Abstract
Maternal infection during pregnancy elevates risk for schizophrenia and related disorders in offspring. Converging evidence suggests the maternal inflammatory response mediates the interaction between maternal infection, altered brain development, and behavioral outcome. The extent to which individual differences in the maternal response to immune challenge influence the development of these abnormalities is unknown. The present study investigated the impact of individual differences in maternal response to the viral mimic polyinosinic:polycytidylic acid (poly I:C) on offspring behavior. We observed significant variability in body weight alterations of pregnant rats induced by administration of poly I:C on gestational day 14. Furthermore, the presence or absence of maternal weight loss predicted MK-801 and amphetamine stimulated locomotor abnormalities in offspring. MK-801 stimulated locomotion was altered in offspring of all poly I:C treated dams; however, the presence or absence of maternal weight loss resulted in decreased and modestly increased locomotion, respectively. Adult offspring of poly I:C treated dams that lost weight exhibited significantly decreased amphetamine stimulated locomotion, while offspring of poly I:C treated dams without weight loss performed similarly to vehicle controls. Social isolation and increased maternal age predicted weight loss in response to poly I:C but not vehicle injection. In combination, these data identify environmental factors associated with the maternal response to immune challenge and functional outcome of offspring exposed to maternal immune activation.
Keywords: poly IC, body weight, prenatal immune activation, inflammation, schizophrenia, psychosocial stress
1. Introduction
Prenatal exposure to maternal infection is a suspected etiological risk factor for neurodevelopmental disorders including schizophrenia and autism [1, 2]. Epidemiological investigations have reported increased incidence of schizophrenia spectrum disorders in adult offspring after maternal viral and bacterial infections during pregnancy [3-10]. While the mechanisms underlying elevated schizophrenia risk following these gestational exposures are unknown, the involvement of multiple infectious pathogens suggests that the maternal immune response, rather than the specific pathogen, adversely impacts neurodevelopment [11]. Several processes common to the acute phase immune reaction are known to disrupt fetal brain development including pro-inflammatory cytokine induction [11, 12], glucocorticoid elevation [13-15], hyperthermia [16-18], and decreased food intake [19]. These factors are not only common to the immune response, but to other environmental insults implicated in schizophrenia etiology such as psychosocial stress, fetal hypoxia, obstetric complications and famine [20]. Therefore, converging evidence suggests that maternal inflammatory processes mediate elevated schizophrenia risk after diverse prenatal insults.
Animal models of maternal immune activation support a causal relationship between the maternal immune response and the development of behavioral, neurochemical and neuroanatomical abnormalities in adult offspring. Specific pathogens including human influenza virus [21, 22], pathogen-free inflammatory agents such as the bacterial endotoxin lipopolysaccharide [23-28] and the viral mimic polyinosinc:polycytidylic acid [11, 29-31], as well as pro-inflammatory cytokines [11, 32] have been administered systemically to stimulate or mimic immune activation in pregnant rodents. These models have proven useful in elucidating the consequences of maternal immune activation and the mechanisms underlying altered neurodevelopment. As reviewed extensively [1, 2, 33, 34], offspring of immune-challenged dams exhibit alterations in multiple neurotransmitter systems and behavioral abnormalities that are relevant to symptoms of schizophrenia.
The synthetic double-stranded RNA polyinosinic:polycytidylic acid (poly I:C) activates Toll-like receptor (TLR) 3, stimulating cytokine expression [35] to stimulate the innate immune response and mimic acute phase viral infection. Systemic poly I:C administration increases plasma levels of the pro-inflammatory cytokines interleukin (IL)-6, tumor necrosis factor (TNF)-α [36, 37], and IL-1β [36] within 2 hours of injection. poly I:C-induced fever peaks within 3 hours of injection in rats and declines to baseline by 8 hours. Sickness behaviors following poly I:C administration include decreased locomotor activity and decreased food intake resulting in weight loss for a duration of approximately 24 hours [37]. Evidence suggests maternal pro-inflammatory cytokine induction in response to poly I:C mediates the development of behavioral abnormalities in offspring [11].
In monitoring maternal weight alterations following poly I:C injection we observed significant individual differences in maternal response to immune challenge. We hypothesized that individual differences in maternal weight loss following poly I:C administration would predict the behavioral outcome of offspring, and sought to identify environmental factors contributing to these individual differences. Here we describe identification of two environmental factors associated with the maternal response, as well as the behavioral outcome of offspring, following maternal immune activation.
2. Materials & Methods
2.1. Animals
Nulliparous female Sprague-Dawley rats for use as breeders were obtained in multiple shipments from Harlan Laboratories (Indianapolis, IN) at 8 weeks of age and housed under controlled temperature and humidity on a 12:12 hour light-dark cycle (lights on at 06:00) with ad libitum food and water. All procedures were performed during the light phase. Male breeders were generated within the facility and were housed in cages of 2-3. Female breeders were housed individually or in pairs in standard shoebox cages upon arrival to the facility and remained undisturbed with the exception of regular cage changes until day of mating. All animal procedures were conducted in agreement with the Guide for the Care and Use of Laboratory Animals in accordance with NIH guidelines, and were approved by the Cincinnati Department of Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
2.2. Maternal immune activation
Following a minimum of two weeks acclimatization to the housing facility, males and females were co-housed overnight. Males were removed the following morning, defined as gestational day (GD) 0. On GD 14 dams were weighed and pregnant dams (weight gain ≥ 40 grams) received a single intraperitoneal injection of either 8 mg/kg polyinosinic:polycytidylic acid (poly I:C; Sigma P1530 sodium salt) dissolved in saline or saline (1 ml/kg). Assignment of pregnant dams to poly I:C or vehicle treatment was randomized across all breeder shipment cohorts. The poly I:C dosage was chosen based upon dosage ranges used by other investigators for rat intraperitoneal injection (range of dosages reported 0.75 mg/kg to 20 mg/kg; mean dose 10 mg/kg [37-39]). Injections were performed between 10:00 and 12:00 hours to limit variability associated with diurnal rhythms. Based on a previous study describing anorexia and weight loss associated with immune response to poly I:C [37], pregnant dams were weighed 24 hours following poly I:C or vehicle administration to determine presence or absence of weight loss. Injected dams were between 10 and 31 weeks of age. All dams were housed individually following injection and left undisturbed with the exception of regular cage changes until weaning. Day of birth was defined as postnatal day (PD) 0. Pups were weaned on PD 21 and housed 2-3 per cage by sex and litter. Due to the infrequent occurrence of weight loss in vehicle-treated dams, there were insufficient offspring from vehicle-treated dams with weight loss available for inclusion in behavioral studies.
2.3. Behavioral testing equipment
Behavioral testing was performed in 30 Residential Activity Chambers as previously described [40]. Each chamber comprises a lighted, ventilated, sound-attenuated cabinet housing a 16″ × 16″ × 15″ Plexiglas enclosure. Locomotion was monitored with a 16 × 16 photo beam array (San Diego Instruments, San Diego, CA) located 0.5 inches above the floor of the enclosure. Locomotion was expressed as crossovers, defined as entry into any of the active zones of the chamber, as previously described [40]. Data were collected in ten-minute intervals for behavioral analyses. Behavioral testing was conducted during the lights-on portion of the light cycle as described below.
