Abstract
An open-label phase II study (ACNS0126) testing the efficacy of chemoradiotherapy with temozolomide (TMZ) followed by adjuvant TMZ was conducted by the Children's Oncology Group. During the period from July 6, 2004 through September 6, 2005, 63 children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) were enrolled in the study. All patients received TMZ at a dosage of 90 mg/m2/day for 42 days to a dose of 59.4 Gy. Four weeks following irradiation, TMZ was given at a dosage of 200 mg/m2/day for 5 days every 28 days, for a total of 10 cycles. The primary objective of the statistical analysis was to determine whether the current treatment produced a 1-year event-free survival (EFS) rate higher than the historical baseline of 21.9% observed in CCG-9941. The mean 1-year EFS (± standard deviation) was 14% ± 4.5%, compared with 21.9% ± 5% for CCG-9941. The P value of the test of comparison of 1-year EFS, based on a 1-sided, 1-sample test of proportions, was .96. There was no evidence that temozolomide produced a 1-year EFS rate higher than 21.9%. The mean 1-year OS (± standard deviation) was 40% ± 6.5%, compared with 32% ± 6% for CCG-9941. The median time to death was 9.6 months. Chemoradiotherapy with TMZ followed by adjuvant TMZ is not more effective than previously reported regimens for the treatment of children with DIPG.
Keywords: temozolomide, DIPG, chemoradiotherapy
The management of children with the diagnosis of a diffuse intrinsic pontine glioma (DIPG) remains one of the great challenges within pediatric oncology. Children often present with relatively modest neurologic findings only to have MRI reveal classic imaging features of an enlarged pons, with minimal contrast enhancement after gadolinium administration, diffuse T2/FLAIR signal, and engulfment of the basilar artery.1 Biopsy of this lesion, although rarely obtained, demonstrates a diffuse, infiltrating astrocytoma World Health Organization (WHO) grades 2, 3, or 4.2 Regardless of histologic grade, with classic imaging features, death is anticipated with most children dying of their disease within 6 months to 2 years from the time of initial diagnosis.3,4
The mainstay of treatment for DIPG remains radiation therapy (XRT), which is essentially palliative, delaying the inevitable progression of disease for a period of some months.5 In an effort to alter the dismal prognosis anticipated for most children, numerous studies have been undertaken in the hopes of improving the outcome for these children.6 Recent strategies have included neoadjuvant chemotherapy prior to XRT,7–9 chemoradiotherapy (often followed by adjuvant therapy),10–17 adjuvant therapy alone,18 high-dose chemotherapy with stem cell rescue,19,20 hyperfractionated radiation therapy,9,21 anti-hypoxic treatment during radiation therapy,22,23 and salvage therapy at the time of tumor progression.24,25 The previous Children's Cancer Group treatment protocol for DIPG (CCG 9941) that serves as the historical control for the present study consisted of 3 cycles of induction multiple-agent chemotherapy followed by radiation therapy given twice daily to a total dose of 7200 cGy.9 None of these approaches has proven satisfactory, leaving once-daily XRT alone as the only routinely recommended “standard of-care.”
Temozolomide (TMZ) is an orally bioavailable prodrug that is metabolized at physiologic pH to its active metabolite monomethyl 5-triazeno imidazole carboxamide. The primary mechanism of action is methylation at the O6 position of guanine with a minor contribution at the N7 position. TMZ is lipophilic facilitating gastrointestinal absorption and CNS penetration. TMZ is widely utilized in the treatment of high-grade gliomas (HGGs), including anaplastic astrocytoma (AA) and glioblastoma (GBM). In the United States, TMZ is approved for the treatment of newly diagnosed GBM in adults concomitantly with radiotherapy and then as maintenance therapy. It is also approved for the treatment of adults with refractory AA following progression on a nitrosourea/procarbazine regimen. Given its apparent activity in the treatment of patients with infiltrating astrocytomas, the Children's Oncology Group (COG) undertook a study (ACNS0126) to determine the utility of TMZ in the treatment of children with infiltrating astrocytomas. Stratum A was restricted to children with a diagnosis of AA, GBM, or gliosarcoma; stratum B was restricted to children with DIPG, the results of which are reported here.
