Abstract
The high comorbidity of anxiety and depression suggests a potential degree of commonality in their etiologies. The chronic unpredictable stress (CUS) model effectively replicates depressive-like phenotypes; however, the ability of CUS to produce anxiety-like behaviors has not been adequately addressed. Using the CUS paradigm (2 stressors per day for 10 days) in adult Sprague Dawley rats we identified behavioral, hormonal, and neurochemical changes one day after the cessation of treatment. Stress attenuated weight gain throughout the study and increased locomotor activity one day after treatment, but had no effect on anxiety-behavior as measured by the elevated plus maze. In addition, plasma corticosterone levels were positively correlated with hypothalamic serotonin (5-HT) activity one day after stress treatment as determined by the ratio of the metabolite 5-hydroxyindoleacetic acid (5-HIAA) to the parent compound (5-HIAA/5-HT ratio). These data suggest behavioral phenotypes associated with depression, but not comorbid anxiety, emerge in the immediate period after cessation of stress and that stress related physiology is related to 5-HT activity in the hypothalamus.
Keywords: Rat, chronic unpredictable stress, depression, anxiety, locomotor activity, hypothalamus, serotonin, corticosterone
1. Introduction
Psychiatric disorders pose a significant risk to human health and burden the health care system in the United States and throughout the world. Major depressive disorder and generalized anxiety disorder are highly comorbid (71%) in the United States; however, the basis for this association remains to be determined [1]. The occurrence of stressful life events is a common risk factor associated with both disorders [2–4]. The present study examined the effect of 10 days of mild variable stressors on the expression of both depression and anxiety-related phenotypes in Sprague Dawley rats.
Katz and colleagues were the first to use a chronic stress model to test antidepressant drugs in the early 1980’s [5]. Although these original studies used severe stressors over a period of weeks, the paradigm was later modified to include only mild stressors and was termed chronic mild stress (CMS). Such daily exposure to various environmental stressors models phenotypes of clinical depression including depressed mood, anhedonia, significant weight changes, and insomnia or hypersomnia [6–9]. Additionally, the external validity of CMS as an animal model of depression has been bolstered by numerous studies highlighting that chronic administration of clinically effective antidepressant agents reverses the CMS-induced depressive-like behaviors [5, 10–12]. A more recent variation, the chronic unpredictable stress (CUS) paradigm, uses the same variable mild stressors as CMS but has a shortened period of time (e.g. ten days) and successfully produces similar depressive phenotypes as its previous iterations [13, 14].
Decreased weight gain [8, 15–17], altered locomotor activity [18, 19], increased corticosterone [20, 21], and altered monoamine levels [22, 23] are all phenotypes of depression that have been reported after chronic stress. Therefore, we measured these phenotypes to assess the validity of our model. Rat weight was assessed daily throughout the experiment. Locomotor activity was measured at the beginning of the light phase of the light-dark cycle when the rats would typically be falling asleep. Locomotor activity has been shown to decrease during the dark phase when the rats are normally active following CUS [18]; however, this measure has not been assessed during the light phase when the animals typically sleep. Corticosterone levels were measured because stress disrupts the control of glucocorticoid release at various points on the hypothalamic-pituitary-adrenal (HPA) axis [24] and acute or chronic stress increases the levels of plasma corticosterone in rats [20, 21], similar to the human cortisol response [25]. Finally, tissue monoamine levels were measured one day after CUS treatment. Previous studies have shown that stress can augment the levels of dopamine, serotonin and their metabolites in certain brain regions [22, 23]. We chose a time point of 1 day after stress treatment to examine neurochemical changes in the hypothalamus, which regulates stress-related physiology.
Although both long and short stress paradigms produce robust expression of depressive-like phenotypes in rodents, anxiety-like behaviors have been inconclusive. Following 3 or more weeks of CMS treatment both increased [10, 26] and decreased [27–29] anxiety-like phenotypes have been found; however, only one study has investigated anxiety after shorter durations of stress showing increased anxiety 4 days after CUS [30]. Since there is conflicting scientific literature on the presence of anxiety like phenotypes after CUS, we chose a 1 day cessation period as a first step to determine emergence of a particular phenotype.