2.4. Experiment 1: Locomotor response to MK-801
Experimentally naïve male offspring of poly I:C with weight loss (n = 15, 5 litters), poly I:C with weight gain (n = 23, 5 litters), and vehicle treated (n = 27, 9 litters) dams were used to evaluate the locomotor response to MK-801 on PD 56. This postnatal age represents late adolescence/young adulthood in the rat (see [41]). Offspring were left undisturbed in the home cage from weaning until the day of behavioral testing. Testing was limited to male offspring in the current study because of previous observations of sex differences in behavioral response to MK-801 [42, 43]. On PD 56 offspring were placed into the Residential Activity Chambers between 09:00 and 10:00, and the locomotor response during the first hour in the novel environment was determined. Animals were then injected subcutaneously (s.c.) with 0.2 mg/kg MK-801 (Sigma, St. Louis, MO) dissolved in 0.9% saline, and locomotion monitored for an additional 3 hours. The MK-801 dose was chosen because of data in our lab and others [44] demonstrating locomotor stimulant effects of this MK-801 dosage.
2.5. Experiment 2: Locomotor response to amphetamine
A separate cohort of offspring derived from poly I:C with weight loss (n = 13: 7 males, 6 females from 3 litters), poly I:C with weight gain (n = 13: 6 males, 7 females from 3 litters), and vehicle treated (n = 29, 12 males, 17 females from 4 litters) dams was used to evaluate the locomotor response to amphetamine in adulthood. Male and female offspring were weighed at regular intervals from PD 35-70, but were otherwise experimentally-naive. On PD 90 offspring were placed into the Residential Activity Chambers as described above. The locomotor response to novelty was determined for 1 hour prior to subcutaneous injection with 1.0 mg/kg D-amphetamine sulfate (Sigma, St. Louis, MO) dissolved in 0.9% saline. Animals were monitored for an additional 3 hours after amphetamine injection. Amphetamine concentration is described as free base and was injected in a final volume of 1 ml/kg. The amphetamine dose was selected based on data from our lab and others demonstrating induction of locomotor activity without the emergence of a distinct stereotypy phase [45].
2.6. Statistical analyses
Statistical analyses were performed using the statistical software package SAS System for Windows (SAS Institute, Cary, NC). Statistical significance was set at p < 0.05. Data are shown as mean ± SEM. The difference between the means of maternal body weight change was compared between treatment groups (poly I:C and Vehicle) by Satterthwaite's approximate t test, as significant unequal variance was identified by F-test [F(202/114) = 1.41, p = 0.042]. Significant differences between the variances in maternal body weight change between treatment groups were further verified by Brown and Forsythe's Test. Group differences in offspring body weight were analyzed by one-way ANOVA and post hoc Tukey-Kramer test.
Locomotor data were log transformed in order to reduce the heavy right-skewness observed as previously described [46]. Data from experiment 1 were analyzed by two-way ANOVA with repeated measures, with treatment (poly I:C with Weight Loss; poly I:C with Weight Gain; and Vehicle) and time as the main factors and the number of zone crossovers per interval as the dependent measure. Data from experiment 2 were analyzed by three-way ANOVA with repeated measures, with treatment (poly I:C with Weight Loss; poly I:C with Weight Gain; and Vehicle), time, and sex as the main factors and crossovers as the dependent measure using the MIXED procedure. Subsequent multiple comparisons of main effects between treatment groups were conducted by post hoc analysis with Tukey-Kramer test. Total crossovers, defined as the sum of zone crossovers in all intervals following MK-801 or amphetamine administration, were analyzed by one-way ANOVA and post hoc Tukey-Kramer test. Where indicated, further analyses were conducted using the slice ANOVA procedure with False Discovery Rate adjustment for multiple planned comparisons (error rate = 0.05).
Retrospective analyses of factors contributing to individual differences in the maternal response to poly I:C were performed. The contributions of the environmental factors of maternal age and ambient room temperature were analyzed by multiple linear regression with change in weight as the dependent measure. The influence of housing condition (Single vs. Double) on the occurrence of weight loss in poly I:C and vehicle treated dams was analyzed by one-sided Fisher's Exact test. A one-sided test was selected based on our a-priori hypothesis that weight loss would occur more frequently in single-housed dams. This prediction was formulated based on the observations of Gandhi et al. [47] that poly I:C-induced sickness behavior, cytokine induction, and corticosterone elevation were significantly enhanced in socially stressed mice; and isolation housing of rats increases stress [48-51]. In order to rule out potential contributions of batch-specific effects on the maternal response to immune challenge, the relationship between batch (defined by treatment date) and the experimental factors of maternal age and housing condition was examined using Pearson's correlation, multiple linear regression, and Chi Square tests where indicated.
3. Results
3.1. Change in maternal weight
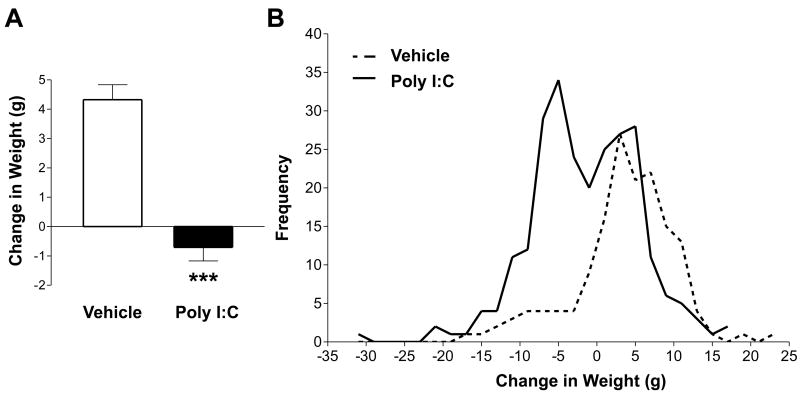
Intraperitoneal injection of 8 mg/kg poly I:C on GD 14 significantly impacted change in maternal body weight at 24 hours post-injection relative to vehicle controls [t(271.47) = 7.31, p < 0.0001]. As shown in Figure 1a, poly I:C treated dams lost an average of 0.71 ± 0.46 g over the 24-hour period after injection, whereas vehicle treated dams gained an average 4.32 ± 0.51 g. Of interest, change in maternal body weight in response to poly I:C injection was markedly variable, and Figure 1b illustrates an apparent bimodal distribution of responses in poly I:C injected dams. Fifty-one percent of dams lost weight in response to poly I:C injection, whereas weight loss occurred in only 16% of vehicle treated dams. The variance in body weight change induced by poly I:C was significantly higher than that induced by vehicle injection as identified by Brown & Forsythe's Test [F(1, 316) = 4.78, p = 0.029], thus indicating significant individual differences in the maternal response to poly I:C.
Figure 1. Effect of poly I:C administration on body weight.