Patients and Methods
Eligibility
Children were eligible for the study provided they had undergone gadolinium-enhanced MRI with imaging features consistent with the diagnosis of DIPG. Biopsy of the lesion was neither required nor recommended unless the treating physician believed that imaging features were not entirely consistent with the diagnosis of DIPG. MRI had to demonstrate that at least two-thirds of the tumor was situated in the pons and that the origin of the tumor was clearly within the pons. Eligibility criteria included that cases be newly diagnosed and that the patients were 3–21 years of age, had received no prior therapy other than corticosteroids, and had a Lansky/Karnofsky performance status ≥50, life expectancy of ≥ 2 months, and adequate hematologic, renal, and hepatic function. Exclusion criteria included the inability to begin treatment within 42 days from the time of diagnosis, DIPG presenting as a second malignancy, or metastatic spread of tumor. Subjects with other brain stem tumors, including exophytic brain stem gliomas, cervicomedullary junction tumors, and focal low-grade gliomas of the midbrain or brain stem, were not eligible. Patients and/or their parents or legal guardians were required to sign institutional review board approved consent prior to enrollment.
Pathology
Pathology was not required for participation on the trial if imaging features were consistent with a DIPG. In cases in which pathologic tests were performed, the institutional pathologist had to confirm that the tumor was an infiltrating astrocytoma of WHO grade 2–4.
Treatment
All subjects enrolled in the study received the same planned therapy. Within 42 days after diagnosis, subjects started radiation therapy with the dose to the planning target volume being 59.4 Gy given in 33 fractions of 1.8 Gy delivered once daily, 5 days per week. The planning target volume encompassed the gross tumor volume on MRI scan with a 1.3–1.5-cm 3-dimensional margin. TMZ was administered at a dosage of 90 mg/m2/day beginning within 5 days after the start of radiation therapy and continued uninterrupted for a total of 42 days. Four weeks after the completion of radiation therapy, maintenance therapy was initiated (200 mg/m2/day for 5 days every 28 days for a total of 10 cycles). Each cycle of TMZ commenced when there had been adequate hematologic recovery from the prior cycle of treatment. All children received Pneumocystis jiroveci pneumomia prophylaxis throughout treatment.
Response evaluation
Evaluation of response to chemoradiotherapy and adjuvant chemotherapy was based on institutional assessment of the change in size of the tumor using the maximal 2-dimensional cross-sectional tumor measurements on T2 or FLAIR sequences. Response was characterized as a complete response (ie, disappearance of all abnormal signal within the brain stem and return to normal size of the brain stem), partial response (ie, ≥ 50% decrease in the sum of the products of the 2 perpendicular diameters of the target lesion), stable disease (ie, neither sufficient decrease in the products of the 2 perpendicular diameters of the target lesion to qualify for a partial response, nor sufficient increase in the target lesion to quality as progressive disease), or progressive disease (ie, a ≥25% increase in the cross-sectional area of the largest 2 diameters of the target lesion). Patients were removed from protocol therapy upon evidence of clinical and/or radiologic progressive disease.
Statistical methods
The primary study endpoint for treatment efficacy was time to treatment failure, measured from the start of treatment, from which event-free survival (EFS) percentage was computed. Failure events included tumor progression, tumor recurrence, or death due to any cause. The secondary efficacy endpoint was time to death due to any cause, from which overall survival (OS) was computed. The primary objective of the statistical analysis was to determine whether the current treatment produced a 1-year EFS rate higher than the historical baseline of 21.9%, which is the maximum likelihood estimates derived from an exponential model during the first year from study CCG-9941. Both ACNS0126 and CCG 9941 were assumed to have exponential distribution. The test of comparison is based on a 1-sided, 1-sample test of proportions,
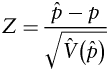 |
where p̂ is the estimated proportion of patients who are event-free at 1 year, p =0.219, and V̂(p̂) is the estimated asymptotic variance of p̂. Because censoring in this disease in the first year of follow-up is minimal, the test statistic is based on the simple estimate of the proportion of patients who are event free at 1 year. The estimated variance in this case is p̂(1−p̂)/n, where n is the total sample size. The test was performed after the last enrolled patient has been followed for a minimum of 1 year. The criteria for significance was the upper 90th percentile of the standard normal distribution, so that the test was performed at a nominal 10% type I error level.