This study was designed to assess the neurochemical, hormonal, and behavioral changes that occur shortly after the cessation of a shortened stress paradigm. Most studies have investigated these effects days after the cessation of the stress treatment, not immediately afterwards; therefore, we utilized a 10 day CUS paradigm to assess depressive- and anxiety-like phenotypes in conjunction with neurochemical measures taken 1 day after the cessation of our stress regimen. Here we sought to determine if depressive and anxiogenic phenotypes emerge in the immediate period after stress treatment and investigated the neurochemical changes that occur in the hypothalamus, which regulates stress related physiology.
2. Materials and Methods
2.1 Animal Care and Welfare
Sixteen male Sprague-Dawley rats (Charles River Laboratories; Wilmington, MA) were housed in groups of four per cage in standard microisolator rat cages upon arrival. Housing conditions, including temperature (~24ºC), light cycle (12 h light / dark cycle; on 7 AM / off 7 PM), and humidity (35–40%) were controlled. Rats were given free access to food and water throughout the study except during select stress treatments and behavioral testing. Rats were acclimated to the new environment for 9 days before experimentation. All animals were handled and weighed daily throughout the experiment and weight gain was calculated by averaging each animal’s weight on a given day minus the average of the weight on the initial day of the study (as shown in figure 1). Rats were randomly split into 2 equal sized experimental groups (control or CUS); sample sizes for each assay are stated in the figure legends. The experimental protocol was approved by the Wayne State University Institutional Animal Care and Use Committee, and the Division of Laboratory Animals maintains AAALAC accredited facilities. Animal care and welfare were maintained according to applicable sections of the Animal Welfare Act and all procedures adhered to guidelines set forth in the Guide for the Care and Use of Laboratory Animals [31].
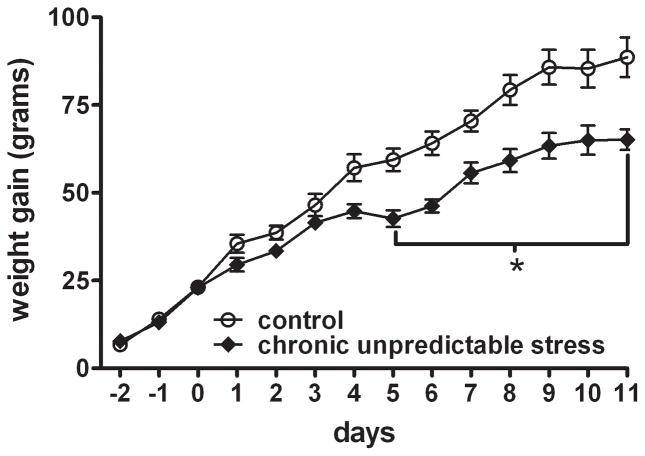
Figure 1. Chronic Unpredictable Stress (CUS) causes decreased weight gain.
The symbols represent weight gain in grams (mean ± SEM) for control and CUS groups over the course of the study. CUS rats had decreased weight gain, and the difference was significant on days 5–11. Data were analyzed by a two-way ANOVA for repeated measures and a Bonferroni posttest: * p< 0.05 when compared to the control group. (n = 8 rats per group)
2.2 Chronic Unpredictable Stress – Experimental Paradigm
CUS rats were exposed to a random pattern of mild stressors twice daily for 10 days (table 1), while the control group was handled once daily. As an additional stressor, CUS rats were single-housed during and after the stress period, while control animals were group-housed (4 per cage) throughout the study. One day after the final CUS treatment, locomotor and elevated plus maze activity were measured. Following behavioral testing rats were euthanized to collect blood and brain samples.
Table 1.