Pregnant rats were administered poly I:C or vehicle on GD 14. Body weight was recorded at the time of injection and 24 hours following injection. (A) Body weight change, shown as the difference between post and pre-injection weight, after poly I:C or vehicle treatment. Administration of poly I:C (n = 203) significantly decreased body weight gain relative to vehicle controls (n = 115) within 24 hours of injection. Data are expressed as means ± SEM. (B) Frequency histogram of body weight change in poly I:C- and vehicle-treated dams. Bin size = 2 grams. The maternal body weight response to poly I:C treatment was bimodal and significantly more variable than the response to vehicle [F(1, 316) = 4.78, p = 0.029]. ***p < 0.0001
3.2. Offspring postnatal weights
No obvious malformations or sensorimotor deficits were found in the offspring of poly I:C or vehicle treated dams. Of interest, body weight of adult female offspring of poly I:C treated dams with weight loss was significantly decreased (Table 1, [F(2, 27) = 10.91, p = 0.0003; poly I:C with Weight Loss vs. Vehicle p = 0.0003; poly I:C with Weight Loss vs. poly I:C with Weight Gain p = 0.0021]. Of note, the body weights of offspring of poly I:C dams with weight gain did not differ from vehicle offspring [p = 0.99]. Prenatal treatment with poly I:C did not have a significant effect on body weight in pubertal and adult male offspring [Table 1, PD 56: F(2, 62) = 2.95, p = 0.06; PD 90: F(2, 22) = 0.01, p = 0.99].
Table 1. Offspring weights at time of behavioral testing.
Body weight was significantly decreased in adult female offspring of poly I:C treated dams with weight loss relative to offspring of both vehicle and poly I:C treated dams with weight gain. Data are expressed as means ± SEM. Body weight was unaltered in male offspring. *** p < 0.001.
| PD56 Males | PD90 Males | PD90 Females | ||||
|---|---|---|---|---|---|---|
| Vehicle | 296.48 | 3.11 | 389.10 | 8.06 | 246.53 | 3.41 |
| Poly I:C with Weight Loss | 284.60 | 4.54 | 388.14 | 8.55 | 228.50 | 4.28 *** |
| Poly I:C with Weight Gain | 295.30 | 3.04 | 389.25 | 7.05 | 246.29 | 3.41 |
3.3. Locomotor response to MK-801
To determine whether changes in maternal weight in response to poly I:C differentially impact offspring behavior, male offspring of poly I:C treated dams with weight loss (poly I:C with Weight Loss; weight change < 0 g), poly I:C treated dams with weight gain (poly I:C with Weight Gain; weight change > 0 g), and vehicle treated dams (Vehicle; weight change > 0 g) were evaluated for sensitivity to the locomotor-activating effects of the noncompetitive NMDA receptor antagonist MK-801. Only offspring of vehicle treated dams that gained weight were used in behavioral analyses due to the low frequency of maternal weight loss in response to vehicle. Following 1 hour of habituation during which novelty-induced locomotion was recorded, the behavioral response to systemic MK-801 (0.2 mg/kg, s.c.) was tested.
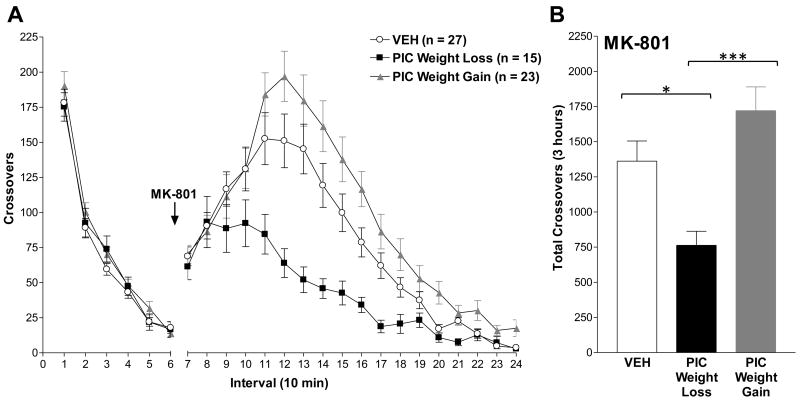
As shown in Figure 2A, maternal immune activation did not significantly effect novelty-stimulated locomotion regardless of maternal weight change [Intervals 1-6: F(2, 62) = 0.70, p = 0.50]. Systemic administration of MK-801 (0.2 mg/kg, s.c.) resulted in an increase in locomotor activity of control animals that peaked at approximately 60 minutes post-injection (Figure 2). The presence or absence of weight loss in response to poly I:C administration dissociated the locomotor behavioral response induced by NMDA receptor antagonism. While MK-801-stimulated locomotion was markedly decreased in offspring of poly I:C treated dams that lost weight relative to control animals, offspring of poly I:C treated dams with weight gain exhibited elevated locomotion relative to controls.
Figure 2. Maternal response to immune challenge predicts the behavioral response to systemic MK-801.
The behavioral sensitivity of male offspring to novelty and to a low dose of the NMDA receptor antagonist MK-801 (0.2 mg/kg, s.c.) was assessed during puberty (PD 56). (A) Number of zone crossovers in 10-minute intervals. The arrow indicates the time of MK-801 administration. The locomotor response to novelty (Intervals 1-6) was unaltered in immune-challenged offspring. Maternal weight loss after poly I:C predicted the locomotor response to MK-801 [F(2, 62) = 36.61, p < 0.0001]. (B) Total number of crossovers for the 3-hour period following MK-801 administration (Intervals 7-24). Male offspring of poly I:C with weight loss exhibited significantly decreased MK-801-stimulated locomotion relative to offspring of vehicle and poly I:C treated dams with weight gain. Data are expressed as means ± SEM. The numbers of litters in each group were: Vehicle, 9 litters; poly I:C with Weight Loss, 5 litters; poly I:C with Weight Gain, 5 litters. VEH=vehicle; PIC=poly I:C; *p < 0.05; ***p < 0.001.
Repeated measures ANOVA identified a main effect of treatment [F(2, 62) = 5.20, p = 0.0082], time [F(1, 62) = 332.35, p < 0.0001], and a treatment by time interaction [F(2, 62) = 36.61, p < 0.0001]. Post hoc analyses of main effects verified that offspring of poly I:C with Weight Loss were significantly different from both Vehicle (p < 0.0001) and poly I:C with Weight Gain (p < 0.0001). In addition, poly I:C with Weight Gain significantly differed from Vehicle offspring (p = 0.018). One-way ANOVA revealed a significant effect of treatment on the total crossovers for the three-hour period following MK-801 administration [Figure 3b: F(2,62) = 8.35, p = 0.0006]. Post hoc Tukey-Kramer analyses confirmed that offspring of poly I:C with Weight Loss significantly differed from Vehicle (p = 0.029) and poly I:C with Weight Gain (p = 0.0004). In contrast to the repeated measures ANOVA, the total crossovers of poly I:C with Weight Gain did not detect a difference from Vehicle (p = 0.18). Based on these outcomes and the observed significant treatment by time interaction described above, further analyses of simple effects were conducted using the slice ANOVA procedure with False Discovery Rate adjustment for multiple planned comparisons. Slice ANOVA identified a significant effect of treatment [F(2, 1116) = 95.80, p < 0.0001], time [F(17, 1116) = 84.49, p < 0.0001], and a significant treatment by time interaction [F(34, 1116) = 2.34, p < 0.0001]. As described in Table 2, group differences in crossovers per 10-minute interval were identified in intervals 11-24. The majority of these differences were due to decreased MK-801-stimulated locomotion in offspring of poly I:C with Weight Loss relative to poly I:C with Weight Gain and Vehicle. Significant increases in MK-801-stimulated locomotion in poly I:C with Weight Gain relative to Vehicle were identified in intervals 20, 22, 23, and 24.