Results
Patient characteristics
During the period from July 6, 2004 through September 6, 2005, 63 children were registered on ACNS0126 BSG stratum. Three children were ineligible because their tumors were coded as being not centered in the pons (did not have DIPG) and two children were ineligible because they failed to meet other eligibility requirements (one had RT at a non-COG institution and the other did not begin treatment in the required time frame). Patient characteristics for the 58 eligible patients are shown in Table 1. The median age for the 58 patients is 7.7 years (range, 3.3–16.2 years). Forty-eight percent were male; 57% were white and 29% were black. Three children had biopsies of their tumor at presentation, with 1 tumor being AA and 2 being GBM.
Table 1.
Characteristics of patients with diffuse intrinsic pontine glioma.
| Characteristic | Frequency | Percentage | |
|---|---|---|---|
| Sex | Male | 28 | 48.3 |
| Female | 30 | 51.7 | |
| Age | <5 years | 13 | 22.4 |
| 5–10 | 32 | 55.2 | |
| 10–15 | 12 | 20.7 | |
| ≥15 years | 1 | 1.7 | |
| Race | White | 33 | 56.9 |
| Black | 17 | 29.3 | |
| Other/unknown | 8 | 13.8 | |
| Primary site | Midbrain | 3 | 5.2 |
| Pons | 55 | 94.8 | |
Therapies administered, response, and toxicity
For the 58 eligible children, 49 (84%) completed the prescribed chemoradiotherapy and went on to receive adjuvant chemotherapy. Of the 9 patients who went off protocol therapy before maintenance, 7 progressed, 1 was withdrawn by the parents who declined further treatment secondary to significant changes in mental and motor status, and 1 died. The responses of the 49 remaining patients at the end of chemoradiotherapy included 18 with partial response, 20 with stable disease, and 1 with progressive disease who did not discontinue protocol therapy. Ten other patients were not evaluated at that time but continued to undergo treatment; their responses in the next course in which they were evaluated included 2 with partial responses, 5 with stable diseases, and 3 with progressive disease. The median number of adjuvant maintenance courses was 2 (range, 1–10). Only 2 patients completed all 10 courses of maintenance, and 1 of them progressed during the last course.
There was a break in the course of radiation therapy ranging from 1 to 7 days in 9 patients due to unstable medical condition precluding delivery of XRT. Protocol-directed dose modification occurred during the first course of chemotherapy due to toxicity for 7 patients (3 cases of thrombocytopenia and 1 each of pneumonia, tachypnea, increased intracranial pressure/unresponsiveness, and radiation-induced edema). Two patients had an allergic reaction to TMZ during the first course. Over all courses of treatment, there were 20 times (17 patients) when the drug was modified due to toxicity; 11 modifications were due to low platelet counts.
Targeted toxicities are shown in Table 2 for the chemoradiotherapy course and the cumulative rates over all the maintenance courses (with number of patients ranging from 49 in course 1 to only 2 after course 7). Hematologic toxicities predominated. One child was removed from study therapy because of prolonged thrombocytopenia. Nontargeted toxicities were rare, with the majority being neurologic toxicities with unlikely attribution to TMZ; during chemoradiotherapy, the nontargeted toxicities included ataxia (n = 3), depressed level of consciousness (n = 3), and speech impairment (n = 3).
Table 2.