Chronic unpredictable stress paradigm
| Day | Time of 1st stressor | 1st stressor | Time of 2nd stressor | 2nd stressor |
|---|---|---|---|---|
| 1 | 10:00 AM | Swim stress (4 min) | 4:00 PM | Lights off (2 h) |
| 2 | 12:30 PM | Lights off (3 h) | 7:00 PM | Lights on overnight |
| 3 | 10:00 AM | Cold isolation (20 min) | 4:00 PM | Cage rotation (50 min) |
| 4 | 11:00 AM | Cold isolation (1 h) | 2:00 PM | Restraint stress (40 min) |
| 5 | 3:00 PM | Lights off (2 h) | 5:00 PM | Cage rotation (30 min) |
| 6 | 3:30 PM | Swim stress (15 min) | 7:00 PM | Lights on overnight |
| 7 | 4:00 PM | Restraint stress (1 h) | 5:00 PM | Lights off (1 h) |
| 8 | 10:00 AM | Cage rotation (20 min) | 3:30 PM | Cold isolation (15 min) |
| 9 | 12:00 PM | Lights off (2 h) | 7:00 PM | Lights on overnight |
| 10 | 10:00 AM | Restraint stress (1 h) | 2:00 PM | Swim stress (5 min) |
| 11 | Behavioral testing followed by euthanasia and collection of blood and brain samples. | |||
2.3 Locomotor Activity
Total locomotor activity was tested one day after the final treatment near the beginning of the light cycle (i.e. on day 11 from 7:30 – 8:30 AM). Activity was measured using the Opto-M3 Activity Meter (Columbus Instruments, Columbus, OH), a computerized monitoring system consisting of 16 parallel infra-red emitter/detector pairs mounted on a metal assembly into which a clear standard microisolator rat cage (45 X 26 X 21 cm) is placed. The system records the number of infra-red beam breaks by a rat and the data are stored on a computer linked to the activity monitors. Spontaneous locomotor activity data were collected for an hour. The data were split into two phases, the first 20 minutes called the habituation phase and the remaining 40 minutes called the rest phase, and summed during these phases. The division of time into habituation and rest phases was based on the observation that rats placed into a novel environment spend their initial time exploring, and once habituated, they stop ambulating and rest. Our locomotor activity exemplifies this observation (supplemental figure 1).
2.4 Elevated Plus Maze
One day following the last CUS treatment (i.e. on day 11 between 3:00 – 6:00 PM), rats were placed on the elevated plus maze to test for anxiety-like behaviors. The maze was commercially made (Coulbourn Instruments, Allentown, PA) and the dimensions can be found in published work [32]. Each rat was placed on an open arm of the elevated plus maze and allowed to explore the maze for 5 minutes. The activity was recorded by a digital camcorder placed above the maze and analyzed by EthoVision XT version 6 (Noldus Information Technology, Leesburg, VA). After each trial the maze was cleaned with 70% ethanol in distilled water. EthoVision tracks three points on the animal, the nose, center, and base of the tail, using a monochrome contrast component. An entry was defined only when all three points on the animal entered a designated arm. The initial placement onto the open arm was not counted as an entry. EthoVision analysis included: number of entries and duration of time on open and closed arms. The percent of open arm entries was calculated by dividing the number of entries on the open arms by the total number of entries on all arms. The percent time on open arms was calculated by dividing the time spent on the open arms by the total time spent on all arms. Time spent in the middle portion of the maze, termed the center square, was not included in the analyses.
2.5 Blood collection and brain dissections
Animals were rapidly decapitated without anesthesia immediately after elevated plus maze testing. Trunk blood was collected in chilled 7 ml blood collection tubes containing heparin (BD Vacutainer; Franklin Lakes, NJ). Samples were stored on ice for 30 minutes before being centrifuged for 15 minutes at low speed to separate the plasma from the cellular fraction. Plasma was transferred to microcentrifuge tubes by glass Pasteur pipettes and samples were stored at −80ºC until biochemical testing.
Brains were quickly removed and placed into a chilled brain matrix (Zinc Instruments, Pittsburgh, PA). Coronal slices (2 mm) containing the medial prefrontal cortex, cingulate cortex, nucleus accumbens, dorsal posterior striatum, amygdala, and hypothalamus were made and placed on a block of dry ice (solid CO2). Using a tissue biopsy-punch (1.5 mm diameter), a tissue sample of the region of interest was taken based on a rat brain atlas [33] and stored untreated at −80ºC until neurochemical testing.
2.6 Enzyme Linked Immuno-Sorbent Assay
Corticosterone was measured using a standard commercially available Enzyme Linked Immuno-Sorbent Assay (ELISA) kit following the manufacturer’s instructions (Neogen Corp., Lexington, KY). Plasma samples were extracted with ethyl ether, and the organic fraction was dried with a stream of nitrogen and reconstituted in assay buffer provided with the kit. The tissue samples and the corticosterone horseradish peroxidase conjugate were added to the antibody-coated plate and incubated for 1 hour at room temperature (~24ºC). Following incubation, the plate was washed (3 times) with wash buffer provided with the kit. The bound conjugate was detected by the addition of tetramethylbenzidine plus H2O2 and measurement of absorbance at 650 nm. Data were quantified by comparison of unknown absorbance readings against standards that were run in parallel.