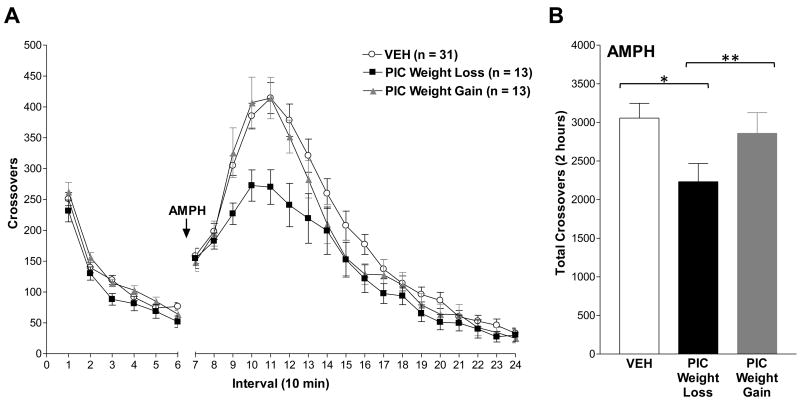
Figure 3. Maternal response to immune challenge predicts the behavioral response to systemic amphetamine.
The behavioral sensitivity of adult male and female offspring to novelty and to a low dose of amphetamine (1 mg/kg, s.c.) was assessed on PD 90. (A) Number of zone crossovers in 10-minute intervals. The arrow indicates the time of amphetamine administration. The locomotor response to novelty (Intervals 1-6) was unaltered in prenatally poly I:C- and vehicle-exposed offspring. Increased locomotor activity following amphetamine treatment was attenuated in adult offspring born to poly I:C treated dams with weight loss [F(2, 43.8) = 4.06, p = 0.024]. (B) Total number of crossovers for the 2-hour period following amphetamine administration (Intervals 7-18). Male and female offspring of poly I:C treated dams with weight loss exhibited significantly decreased amphetamine-stimulated locomotion relative to offspring of poly I:C treated dams with weight gain and vehicle offspring. In contrast, offspring of poly I:C treated dams with weight gain did not differ from controls (p = 0.95). Data are expressed as means ± SEM. The distribution of males and females and numbers of litters in each group were: Vehicle, 12♂ 17♀ (4 litters); poly I:C with Weight Loss, 7♂ 6♀ (3 litters); poly I:C with Weight Gain, 6♂ 7♀ (3 litters). PIC=poly I:C; AMPH=amphetamine; *p < 0.05; **p < 0.01.
Table 2. Analysis of MK-801-stimulated locomotion by slice ANOVA procedure.
Simple group effect differences in the locomotor-activating effects of MK-801 were analyzed by slice ANOVA with False Discovery Rate adjustment for multiple planned comparisons. Tests of effect slices revealed significant group differences in intervals 12-24. Prenatal exposure to poly I:C with weight loss resulted in significantly decreased MK-801 locomotor activity in the second hour relative to vehicle-exposed offspring. MK-801-stimulated locomotion was elevated in the last hour of testing in offspring of poly I:C treated dams with weight gain relative to vehicle offspring. NS=Not significant as determined by False Discovery Rate; PIC=poly I:C.
| Adjusted P Value | ||||
|---|---|---|---|---|
| Interval | Slice | PIC Weight Loss vs. Vehicle | PIC Weight Gain vs. Vehicle | PIC Weight Loss vs. PIC Weight Gain |
| 7 | 0.7448 | - | - | - |
| 8 | 0.6194 | - | - | - |
| 9 | 0.4588 | - | - | - |
| 10 | 0.372 | - | - | - |
| 11 | 0.0263 | NS | NS | 0.02 |
| 12 | 0.0018 | 0.02 | NS | 0.015 |
| 13 | 0.0002 | 0.017 | NS | 0.0028 |
| 14 | 0.0011 | 0.022 | NS | 0.011 |
| 15 | 0.0005 | 0.019 | NS | 0.0009 |
| 16 | 0.0011 | NS | NS | 0.012 |
| 17 | <.0001 | 0.0083 | NS | 0.0037 |
| 18 | <.0001 | 0.0093 | NS | 0.0046 |
| 19 | 0.0042 | NS | NS | 0.0157 |
| 20 | <.0001 | NS | 0.018 | 0.0056 |
| 21 | <.0001 | 0.01 | NS | 0.0065 |
| 22 | 0.0033 | NS | 0.021 | 0.019 |
| 23 | <.0001 | NS | 0.013 | 0.0019 |
| 24 | <.0001 | NS | 0.0074 | 0.014 |
3.4. Locomotor response to amphetamine
Adult male and female offspring were evaluated for sensitivity to the locomotor-activating effects of the indirect dopamine receptor agonist amphetamine. Testing was conducted in adult offspring based on reports of the post-pubertal emergence of amphetamine hypersensitivity after maternal immune activation [31]. The behavioral response to systemic low-dose amphetamine (1 mg/kg, s.c.) was recorded following 1 hour of habituation.
Consistent with Experiment 1, maternal poly I:C had no measurable effect on novelty-stimulated locomotion of adult offspring regardless of maternal weight change [Figure 3, Intervals 1-6: F(2, 51) = 2.10, p = 0.13]. Amphetamine administration resulted in a general increase in locomotor activity in all animals that peaked at approximately 40 minutes post-injection. Amphetamine-stimulated locomotion was decreased in male and female offspring of poly I:C treated dams that lost weight relative to offspring of both vehicle treated dams and poly I:C treated dams with weight gain. Notably, offspring of poly I:C treated dams with weight gain did not differ from offspring of vehicle controls. ANOVA did not identify a significant effect of sex [F(1, 51) = 2.84, p = 0.10], or a significant interaction [F(2,51) = 0.62, p = 0.54] of sex with treatment effects on amphetamine-stimulated locomotion, and therefore behavioral responses of both sexes were combined for analysis of group effects. ANOVA identified a main effect of treatment [F(2, 43.8) = 4.06, p = 0.024], and a significant treatment by time interaction [F(2, 54) = 4.52, p = 0.015]. Post-hoc Tukey-Kramer tests confirmed that the decreased amphetamine-induced locomotion was significantly different between offspring of poly I:C with Weight Loss and poly I:C with Weight Gain (p = 0.001) as well as Vehicle (p < 0.0001), while offspring of poly I:C with Weight Gain did not differ from Vehicle (p = 0.99). Post-hoc comparison between males and females by Tukey-Kramer test did not reach statistical significance (p = 0.098). Two-way ANOVA analysis of total crossovers for the 2-hour period following amphetamine administration was consistent with the repeated measures ANOVA. Two-way ANOVA identified a significant effect of treatment [F(2, 55) = 5.52, p = 0.0068] and sex [F(1, 56) 5.92, p = 0.019]. Because ANOVA did not identify a significant interaction between treatment and sex [F(2, 55) = 0.07, p = 0.94], data from males and females were combined for post hoc Tukey-Kramer analyses of the main effect of treatment (Figure 3B).
