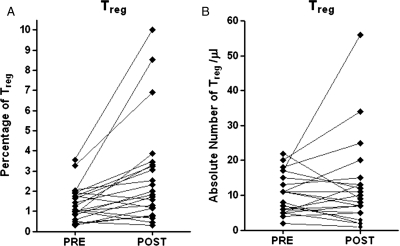
Twenty-two patients were available for Treg analysis. The Treg cell fraction as defined by determining the proportion of CD3+ CD4+ cells that are CD25+FOXP3+ was significantly higher 4 weeks after completion of treatment (P = .0012) (Figure 2A). When the absolute number of Treg was analyzed, there was no significant difference between PRE and POST time points (Figure 2B). When we included only the 21 patients who had not received steroids for at least 6 weeks before the POST blood draw, the Treg cell fraction was again significantly higher, but there was no difference in the absolute cell count (data not shown). A Treg suppression assay was performed for 2 of the POST time point cells, and these Treg cells were able to suppress CD4 responder cell proliferation, confirming that they were functional. Furthermore, there was no change in the expression of chemokine-receptor (CCR4) on the Treg cells after treatment (data not shown).
Fig. 2.
Effect of concomitant radiation therapy and temozolomide therapy on percentage (A) and absolute number (B) of CD4+ regulatory T (Treg) cells at PRE and POST time points (n = 22). The Wilcoxon matched pairs test P values were (A) P = .0012 and (B) P = .7739.
Effector lymphocytes
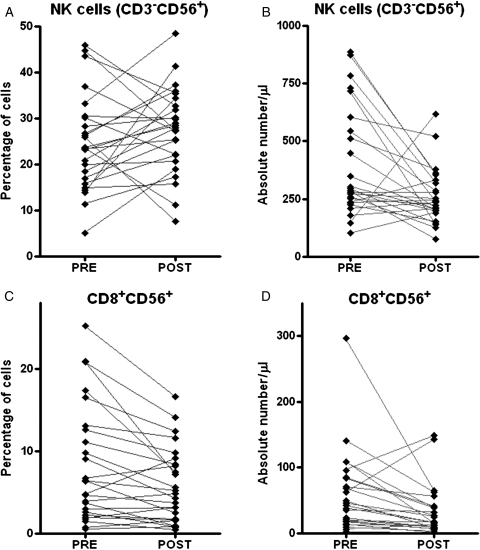
RT-TMZ effects on NK (CD3−CD56+) and CD8 + CD56+ lymphocyte subpopulations was examined. There was a significant drop in the absolute number (P = .004) but not in the proportion of lymphocytes with an NK phenotype (P = .2502) after treatment. The absolute cell count and the percentage of CD3+ T cells that are CD8 + CD56+ significantly decreased at the POST time point (Figure 3).
Fig. 3.
Effect of concomitant radiation therapy and temozolomide therapy on percentage (A and C) and absolute number (B and D) of natural killer (NK) cells and CD8+CD56+ T cells at PRE and POST time points (n = 25). The Wilcoxon matched pairs test P value were (A) P = .2502, (B) P = .004, (C) P = .0047, and (D) P = .0024.
Other lymphocyte populations
The number of CD8+ naive, effector (CD3+CD8+CD25+), and central memory T cells only represented a small proportion of the CD8+ lymphocytes circulating in the peripheral blood— <1.5% in all cases—and there was no difference after RT-TMZ. There were no significant changes in the absolute numbers from PRE to POST RT-TMZ for any of the memory subsets examined. Nevertheless, the percentage of the CD8+ cells that had an effector memory TEMRA phenotype decreased significantly (P < .001), whereas the proportion of CD8+ effector memory lymphocytes increased significantly (P = .0220). Treatment with RT-TMZ induced a significant decrease in both the percentage (P = .0007) and absolute number (P = .0001) of lymphocytes expressing the B cell marker CD19.
Monocyte-derived DCs
For 10 of 25 patients, a panel of antibodies was used to compare the fluorescence intensity of key DC maturation markers on day 0 enriched monocyte precursors and day 7 DCs. The resulting phenotype profile was used to monitor DC maturation. A summary of the change in expression of CD14, CD40, CD80, CD83, CD86, and HLA-DR for these 2 cell types is presented in Table 2. Results of the comparison measured as a fold change in mean fluorescence intensity revealed increase in expression of the DC maturation markers (CD40, CD80, CD83, CD86, and HLA-DR) and a decrease in CD14.
Table 2.