2.7 High Pressure Liquid Chromatography
High Pressure Liquid Chromatography (HPLC) was used to measure monoamine tissue levels from brain regions of interest. The tissue samples were weighed and sonically disrupted in 200 μl of 200 mM HClO4. To remove cellular debris, the samples were centrifuged for 5 min at 4° C. Using an ESA 542 autoinjector (ESA – A Dionex Company, Chelmsford, MA) measurements were made by injecting 10 μl of the supernatant reconstituted in ESA mobile phase (micro-dialysis three metabolite, MD-TM) onto an ESA C18-reverse phase column (dimensions 150 x 3.2 mm, particle size 3 μm) maintained at 30° C. A flow rate of 0.5–0.6 ml/min was maintained. For coulometric (electrochemical) detection an ESA 5011A dual electrode cell was used. The signal was analyzed by EZChrom Elite data processing software. Whole tissue levels of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) as well as 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were determined by comparison to external standards run in parallel and adjusted based on tissue weight. Ratios of metabolite to parent monoamine were also calculated; these data indicate increase 5-HT or dopamine metabolism and reflect serotonergic or dopaminergic activity (respectively).
2.8 Statistical Analysis
All data were analyzed, graphed and tested for statistical significance using Microsoft Excel 2003 and Prism 4 (Graphpad, La Jolla, CA). Statistical significance was set at p<0.05 with a 95% confidence interval. Weight data were analyzed by two-way analysis of variance (ANOVA) with treatment and days as factors, and Bonferroni post hoc analysis was used to compare data between groups on a given day. Locomotor activity, elevated plus maze behavior and HPLC data were analyzed by independent two-tailed t-tests. ELISA data were analyzed by an independent one-tailed t-test, given that we expected an increase in corticosterone after chronic stress based on published reports [20, 21, 34]. A Pearson correlation was performed to compare hypothalamic serotonin activity (i.e. 5-HIAA / 5-HT ratio) to plasma corticosterone. All data are presented as the mean ± standard error of the mean (SEM).
3. Results
3.1 CUS treatment attenuates weight gain
Weight data were collected daily throughout the duration of the study. Initial weights (day 0) were not statistically different between control (272 ± 5 g) and CUS groups (263 ± 5 g). As shown in figure 1, two-way ANOVA revealed a statistical difference across days (F13,194=116.7, p<0.0001), between groups (F1,194=106.3, p<0.0001), and day X group interaction (F1,194=4.3, p<0.0001). Bonferroni post hoc analysis showed that CUS rats had a blunted weight gain compared to controls beginning on day 5 and continuing throughout the study (p<0.05).
3.2 CUS increases total locomotor activity during the rest phase of testing
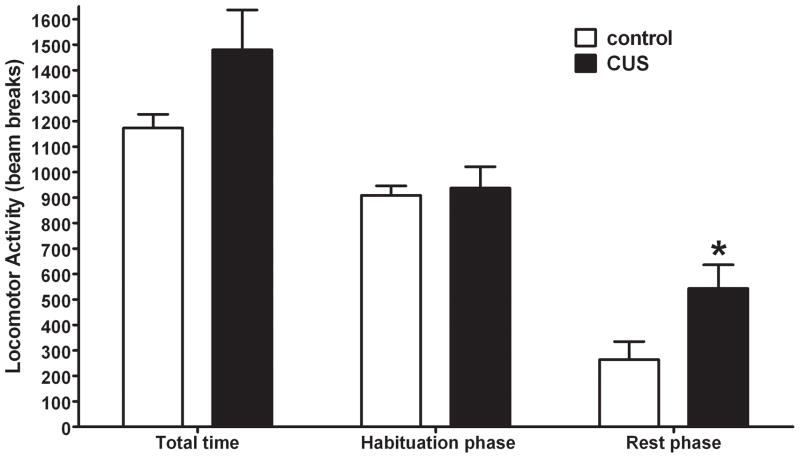
One day following the last day of CUS total locomotor activity was recorded for one hour at the beginning of the light cycle to determine the effects of CUS on exploratory behavior (i.e. habituation phase; first 20 minutes) and rest behavior (rest phase; last 40 minutes). As illustrated in figure 2, no statistical difference in total activity during the entire hour was observed between groups (see supplemental figure 1 for a time course); however, a non-significant trend was seen with the CUS animals having increased activity compared to controls (t14=1.856; p=0.085). Upon splitting the data into habituation and rest phases, it became evident that the trend in activity during the hour was driven by differences between groups during the rest phase. There was no statistical difference in the habitation phase (first 20 minutes) between the two groups (t14=0.305; p>0.05), but the activity during the rest phase (last 40 minutes) was statistically different with the CUS animals having increased activity compared to controls (t14=2.383; p<0.05).