Summary of targeted toxicities for diffuse intrinsic pontine glioma stratum
| Toxicity | Chemoradiotherapy (n = 58) | Maintenance (n = 49) |
|---|---|---|
| Allergic reaction/hypersensitivity | 0 | 8 |
| Anemia | 5 | 13 |
| Leukocytes (total white blood cell count) | 10 | 35 |
| Lymphopenia | 38 | 39 |
| Neutrophils/granulocytes | 10 | 47 |
| Thrombocytopenia | 9 | 34 |
| Nausea | 3 | 8 |
| Vomiting | 3 | 7 |
| Alanine aminotransferase level | 0 | 4 |
| Febrile neutropenia (fever of unknown origin) | 0 | 4 |
| Infection with grade 3 or 4 neutropenia | 2 | 5 |
| Infection with unknown ANC | 5 | 0 |
| Infection without neutropenia | 9 | 8 |
| Neuropathy | ||
| Cranial | 12 | 35 |
| Motor | 17 | 46 |
| Neurology | ||
| Sensory | 5 | 7 |
| Seizures | 0 | 3 |
Data are percentage of grade 3 or 4 toxicities for chemoradiotherapy and maintenance courses.
Outcome
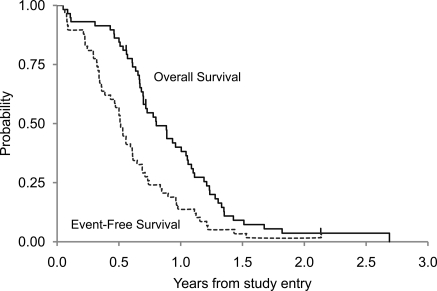
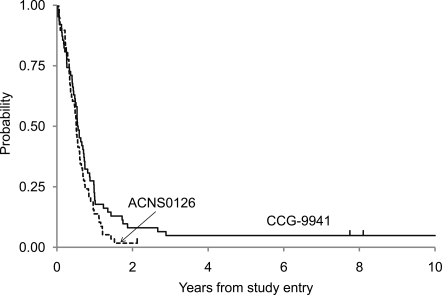
Figure 1 demonstrates the EFS and OS for all eligible patients with DIPG. Figure 2 presents an event-free survival plot for the 58 eligible DIPG patients on ACNS0126 and compares them with a similar cohort of 63 patients from the historical control study CCG-9941. The primary objective of the statistical analysis was to determine whether the current treatment produced a 1-year EFS rate higher than the historical baseline of 21.9%. On ACNS0126, 46 patients progressed and an additional 4 patients died with presumptive progression in the absence of an MRI within 1 year. Fourteen percent (8 of 58) of patients were event-free at 1 year. The 1-sided P value of the test of comparison based on a 1-sided, 1-sample test of proportions was .96. Therefore, we failed to collect evidence to show that the 1-year EFS rate on the current treatment is higher than the historical baseline of 21.9% at the .1 significance level.
Fig. 1.
ACNS0126 DIPG Event-Free and Overall Survival.
Fig. 2.
Event-Free Survival Comparison of ACNS0126 and CCG-9941.
The mean Kaplan-Meier estimate (±standard deviation) of 1-year EFS was 14% ± 4.5% for ACNS0126, compared with 21.9% ± 5% for CCG-9941; the mean 1-year OS (±standard deviation) was 40% ± 6.5% for ACNS0126, compared with 32% ± 6% for CCG-9941. The median times to progression and death were 6.1 and 9.6 months, respectively, for patients with DIPG in ACNS0126. The mean 2-year EFS and OS (±standard deviation) were 1.7% ±1.7% and 3.6% ±2.5%, respectively. The only patient not known to have died was lost to follow-up after 25 months.
Discussion
Results of this study demonstrated no apparent survival benefit associated with the addition of TMZ to conventional radiation therapy among children with DIPG. In this study and in CCG-9941, the majority of children died with disease progression within 1 year of diagnosis. This finding is particularly disappointing given the encouraging results seen with a similar chemoradiotherapy and maintenance regimen using TMZ in adult patients with supratentorial HGG.26 Two smaller pediatric trials27,28 recently reported similarly poor outcomes, albeit with slightly reduced doses of TMZ. The addition of chemoradiotherapy in the current study appeared to offer no therapeutic advantage, with a dismal outcome similar to that seen using maintenance TMZ following radiation therapy alone.29 Seven children progressed following chemoradiotherapy prior to any courses of maintenance therapy. One explanation could have been pseudoprogression, although all 7 children were dead of disease wtihin 1 year after diagnosis, suggesting that this phenomenon, if present, did not alter the natural history of this tumor.