3.5. Factors predictive of maternal weight change
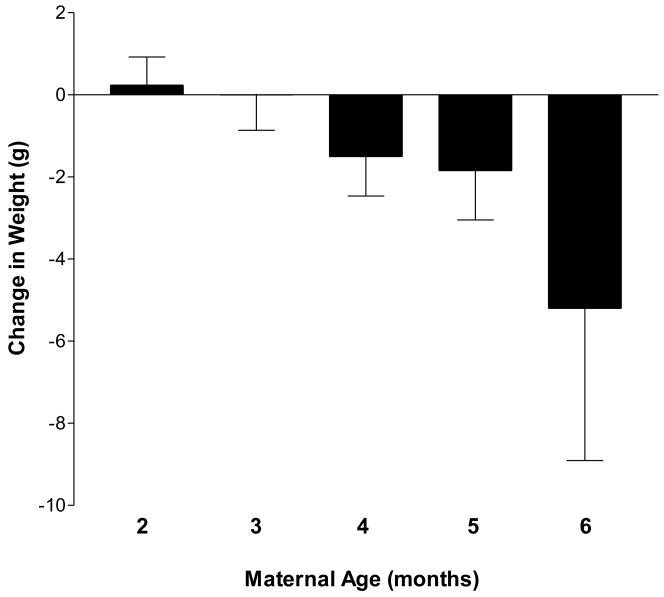
Retrospective analyses of environmental factors were conducted to identify the relative contributions of these variables to individual differences in maternal weight change. The influence of maternal age and injection day room temperature on maternal weight change following poly I:C or vehicle injection were analyzed by multiple linear regression. Regression identified a significant treatment (poly I:C vs. Vehicle) by maternal age interaction [F(1, 296) = 11.92, p = 0.0006] on change in maternal weight. Subsequent analyses identified a significant effect of maternal age on weight change in response to poly I:C [F(1, 200) = 13.54, p = 0.0003] but not vehicle administration [F(1, 112) = 2.12, p = 0.15]. Body weight gain after poly I:C injection decreased with increasing maternal age by approximately 0.05 grams for each day of age (SEM = 0.014, p = 0.0003). As depicted in Figure 4, the incidence and magnitude of poly I:C-induced weight loss increased as pregnant dams age. Pearson's correlation did not identify a statistically significant relationship between batch and maternal age (r(316) = 0.08, p = 0.16). Furthermore, no significant interactions between maternal age and season of treatment administration were identified by multiple linear regression [F(3, 310) = 0.62, p = 0.60]. Thus, the influence of batch on the observed effect of age in response to immune challenge is unlikely. Regression did not identify a significant effect of injection day room temperature [F(1, 296) = 0.04, p = 0.84] or a treatment by temperature interaction [F(1, 296) = 2.75, p = 0.10] on change in maternal weight.
Figure 4. Impact of maternal age on response to immune challenge.
Maternal age at the time of poly I:C exposure predicts the direction and magnitude of poly I:C-induced changes in body weight. Retrospective analyses identified a significant effect of maternal age on weight change in response to poly I:C [F(1, 200) = 13.54, p = 0.0003]. Body weight gain after poly I:C injection decreases with increasing maternal age by approximately 0.05 grams for each day of age. Data are expressed as means ± SEM by maternal age in months. The number of dams in each age group were: 2 months (PD 60-89); n = 74; 3 months (PD 90-119), n = 47; 4 months (PD 120-149), n = 46; 5 months (PD 150-179), n = 31; 6 months (PD 180-209), n = 10.
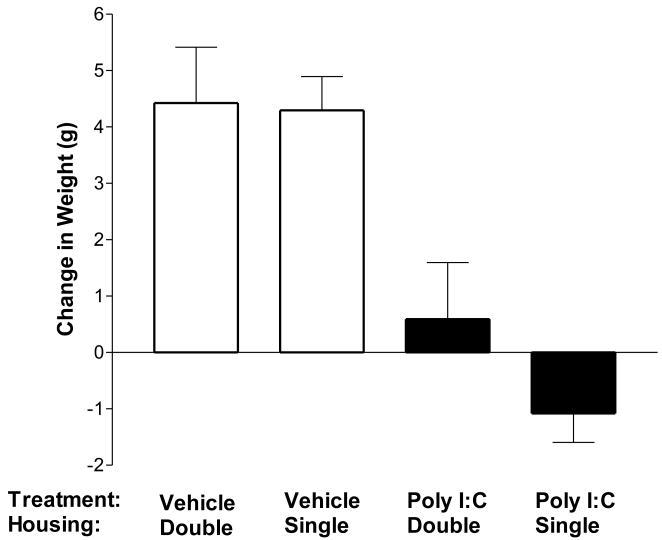
Based on the reported moderation of responsiveness to poly I:C by social stressors [47], the change in maternal weight was compared between dams housed individually or in pairs upon arrival to the facility until 24 hours following poly I:C or vehicle injection. As shown in Figure 5, single housing was associated with a mean decrease in maternal body weight in response to poly I:C injection. poly I:C induced weight loss in 54% of single-housed dams and 38% of double-housed dams. Fisher's Exact test (one-tailed) confirmed that weight loss occurred more frequently in single-housed dams (p = 0.042). The occurrence of weight loss in vehicle treated dams did not differ between single- and double-housed dams (20% versus 12% respectively, p = 0.89). Because experimental housing conditions were not equally distributed across seasons in our study [Chi Square (3, N = 323) = 132.39, p < 0.0001], the frequency of weight loss was compared between housing conditions for each season separately. Fisher's Exact test did not identify differences in the frequency of weight loss in single-housed versus pair-housed dams in any season. Regression did not identify a significant effect of season [F(1, 246) = 0.11, p = 0.74] or a season by prenatal treatment interaction [F(1, 246) = 1.66, p = 0.20] on change in weight in single-housed dams. Together these data suggest that season alone does not account for the increased weight loss in isolated dams.
Figure 5. Influence of social isolation on maternal response to immune challenge.
The maternal body weight response to poly I:C or vehicle was compared between dams that were housed either individually or in pairs upon arrival to the animal facility and until 24 hours following treatment. Weight loss occurred more frequently in single-housed dams treated with poly I:C (p = 0.042, one-tailed by Fisher's Exact test). Weight loss occurred at similar rates in single- and double-housed dams following vehicle treatment (20% versus 12% respectively, p = 0.89). Data are expressed as means ± SEM. The number of dams in each group were: Vehicle Double n = 26; Vehicle Single n =130; poly I:C Double n = 45; poly I:C Single n = 205.
4. Discussion
The present study investigated the impact of individual differences in maternal response to acute immune challenge on offspring behavior. First, it identified marked variability in the body weight alterations induced by poly I:C administration. Second, it demonstrates that maternal body weight change differentiates offspring behavioral response to pharmacologic challenge. Finally, it suggests that individual differences in maternal response to poly I:C are moderated in part by environmental conditions. These findings demonstrate that the maternal response to immune challenge is a critical determinant of the behavioral outcome of offspring.