Figure 2. Chronic Unpredictable Stress (CUS) affects locomotor activity during the rest phase.
Total locomotor activity represents the averaged sum of the data collected for each animal during the one hour of locomotor testing. Habituation phase (first 20 min) and rest phase (last 40 min) represent the total locomotor data as two phases. There was a significant difference in locomotor activity during the resting phase. Data were analyzed by an independent two-tailed t-test * p< 0.05 when compared to the control group. (n = 8 rats per group)
3.3 CUS does not increase anxiety-like behavior
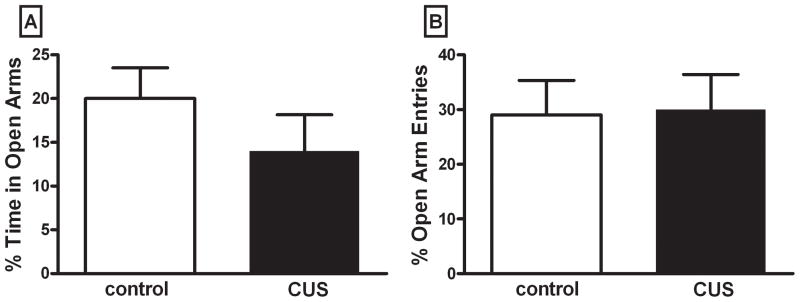
Anxiety-like behavior was measured using the elevated plus maze one day after CUS treatment (figure 3). Figure 3A shows the mean data for percent time spent on the open arms, and statistical analysis revealed no significant difference between the two groups, (t13=1.107, p>0.05). Figure 3B illustrates the mean number of entries onto the open arms as a percent of the total number of entries. There was no significant difference between groups for this measure (t13= 0.110, p>0.05). Total distance traveled was not different between groups (t13= 1.318, p>0.05, data not shown), nor was the difference in total arm entries between groups (t13= 0.708, p>0.05, data not shown).
Figure 3. Chronic Unpredictable Stress (CUS) does not affect anxiety-like behavior measured on the elevated plus maze.
Figure 3A represents percent open arm time = total time in open arms / total time in all arms. Figure 3B represents percent of open arm entries = open arm entries / total entries in all arms. There was no significant difference in either measure. Data were analyzed by an independent two-tailed t-test. (n = rats 7–8 per group)
3.4 CUS increases plasma corticosterone levels
Plasma corticosterone was measured by ELISA one day after cessation of CUS. As depicted in figure 4, CUS animals had higher levels of plasma corticosterone compared to control animals (t11=1.855, p<0.05).
Figure 4. Chronic Unpredictable Stress (CUS) increases plasma corticosterone one day after treatment.