Fold change in mean fluorescence intensity from day 0 monocytes to mature dendritic cells (n = 10)
| Fluorescence determination | Mean ± sd | Range |
|---|---|---|
| CD14 decrease | 10 ± 7 | 4–24 |
| CD83 increase | 38 ± 37 | 2–136 |
| HLA-DR increase | 57 ± 49 | 7–151 |
| CD80 increase | 86 ± 48 | 33–242 |
| CD86 increase | 9 ± 5 | 1–21 |
| CD40 increase | 59 ± 33 | 10–117 |
SD indicates standard deviation.
Discussion
The modulation of the mononuclear cell populations by chemotherapy and radiation in patients with GBM has important implications for optimal coadministration with immunotherapy. Six weeks of treatment with radiation and TMZ significantly increased the proportion, but not the absolute number, of CD4+ lymphocytes expressing a Treg phenotype 4 weeks after therapy. RT-TMZ therapy did not appear to impair Treg ability to suppress the proliferation of other T cells or to express trafficking chemokine receptor. We found similar results when the Treg cells for the 21 patients who had not received corticosteroids for at least 6 weeks before the posttreatment time point were examined. Our results also indicate that RT-TMZ therapy decreases NK and CD8+CD56+ effector cell counts but not CD8+ cell counts in peripheral blood.
Our results show that Treg cells are less sensitive than the remainder of CD4+ T cells to the cytotoxic effect of radiation and chemotherapy in patients with GBM and support earlier findings.19,20 In one study examining the role of vaccination with an epidermal growth factor receptor variant III (EGFRvIII) peptide in patients with newly diagnosed GBM, a similar increase in the proportion of Treg cells after RT-TMZ was found.19 Although humoral immune responses were noted in 6 of 14 subjects, there was no correlation with clinical outcome or with DTH response to EGFRvIII, which were observed in only 3 of 17 patients.21 This suggests that immune regulation may play an important role in the effectiveness of this strategy.19 Another study found a significant increase in the proportion of CD4+ cells with a Treg phenotype after RT and TMZ in patients with high-grade glioma but no significant change in the number of Wilms tumor 1 (WT1) tetramer–positive circulating T cells or NK cells.20 The function of T cells in the presence of Treg cells was not evaluated. In our study, we confirmed that after treatment Treg cells were functionally suppressive and exhibited no change in their chemokine-receptor expression (CCR4) that could be responsible for blocking their trafficking into the brain.22,23 Therefore, the lack of correlation between the increase in the proportion in Treg and tumor-specific immune response measured after therapy is not the result of changes induced by the treatment that renders Treg functionally inefficient or unable to traffic. Recent data suggest that the presence of Treg in the tumor microenvironment may not have prognostic implications in patients with GBM.24 Collectively, these data would indicate that the Treg cells are less sensitive to chemotherapy than other lymphocyte populations and that the resulting change in ratio induced by treatment may not significantly affect immune therapy. It is unclear whether adding Treg-depleting interventions to RT and TMZ could enhance the effect of adjuvant immune therapy.
In our study, the PBMC count was not affected by treatment. Although in vitro peripheral blood monocytes are sensitive to the cytotoxic effects of methylating agents such as TMZ,13,25 we were unable to confirm this in our study. These monocytes were able to mature into DCs under ex vivo culture conditions and thus may be used as a source of DCs in immune therapies against GBM.26,27
We observed a decrease in the absolute numbers of NK cells that inversely correlated with an increase in the proportion of Treg 4 weeks after stopping RT-TMZ. Chiba et al.20 report a similar shift in absolute number and proportion of NK cells after RT-TMZ therapy. Another potentially negative effect of RT-TMZ therapy on the immune system is the reduction in CD3+ cells that are CD8+ CD56+—cells considered important in tumor immunity because they can induce both MHC-restricted and -unrestricted cytotoxicity.28 Others have found that ex vivo expansion of cytotoxic effector cells results in a CD8+CD56+ cell population that can kill tumor cells by an MHC class I unrestricted mechanism, which could enhance adoptive immunotherapy for tumors that evade the immune system by low or no expression of MHC class I molecules.29 Although we found RT-TMZ treatment resulted in significant decreases in circulating NK and CD8+CD56+ effector cells, this finding has not been universal and remains an area in need of further exploration.