It is unclear why TMZ was ineffective in this patient population. One possible explanation is that DIPG contains an active, presumably unmethylated, O6-methylguanine DNA methyltransferase (MGMT) promoter, as is often seen in supratentorial HGGs. MGMT activity would be anticipated to rapidly remove methyl and alkyl groups from the O6 position of guanine abrogating the cytotoxic impact of TMZ. This hypothesis is speculative, because a biopsy of the pons was not required for enrollment on this stratum. A small number of children underwent biopy, presumably when the treating physician felt that the imaging features at the time of presentation were not classic for a diagnosis of DIPG. However, these specimens were not tested for MGMT expression. Agents that inhibit MGMT activity, such as O6-benzylguanine,30 are being studied as potential therapeutic strategies to enhance the sensitivity of tumor cells to TMZ exposure.31 A major concern with this approach has been marked bone marrow suppression caused by the alkylating agent.32
A second possible explanation is that, despite the central nervous system penetration of TMZ, it fails to adequately reach the target tissue. At presentation, most DIPGs show limited or no contrast enhancement after the administration of gadolinium, raising the possibility that the blood-brain barrier is relatively intact in that region. Whether TMZ effectively penetrates the pons is unknown.
Many practitioners have suggested that improvements in the outcome for children diagnosed with a DIPG will require tissue acquisition so that appropriate molecular analyses can be undertaken in an effort to better understand the biology of this tumor. Central to this perspective is the presumption that the biology of DIPG is fundamentally different from that of other infiltrating astrocytomas, such as supratentorial AA and GBM, for which numerous specimens are available for analysis. Small series have recently suggested that there are fundamental biological differences in DIPG versus HGG.33, 34 However, biopsy of the brain stem is a contentious issue because such a procedure poses some surgical risk to the child, although in capable hands, this appears to be a relatively low-risk procedure35, 36 with limited, or no likelihood of direct benefit.37 In selected cases, an upfront biopsy is justified for clinical purposes when imaging features are not typical for the diagnosis. One recent study reported that, if biopsies are obtained from newly diagnosed children with presumed DIPG, an alternate histology will occasionally be identified. Whether this finding justifies the routine use of biopsies in all children is doubtful. Currently, if a biopsy is clinically indicated, every effort should be made by the practitioner to bank such specimens after histologic confirmation of the diagnosis, provided appropriate regulatory requirements are met for such banking. Alternatively, if the specimen were used to influence the plan of care for the child undergoing a biopsy, then the prospect of direct benefit could be argued, allowing for classification of the research as more than minimal risk but with the prospect of direct benefit. Alternatives have been recently reported, including the use of so-called “warm autopsy” specimens, in which pontine tissue is acquired as soon after the death of the child as possible. Whether such tissue reflects the pretreatment biology of a DIPG is unclear, but potential molecular targets are being identified by these methods. Another possibility would be to use specimens obtained from children who present with a classic bithalamic infiltrating astrocytoma, a lesion type that is commonly biopsied. These lesions tend to image in a manner quite similar to that of a DIPG, and their natural history is equally unfavorable.38
Numerous strategies for the treatment of DIPG are being studied in both the preclinical and early clinical setting. Chemotherapeutics that show promise against supratentorial AA and GBM are often proposed as rational candidates for study in children with DIPG. Exploiting this approach has been ineffective in the past, but perhaps newer agents will provide greater benefit. Molecularly targeted therapies hold some conceptual promise, but the utility of these agents is, as yet, unproven in the setting of a DIPG, in part because of the lack of available tissue to analyze. Local delivery strategies (eg, convection-enhanced delivery) are being explored on the presumption that the current failure of systemic therapy is related to inefficient delivery of drug to the target tissue.