4.1. Effect of poly I:C administration on body weight of pregnant dams
Consistent with previous investigations [31, 36, 37], systemic poly I:C administration induced significant weight loss over the 24 hours following treatment. These findings are in contrast to a recent study by Wolff and Bilkey [52] in which there was no measurable impact of poly I:C administration on maternal weight. It is possible that these conflicting outcomes may be due in part to differences in poly I:C dose and/or injection route; however, poly I:C-induced weight loss has been reported to occur following intravenous doses of 4 mg/kg [31] as well as intraperitoneal doses ranging from 0.75 – 12 mg/kg [36, 37]. Decreased body weight within 24 hours of poly I:C administration has been previously associated with reduced food intake, and occurred together with body temperature fluctuation and transient elevation of pro-inflammatory cytokines [37]. Weight loss and cytokine induction have not been observed in control animals administered equivalent volumes of vehicle. Thus, weight change may serve as a non-invasive indicator of poly I:C responsiveness.
While poly I:C induced significant weight loss relative to vehicle treated controls, the effect of poly I:C on body weight was unexpectedly variable. To our knowledge, significant variability in response to poly I:C has not been previously reported in pregnant female, non-pregnant female, or male rodents. Cunningham and colleagues [36] reported weight loss in all poly I:C challenged non-pregnant female mice at all doses tested. Body weight loss in pregnant dams after poly I:C administration was also observed in rats [31]; however, the range of weight change and impact on offspring behavior were not discussed. Ozawa and colleagues [30] reported a general decrease in body weight gain of pregnant mice, and significant weight loss in dams that miscarried after subchronic poly I:C administration. Miscarriage did not contribute significantly to the variability observed in the present study, as the poly I:C dose administered produced a 6.4% miscarriage rate (13 of 203 dams). Furthermore, the effects of housing status and maternal age on likelihood of maternal weight loss suggest that variability in body weight response was not likely a result of injection differences impacting bioavailability.
In the present study, we analyzed body weight change as a non-invasive measure of the maternal response to poly I:C injection. Other indicators of immune activation capable of revealing individual differences include cytokine induction, febrile responses, and sickness behavior [36, 37, 53-55]. The relationship between variability in body weight change in the present study and the potential involvement of circulating cytokines is of great interest. In general, pro-inflammatory cytokines suppress food intake [56-58]; however, the direct involvement of cytokines and other mediators of inflammation in poly I:C induced anorexia and subsequent weight loss is unknown. Evidence suggests weight loss occurs independently of IL-1 [37]. The relative contributions of IL-6 and TNF-α, as well as hyperthermia and corticosterone alterations have not been examined.
4.2. Differences in offspring body weight
We observed significantly decreased body weight in adult female offspring of poly I:C treated dams with weight loss. These data suggest further analyses of metabolic function in offspring exposed to maternal immune activation. Of interest, metabolic abnormalities have been identified in patients with schizophrenia [59-61].
4.3. Locomotor response to MK-801
Prenatal exposure to maternal immune activation results in abnormalities in NMDA type glutamate receptor expression and NMDA receptor-mediated behavior [21, 32, 62-64]. Specifically, sensitivity to the locomotor-activating effects of NMDA receptor antagonism in prenatally immune challenged-offspring is dependent on both the gestational age at prenatal exposure (potentiated after GD 17 exposure, but not GD 9 [62]), as well as the age of offspring at testing (potentiated at PD 100-110, but not PD 35 [63]). We hypothesized that abnormalities in NMDA receptor-mediated behavior would emerge in prenatally immune-challenged offspring during late adolescence/young adulthood. This hypothesis was based on the NMDA receptor hypofunction model of schizophrenia, which posits that decreased NMDA-subtype glutamate receptor activity may lead to secondary mesolimbic dopaminergic hyperfunction [65-67], which emerges in adulthood following prenatal immune activation [30, 31]. Here, we examined the impact of individual differences in maternal response to poly I:C on sensitivity to NMDA receptor antagonism on PD 56. Systemic administration of the non-competitive NMDA receptor antagonist MK-801 elevates locomotor activity at low doses [42], and similar elevation was observed here in control offspring. The present study identified a dissociation of the behavioral consequences of systemic MK-801 challenge by the maternal body weight response to poly I:C. Although offspring of poly I:C treated dams exhibited abnormal behavior regardless of change in maternal weight, the presence or absence of weight loss determined the direction and magnitude of the behavioral deficit. Specifically, rats born to poly I:C treated dams with weight loss exhibited markedly attenuated MK-801 sensitivity. In contrast, offspring of poly I:C treated dams with weight gain exhibited modestly elevated MK-801-stimulated locomotion that reached statistical significance in the third hour following drug administration, thereby suggesting alterations in the duration of MK-801 response in these animals. Our data indicate that maternal body weight change in response to poly I:C injection predicts the nature of NMDA receptor-mediated behavior dysfunction.
Previous investigations have reported enhanced sensitivity in adult rodents to the locomotor-activating effects of NMDA receptor antagonism after gestational exposure to maternal immune challenge [62-64]. Such elevated locomotor responses to NMDA receptor blockade have been attributed to mesolimbic dopaminergic hyperactivity based on the ability of MK-801 to activate dopamine neurotransmission; however, the involvement of mesolimbic dopamine in the locomotor-stimulating effects of NMDA receptor antagonism is unclear [68, 69]. Additionally, poly I:C-induced abnormalities in MK-801 sensitivity and NMDA receptor subunit 1 expression are dependent on the gestational timing of poly I:C exposure [62]. In the present study, offspring of poly I:C dams with weight loss exhibited markedly attenuated MK-801 sensitivity during adolescence/young adulthood. We hypothesize that the neurochemical mechanism(s) underlying this decreased behavioral sensitivity to NMDA receptor antagonism may be related to hypofunction of the NMDA receptor. This hypothesis is further supported by in vivo microdialysis data demonstrating attenuated MK-801 stimulated prefrontal cortical extracellular glutamate in these offspring [70]. These findings are directly relevant to the NMDA receptor hypofunction model of schizophrenia pathogenesis [67, 71, 72] and therefore link a known schizophrenia risk factor, prenatal immune activation, with alterations in NMDA receptor function.
4.4. Locomotor response to amphetamine
In addition to disruptions in NMDA receptor-mediated behavior, prenatal exposure to maternal immune activation results in abnormal dopaminergic development and dopamine-mediated behavior [25, 29-31, 73-76]. We therefore sought to determine whether maternal body weight alterations in response to immune challenge also predict dopamine-mediated behavior of offspring. Rodents respond behaviorally to low-dose amphetamine with an increase in locomotor activity [77], a response that is mediated by mesoaccumbens dopaminergic projections [78]. Consistent with our results for MK-801-stimulated behavior, the maternal body weight response to poly I:C predicted the behavioral response to amphetamine in male and female offspring. Specifically, male and female offspring of poly I:C treated dams that lost weight exhibited significantly attenuated amphetamine-stimulated locomotion, whereas offspring of poly I:C treated dams with weight gain did not differ from vehicle offspring.