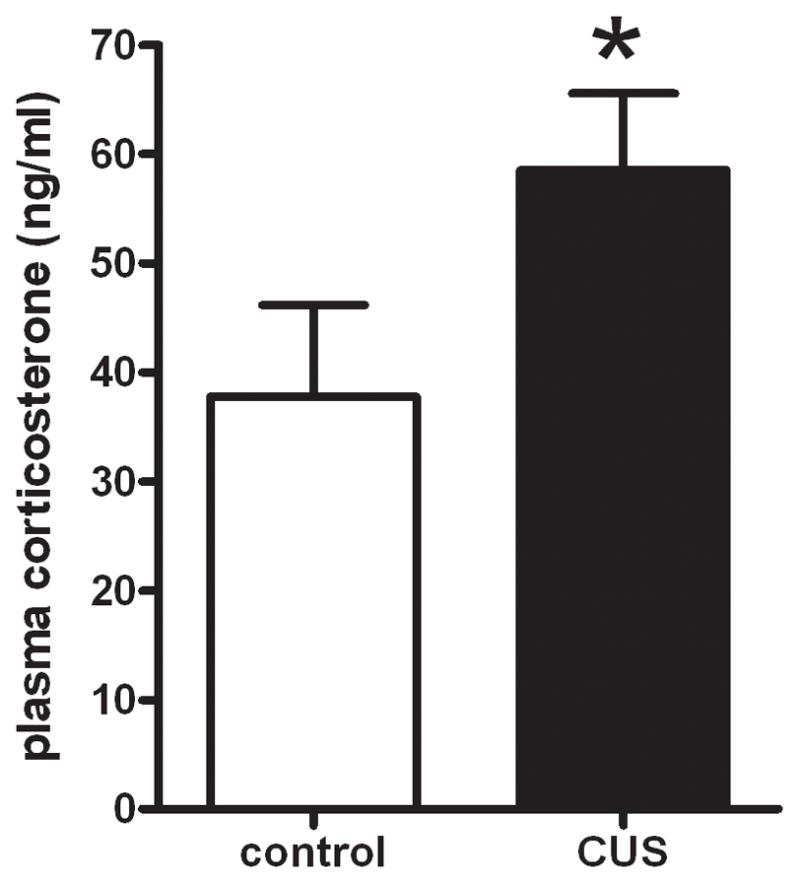
CUS rats had increased levels of plasma corticosterone (ng/ml). These data were analyzed by an independent one-tailed t-test (*p < 0.05). (n = rats 6–7 per group)
3.5 CUS increases 5-HIAA levels and serotonergic activity in the hypothalamus, but does not change monoamine levels in other brain regions
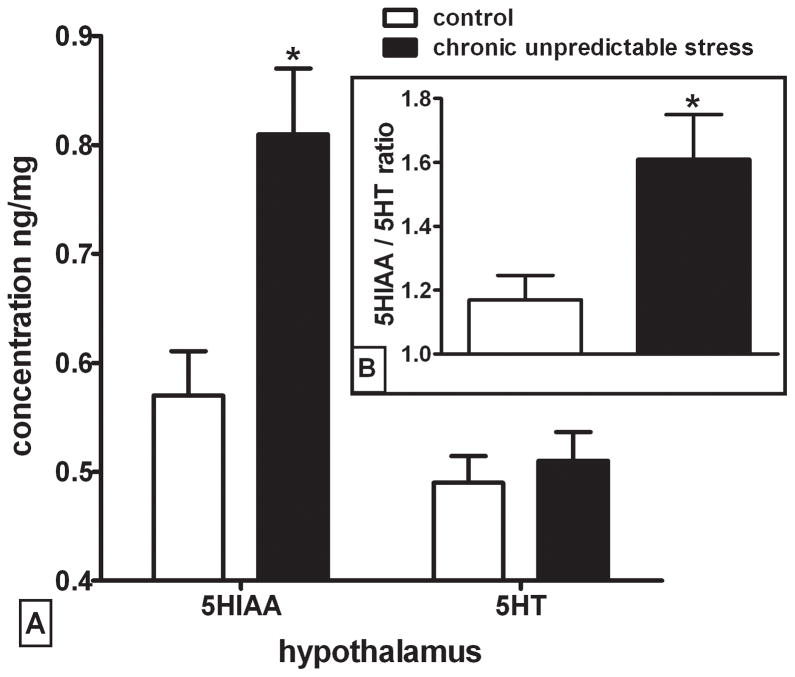
Whole tissue levels of dopamine, serotonin, and their major metabolites were measured in select brain regions by HPLC one day after the final day of CUS (figure 5 and supplemental table 1). Levels of 5-HIAA were significantly higher in the hypothalamus of CUS animals compared to the controls (figure 5, t14=3.065, p<0.01), but no change in 5-HT levels were seen in this region. The increase in 5-HIAA in the CUS group resulted in an increased 5-HIAA / 5-HT ratio (i.e. serotonergic activity) that was significantly different between groups (Figure 5 inset; t10=2.455, p<0.05). The CUS treatment did not significantly affect dopamine or DOPAC in the hypothalamus, nor did it have an effect on any other monoamine or metabolite in medial prefrontal cortex, cingulate cortex, nucleus accumbens, dorsal posterior striatum, or amygdala (supplemental table 1).