Despite anecdotal reports of efficacy, the data are sufficiently poor that there appears to be little justification for the continued use of TMZ in this patient population, even in combination therapy. Currently, the only therapy remains palliative up-front XRT. Other treatments are best explored in the setting of early phase clinical trials.
Acknowledgments
Presented in part at The 12th Annual Meeting of the Society for Neuro-Oncology, Dallas, Texas.
Conflict of interest statement. None declared.
Funding
The Chair's Grant (U10 CA98543-08) and Statistics and Data Center (U10 CA98413-08) of the Children's Oncology Group from the National Cancer Institute, National Institutes of Health.
References
- 1.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24(8):1266–1272. doi: 10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, Schulte-Monting J, Stoeter P, et al. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. J Neurosurg. 2007;106(2 Suppl):111–119. doi: 10.3171/ped.2007.106.2.111. [DOI] [PubMed] [Google Scholar]
- 3.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 4.Laigle-Donadey F, Doz F, Delattre JY. Brainstem gliomas in children and adults. Curr Opin Oncol. 2008;20(6):662–667. doi: 10.1097/CCO.0b013e32831186e0. [DOI] [PubMed] [Google Scholar]
- 5.Langmoen IA, Lundar T, Storm-Mathisen I, et al. Management of pediatric pontine gliomas. Childs Nerv Syst. 1991;7(1):13–15. doi: 10.1007/BF00263825. [DOI] [PubMed] [Google Scholar]
- 6.Frazier JL, Lee J, Thomale UW, et al. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009;3(4):259–269. doi: 10.3171/2008.11.PEDS08281. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmar CS, Tarbell NJ, Barnes PD, et al. Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A phase II study of the Pediatric Oncology Group, Protocol 8833. Cancer. 1993;72(4):1404–1413. doi: 10.1002/1097-0142(19930815)72:4<1404::aid-cncr2820720441>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Doz F, Neuenschwander S, Bouffet E, et al. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Societe Francaise d'Oncologie Pediatrique. Eur J Cancer. 2002;38(6):815–819. doi: 10.1016/s0959-8049(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 9.Jennings MT, Sposto R, Boyett JM, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children's Cancer Group. J Clin Oncol. 2002;20(16):3431–3437. doi: 10.1200/JCO.2002.04.109. [DOI] [PubMed] [Google Scholar]
- 10.Jakacki RI, Jamison C, Mathews VP, et al. Dose-intensification of procarbazine, CCNU (lomustine), vincristine (PCV) with peripheral blood stem cell support in young patients with gliomas. Med Pediatr Oncol. 1998;31(6):483–490. doi: 10.1002/(sici)1096-911x(199812)31:6<483::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Packer RJ, Krailo M, Mehta M, et al. Phase 1 study of concurrent RMP-7 and carboplatin with radiotherapy for children with newly diagnosed brainstem gliomas. Cancer. 2005;104(6):1281–1287. doi: 10.1002/cncr.21301. [DOI] [PubMed] [Google Scholar]
- 12.Walter AW, Gajjar A, Ochs JS, et al. Carboplatin and etoposide with hyperfractionated radiotherapy in children with newly diagnosed diffuse pontine gliomas: a phase I/II study. Med Pediatr Oncol. 1998;30(1):28–33. doi: 10.1002/(sici)1096-911x(199801)30:1<28::aid-mpo9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Allen J, Siffert J, Donahue B, et al. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999;86(6):1064–1069. doi: 10.1002/(sici)1097-0142(19990915)86:6<1064::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Bernier-Chastagner V, Grill J, Doz F, et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: results of a French Society of Paediatric Oncology Phase II Study. Cancer. 2005;104(12):2792–2797. doi: 10.1002/cncr.21534. [DOI] [PubMed] [Google Scholar]