The present study identified decreased sensitivity to the locomotor-activating effects of amphetamine in adult offspring following maternal immune activation with weight loss. In contrast, several previous investigations of poly I:C induced maternal immune activation have identified enhanced sensitivity to amphetamine in adult rodents [29, 31, 62]. Notably, dopamine abnormalities after prenatal immune challenge are highly dependent on the developmental stage of offspring. While dopamine hyperfunction has been demonstrated in adult offspring, decreased dopamine function has been observed in developing rodents following maternal immune activation [27]. A detailed analysis of dopamine system development identifies the presence of cellular mechanisms which would be expected to underlie decreased locomotor response to amphetamine in offspring of poly I:C injected dams in periadolescence. These studies identified decreased immunoreactivity for both tyrosine hydroxylase and dopamine transporter in caudate putamen, nucleus accumbens core, and nucleus accumbens shell in offspring of poly I:C injected dams relative to control mice at PD 35. In contrast, at PD 70 tyrosine hydroxylase immunoreactivity was increased in the core and shell subregions of the nucleus accumbens [75]. The processes mediating the transition from dopamine hypofunction early in development to hyperfunction in adult poly I:C offspring are unknown. The enduring decrease in amphetamine sensitivity observed in the present study is therefore of interest because it provides an opportunity to identify the processes underlying the transition to hyperdopaminergic function. Moreover, the locomotor response to amphetamine is an indirect measure of dopamine function. While our findings suggest alterations in dopamine neurotransmission, the specific nature of the disruption remains unclear. Indeed, Bakos and colleagues [73] demonstrated reduced nucleus accumbens dopamine levels in adult offspring of rats administered a subchronic regimen of lipopolysaccharide during late pregnancy, a finding similar to our observation. Further studies are needed to determine 1) the nature of the observed dopamine dysfunction; and 2) the degree to which primary or compensatory changes in dopamine transporter and dopamine receptor expression and/or function, as well as in dopamine synthesis (tyrosine hydroxylase) and release may underlie the behavioral abnormalities identified here.
Together these findings indicate that the presence or absence of maternal weight loss following poly I:C administration predicts offspring behavioral response to both MK-801 and amphetamine. In contrast, Wolff and Bilkey [52] found no relationship between maternal weight change and pre-pulse inhibition of startle following immune challenge. As noted above, the poly I:C regimen utilized in the previous study did not induce maternal weight loss, and is thus an important difference that may account for the contrasting impact of maternal weight on offspring behavior. It is also possible that the neural circuits mediating psychostimulant-induced locomotion and pre-pulse inhibition have differing vulnerabilities to developmental insult. Therefore, the effect of maternal weight change on pre-pulse inhibition following prenatal immune challenge with the poly I:C regimen reported here is of great interest.
4.5. Factors contributing to the maternal response to poly I:C
In addition to methodological relevance to investigations of the neurodevelopmental consequences of maternal immune activation, the observed individual differences in maternal response present an opportunity to identify environmental factors that may contribute to increased vulnerability. Here, we identified maternal age as a factor contributing to the response to immune challenge. While maternal age had no influence on the body weight alterations in vehicle treated animals, increased maternal age predicted greater reduction in body weight gain after poly I:C administration. Notably, we did not identify significant relationships between maternal age and breeder batch. Thus, the effect of maternal age on poly I:C response is unlikely to be accounted for by batch effects. Because season has been shown to be a factor in immune response [79], we also verified that there was no impact of season, or a season by maternal age interaction on treatment-induced change in weight in the present study. To our knowledge, the impact of maturation/aging on the acute-phase reaction to poly I:C has not been examined. Our findings suggest that maturation is related to greater response to immune challenge and warrant further investigation using prospective experimental paradigms.
Our data also suggest that social housing conditions may contribute to the maternal response to immune challenge. Specifically, individually-housed dams exhibited greater incidence of weight loss following poly I:C treatment. The mechanisms underlying this relationship between poly I:C challenge and isolation housing are uncertain. Social isolation of rodents is stressful, and alters basal and stress-induced activity of the hypothalamic-pituitary-adrenal axis [49, 51]. As discussed by Gandhi and colleagues [47], it is possible that poly I:C-induced immune activation and social stressors may act synergistically. Our findings are consistent with the enhanced sickness behavior and pro-inflammatory cytokine induction observed when poly I:C and acute social stress were co-administered [47].
4.5. Limitations
The data presented above demonstrate that change in maternal weight following immune challenge is predictive of offspring outcome. Of interest, the mechanisms mediating offspring behavior following poly I:C-induced weight loss remain unclear. We were not able to investigate the effect of maternal weight loss itself as a potential mechanism by which poly I:C-induced weight loss alters fetal development, due to an insufficient incidence of vehicle-treated dams with weight loss. Clarification of the impact of the transient weight loss observed in the present study is of great interest, as epidemiological studies link maternal nutritional deficiency with increased schizophrenia risk [80]. While animal models support a causal relationship between prenatal undernutrition and behavioral and neurochemical abnormalities of relevance to schizophrenia, it is not known whether the transient, mild nutritional deficit resulting from prenatal immune activation is in itself sufficient to result in a measurable behavioral effect. For example, it is known that chronic prenatal protein deprivation in rats causes sex-dependent alterations in striatal and hippocampal NMDA receptor binding [81, 82] which correlate with deficits in sensorimotor gating [81] following maturational delay. Prenatal protein deprivation also disrupts dopaminergic development, resulting in sexually dimorphic, post-pubertal emergence of elevated behavioral sensitivity to apomorphine, amphetamine [82], and cocaine [83]. Furthermore, offspring born to protein restricted dams exhibit changes in dopamine-related molecular markers [83]. To our knowledge, there are no published data regarding the impact of transient (i.e., <48 hours) food restriction and/or mild weight loss on fetal development. Future studies comparing offspring of pair-fed vehicle-treated dams are needed in order to more clearly establish the role of transient maternal weight loss in behavioral outcome following prenatal immune challenge.
Finally, the reductions in locomotor response to MK-801 and AMPH observed in the present study are in direct contrast to the behavioral outcomes identified in previous investigations of maternal immune activation. As discussed above, it is possible that methodological differences (i.e., injection dose, route, gestational age at exposure) between the present study and previous investigations contributed to these differences.
5. Conclusion
In summary, the findings described above demonstrate a critical role of the maternal response to immune challenge in the behavioral outcome of offspring. Moreover, we identified environmental factors contributing to the direction and magnitude of the maternal response. These findings may be particularly relevant to human investigations of environmental risk factors that increase vulnerability to neurodevelopmental disorders associated with prenatal immune activation, including schizophrenia and autism. It will be of interest in future studies to determine the relationship between the body weight alterations induced by poly I:C administration and measures of inflammatory processes, such as cytokine induction, and to identify the relative contributions of immune activation versus transient maternal weight loss to the behavioral outcome of offspring. Of interest, our findings suggest NMDA receptor dysfunction following maternal immune activation and are therefore highly relevant to the NMDA receptor hypofunction hypothesis of schizophrenia.
Acknowledgments
This work was supported by the Department of Veterans Affairs Medical Research Service, National Institute of Mental Health (R21MH083192-01), and Ortho McNeil Janssen Scientific Affairs, L.L.C.