Our results reveal snapshots of changes in the peripheral blood but provide incomplete information about the function of these cells and no direct measure of changes in the lymph nodes or the glioblastoma micro-environment. There could be other suppressive and effector mechanisms influenced by treatment that could affect adjuvant immune therapy that were not considered in this study. Treatment may downregulate NK receptors on NK and T cells, which has recently been found to be another mechanism by which GBM may mediate immune suppression.9 As with other types of cancer, myeloid-derived suppressor cells have been reported to be increased in patients with GBM versus healthy control subjects.30
Treatment of GBM with combined RT and TMZ therapy induces a shift toward immune suppression as measured by changes in the peripheral blood compartment. These changes should be considered in designing and analyzing clinical trials which include patients with GBM undergoing standard therapy prior to immune therapy. The patients included in our study are representative of subjects who may go on to be included in immune therapy protocols. Therefore, strategies that combine RT-TMZ treatment with agents that selectively deplete regulatory immune cells, induce recovery of lymphopenia, block immune suppressive cytokines, and expand memory and cytotoxic T cells need to be considered to enhance the overall anti-neoplastic immune environment for successful immunotherapy outcomes in patients with GBM.
Funding
Schering-Plough, National Institutes of Health (RO1 CA095648 to M.E., P20RR016437 to M.E., P42ES007373 to T.H.), and the Medical Oncology Immunotherapy Program.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Rolle CE, Sengupta S, Lesniak MS. Challenges in clinical design of immunotherapy trials for malignant glioma. Neurosurg Clin N Am. 2010;21(1):201–214. doi: 10.1016/j.nec.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(A):133–146. [PMC free article] [PubMed] [Google Scholar]
- 5.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10(16):5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Akasaki Y, Khong HT, et al. Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma for chemotherapy. AACR Meeting Abstracts. 2005 doi: 10.1038/sj.onc.1208519. 2005;2005(1):1003a- [DOI] [PubMed] [Google Scholar]
- 8.Wang HY, Wang RF. Antigen-specific CD4+ regulatory T cells in cancer: implications for immunotherapy. Microbes Infect. 2005;7(7–8):1056–1062. doi: 10.1016/j.micinf.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12(1):7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;1(11) doi: 10.1126/scitranslmed.3000302. 11ps12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62(5):1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 13.Briegert M, Enk AH, Kaina B. Change in expression of MGMT during maturation of human monocytes into dendritic cells. DNA Repair (Amst). 2007;6(9):1255–1263. doi: 10.1016/j.dnarep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 15.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35(6):1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 17.Learn CA, Fecci PE, Schmittling RJ, et al. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin Cancer Res. 2006;12(24):7306–7315. doi: 10.1158/1078-0432.CCR-06-1727. [DOI] [PubMed] [Google Scholar]
- 18.Ernstoff MS, Crocenzi TS, Seigne JD, et al. Developing a rational tumor vaccine therapy for renal cell carcinoma: immune yin and yang. Clin Cancer Res. 2007;13(2 Pt 2):733s–740s. doi: 10.1158/1078-0432.CCR-06-2064. [DOI] [PubMed] [Google Scholar]
- 19.Sampson JH, Aldape KD, Gilbert MR, et al. Temozolomide as a vaccine adjuvant in GBM. J Clin Oncol (Meeting Abstracts). 2007 2007;25(18_suppl):2020- [Google Scholar]
- 20.Chiba Y, Hashimoto N, Tsuboi A, et al. Effects of concomitant temozolomide and radiation therapies on WT1-specific T-cells in malignant glioma. Jpn J Clin Oncol. 2010;40(5):395–403. doi: 10.1093/jjco/hyp196. [DOI] [PubMed] [Google Scholar]
- 21.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W, Jordan JT, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma secreted chemokines can be blocked with chemotherapy (Abstract) Neuro-oncol. 2007;9(4):505. doi: 10.1007/s00262-007-0336-x. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 25.Briegert M, Kaina B. Human monocytes, but not dendritic cells derived from them, are defective in base excision repair and hypersensitive to methylating agents. Cancer Res. 2007;67(1):26–31. doi: 10.1158/0008-5472.CAN-06-3712. [DOI] [PubMed] [Google Scholar]
- 26.Ardon H, Van Gool S, Lopes IS, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99(2):261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 27.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 28.Atanackovic D, Panse J, Schafhausen P, et al. Peripheral T cells of patients with B cell non-Hodgkin's lymphoma show a shift in their memory status. Leuk Res. 2005;29(9):1019–1027. doi: 10.1016/j.leukres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu JY, Ernstoff MS, Hill JM, Cole B, Meehan KR. Ex vivo expansion of non-MHC-restricted cytotoxic effector cells as adoptive immunotherapy for myeloma. Cytotherapy. 2006;8(2):141–148. doi: 10.1080/14653240600620218. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]