- 15.Turner CD, Chi S, Marcus KJ, et al. Phase II study of thalidomide and radiation in children with newly diagnosed brain stem gliomas and glioblastoma multiforme. J Neurooncol. 2007;82(1):95–101. doi: 10.1007/s11060-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 16.Wagner S, Warmuth-Metz M, Emser A, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79(3):281–287. doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 17.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50(2):227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 18.Jenkin RD, Boesel C, Ertel I, et al. Brain-stem tumors in childhood: a prospective randomized trial of irradiation with and without adjuvant CCNU, VCR, and prednisone. A report of the Childrens Cancer Study Group. J Neurosurg. 1987;66(2):227–233. doi: 10.3171/jns.1987.66.2.0227. [DOI] [PubMed] [Google Scholar]
- 19.Dunkel IJ, Garvin JH, Jr., Goldman S, et al. High dose chemotherapy with autologous bone marrow rescue for children with diffuse pontine brain stem tumors. Children's Cancer Group. J Neurooncol. 1998;37(1):67–73. doi: 10.1023/a:1005874508975. [DOI] [PubMed] [Google Scholar]
- 20.Bouffet E, Raquin M, Doz F, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88(3):685–692. doi: 10.1002/(sici)1097-0142(20000201)88:3<685::aid-cncr27>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy [see comments] Int J Radiat Oncol Biol Phys. 1999;43(5):959–964. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 22.Aquino-Parsons C, Hukin J, Green A. Concurrent carbogen and radiation therapy in children with high-risk brainstem gliomas. Pediatr Blood Cancer. 2008;50(2):397–399. doi: 10.1002/pbc.21057. [DOI] [PubMed] [Google Scholar]
- 23.Marcus KJ, Dutton SC, Barnes P, et al. A phase I trial of etanidazole and hyperfractionated radiotherapy in children with diffuse brainstem glioma. Int J Radiat Oncol Biol Phys. 2003;55((5): p):1182–1185. doi: 10.1016/s0360-3016(02)04391-2. [DOI] [PubMed] [Google Scholar]
- 24.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer. 2007;110(11):2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 25.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9(2):145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.Jalali R, Raut N, Arora B, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 77(1):113–118. doi: 10.1016/j.ijrobp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Chiang KL, Chang KP, Lee YY, et al. Role of temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: experience at a single institution. Childs Nerv Syst. 26(8):1035–1041. doi: 10.1007/s00381-010-1106-1. [DOI] [PubMed] [Google Scholar]
- 29.Broniscer A, Iacono L, Chintagumpala M, et al. Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98) Cancer. 2005;103(1):133–139. doi: 10.1002/cncr.20741. [DOI] [PubMed] [Google Scholar]
- 30.Bobola MS, Silber JR, Ellenbogen RG, et al. O6-methylguanine-DNA methyltransferase, O6-benzylguanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res. 2005;11(7):2747–2755. doi: 10.1158/1078-0432.CCR-04-2045. [DOI] [PubMed] [Google Scholar]
- 31.Broniscer A, Gururangan S, MacDonald TJ, et al. Phase I trial of single-dose temozolomide and continuous administration of o6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin Cancer Res. 2007;13(22 Pt 1):6712–6718. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 32.Quinn JA, Desjardins A, Weingart J, et al. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23(28):7178–7187. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 33.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 35.Cartmill M, Punt J. Diffuse brain stem glioma: a review of stereotactic biopsies. Childs Nerv Syst. 1999;15(5):235–237. doi: 10.1007/s003810050379. discussion 238. [DOI] [PubMed] [Google Scholar]
- 36.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 Suppl):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 37.Leach PA, Estlin EJ, Coope DJ, et al. Diffuse brainstem gliomas in children: should we or shouldn't we biopsy? Br J Neurosurg. 2008;22(5):619–624. doi: 10.1080/02688690802366198. [DOI] [PubMed] [Google Scholar]
- 38.Reardon DA, Gajjar A, Sanford RA, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29(1):29–35. doi: 10.1159/000028681. [DOI] [PubMed] [Google Scholar]