Abbreviations
- AMPH
amphetamine
- IL
interleukin
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- MK-801
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine maleate
- poly I:C
polyinosinic:polycytidylic acid
Footnotes
DISCLOSURES: N. Richtand has the following disclosures: Speaker's Bureau: Bristol-Meyers Squibb, Schering-Plough Corporation, Novartis Pharmaceuticals, Sunovion Pharmaceuticals. Consultant: Bristol-Meyers Squibb, Sunovion Pharmaceuticals, Gerson Lehrman Group. Grant/Research Support: Ortho-McNeil Janssen Scientific Affairs, LLC, AstraZeneca Pharmaceuticals, Department of Veterans Affairs Medical Research Service, and National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 4.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 5.Buka SL, Cannon TD, Torrey EF, Yolken RH, Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders Maternal Exposure to Herpes Simplex Virus and Risk of Psychosis Among Adult Offspring. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 7.O'Callaghan E, Sham PC, Takei N, Murray G, Glover G, Hare EH, Murray RM. The relationship of schizophrenic births to 16 infectious diseases. Br J Psychiatry. 1994;165:353–356. doi: 10.1192/bjp.165.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–637. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvisaari J, Haukka J, Tanskanen A, Hovi T, Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry. 1999;156:1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- 10.Watson CG, Kucala T, Tilleskjor C, Jacobs L. Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch Gen Psychiatry. 1984;41:85–90. doi: 10.1001/archpsyc.1984.01790120089011. [DOI] [PubMed] [Google Scholar]
- 11.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, Tsai WY, Schaefer CA, Brown AS. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz R, Ogren SO, Blum M, Fuxe K. Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience. 1995;66:467–473. doi: 10.1016/0306-4522(94)00605-5. [DOI] [PubMed] [Google Scholar]
- 14.Mesquita AR, Wegerich Y, Patchev AV, Oliveira M, Leao P, Sousa N, Almeida OF. Glucocorticoids and neuro- and behavioural development. Semin Fetal Neonatal Med. 2009;14:130–135. doi: 10.1016/j.siny.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Breen JG, Claggett TW, Kimmel GL, Kimmel CA. Heat shock during rat embryo development in vitro results in decreased mitosis and abundant cell death. Reprod Toxicol. 1999;13:31–39. doi: 10.1016/s0890-6238(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 17.Edwards MJ. Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit Anom (Kyoto) 2007;47:84–89. doi: 10.1111/j.1741-4520.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 18.Upfold JB, Smith MS, Edwards MJ. Quantitative study of the effects of maternal hyperthermia on cell death and proliferation in the guinea pig brain on day 21 of pregnancy. Teratology. 1989;39:173–179. doi: 10.1002/tera.1420390209. [DOI] [PubMed] [Google Scholar]
- 19.Shen Q, Li ZQ, Sun Y, Wang T, Wan CL, Li XW, Zhao XZ, Feng GY, Li S, St Clair D, He L, Yu L. The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr Res. 2008;99:48–55. doi: 10.1016/j.schres.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Susser ES, Brown AS, Gorman JM. Prenatal Exposures in Schizophrenia. 1st. Washington, DC: American Psychiatric Press; 1999. p. 275. [Google Scholar]
- 21.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 24.Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 25.Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- 28.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 29.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–56. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 33.Bronson SL, Richtand NM. Developmental consequences of prenatal immune activation. In: Ritsner M, editor. Handbook of Schizophrenia Spectrum Disorders. 1st. I. Springer; 2011. [Google Scholar]
- 34.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–66. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 38.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- 41.McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- 43.Devaud LL. Effects of ethanol or rimcazole on dizocilpine maleate-induced behaviors in male and female rats. Alcohol. 2003;29:69–81. doi: 10.1016/s0741-8329(02)00326-9. [DOI] [PubMed] [Google Scholar]
- 44.Al-Amin HA, Shannon Weickert C, Weinberger DR, Lipska BK. Delayed onset of enhanced MK-801-induced motor hyperactivity after neonatal lesions of the rat ventral hippocampus. Biol Psychiatry. 2001;49:528–539. doi: 10.1016/s0006-3223(00)00968-9. [DOI] [PubMed] [Google Scholar]
- 45.Segal DS, Kuczenski R. Anonymous Amphetamine and its analogues: pharmacology, toxicology, and abuse. Orlando, FL: Academic Press; 1994. Behavioral pharmacology of amphetamine; p. 115. [Google Scholar]
- 46.Welge JA, Richtand NM. Regression modeling of rodent locomotion data. Behav Brain Res. 2002;128:61–69. doi: 10.1016/s0166-4328(01)00311-4. [DOI] [PubMed] [Google Scholar]
- 47.Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 49.Serra M, Pisu MG, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- 50.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- 51.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Cooper KE, Blahser S, Malkinson TJ, Merker G, Roth J, Zeisberger E. Changes in body temperature and vasopressin content of brain neurons, in pregnant and non-pregnant guinea pigs, during fevers produced by Poly I Poly C. Pflugers Arch. 1988;412:292–296. doi: 10.1007/BF00582511. [DOI] [PubMed] [Google Scholar]
- 54.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 55.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 56.Buchanan JB, Johnson RW. Regulation of food intake by inflammatory cytokines in the brain. Neuroendocrinology. 2007;86:183–190. doi: 10.1159/000108280. [DOI] [PubMed] [Google Scholar]
- 57.Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- 58.Plata-Salaman CR, Sonti G, Borkoski JP, Wilson CD, French-Mullen JM. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- 59.Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Am J Prev Med. Vol. 36. 2009. Obesity among those with mental disorders: a National Institute of Mental Health meeting report; pp. 341–350. [DOI] [PubMed] [Google Scholar]
- 60.Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70 3:22–29. doi: 10.4088/JCP.7075su1c.04. [DOI] [PubMed] [Google Scholar]
- 61.Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13:S170–7. [PubMed] [Google Scholar]
- 62.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- 64.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 66.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 67.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Druhan JP, Rajabi H, Stewart J. MK-801 increases locomotor activity without elevating extracellular dopamine levels in the nucleus accumbens. Synapse. 1996;24:135–146. doi: 10.1002/(SICI)1098-2396(199610)24:2<135::AID-SYN5>3.0.CO;2-G. doi: 2-G. [DOI] [PubMed] [Google Scholar]
- 69.Marcus MM, Mathe JM, Nomikos GG, Svensson TH. Effects of competitive and non-competitive NMDA receptor antagonists on dopamine output in the shell and core subdivisions of the nucleus accumbens. Neuropharmacology. 2001;40:482–490. doi: 10.1016/s0028-3908(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 70.Roenker NL, Ahlbrand R, Richtand NM, Gudelsky GA. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Effect of risperidone, L-NAME or prenatal immune activation on the MK-801-induced increase in extracellular glutamate in the prefrontal cortex. Online., 2009:Program Number 443.23/R9. [Google Scholar]
- 71.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 72.Krystal JH, D'Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7:125–143. [PubMed] [Google Scholar]
- 73.Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann N Y Acad Sci. 2004;1018:281–287. doi: 10.1196/annals. 1296.033. [DOI] [PubMed] [Google Scholar]
- 74.Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 75.Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 77.Segal DS. Behavioral characterization of d- and l-amphetamine: neurochemical implications. Science. 1975;190:475–477. doi: 10.1126/science.1166317. [DOI] [PubMed] [Google Scholar]
- 78.Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. doi: 2-8. [DOI] [PubMed] [Google Scholar]
- 79.Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- 80.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 82.Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, Butler PD. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62–74. doi: 10.1016/j.brainres.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168:359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]