Figure 5. Chronic Unpredictable Stress (CUS) increases 5-HIAA but not 5-HT and also increases 5-HIAA / 5-HT ratio in the hypothalamus.
Figure 5A represents a significant difference in hypothalamic 5-HIAA levels between CUS and control animals (p=0.0098). No change in 5-HT levels was observed in this region (n = rats 6–8 per group). Figure 5B represents the 5-HIAA / 5-HT ratio which was significantly different between groups (p=0.034). Data were analyzed by independent two-tailed t-test (*p < 0.05). (n = rats 5–7 per group)
3.6 Hypothalamic serotonin activity correlates with plasma corticosterone levels in CUS treated rats
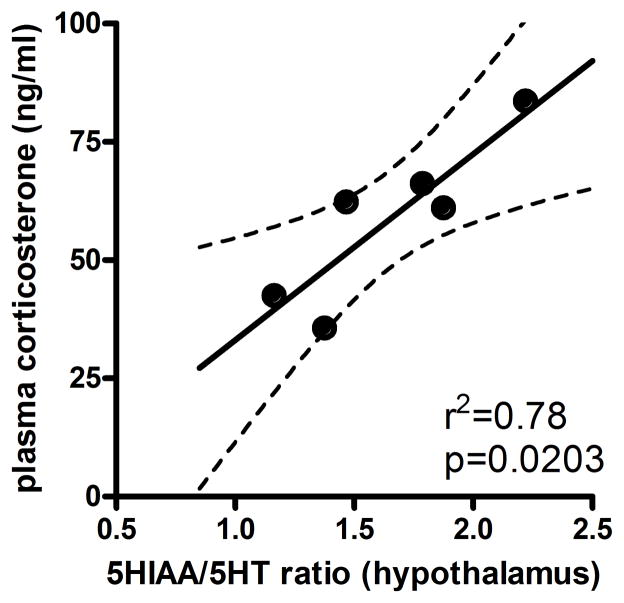
To determine if a relationship between the level of plasma corticosterone and the hypothalamic increase in the 5-HIAA / 5-HT ratio in the CUS animals exists a correlation analysis was performed (figure 6). A significant positive correlation was found in CUS animals between the concentration of plasma corticosterone in individual rats and their hypothalamic 5-HIAA / 5-HT ratio (r=0.881, r2=0.777, p<0.05). No correlation was observed in control animals between plasma corticosterone and hypothalamic serotonergic activity (r=−0.852, r2=0.727, p>0.05, data not shown).
Figure 6. Hypothalamic serotonergic activity correlates with plasma corticosterone levels in stressed animals.
A positive correlation was observed in individual animals treated with Chronic Unpredictable Stress (CUS) between the concentration of plasma corticosterone (ng/ml) and their ratio of 5-HIAA / 5-HT in the hypothalamus. A Pearson correlation was performed to compare these data (n = 4–6 per group).
4. Discussion
The present studies show that CUS treatment caused a significant reduction in weight gain, increased locomotor activity during the resting phase, increased plasma levels of corticosterone, and increased 5-HT turnover in the hypothalamus with a correlation between corticosterone levels and serotonergic activity. The phenotypic changes observed here parallel symptoms of clinical depression, which demonstrates that 10 days of CUS has good face and construct validity for modeling depression in animals. Overall, the results show that depressive phenotypes predominate in the early period after 10 days of repeated mild stress. Similarly, we found evidence to suggest that repeated mild stress increases hypothalamic 5-HT turnover with a concomitant decrease in the rate of weight gain and disrupted control of glucocorticoid homeostasis.
In accordance with other CUS studies [10, 16], we measure a significant decrease in weight gain in CUS treated versus control animals (figure 1). However, unlike many CUS paradigms, which use food and water deprivation as stressors, the CUS paradigm used here does not include these stressors. Therefore, the blunted weight gain observed cannot be attributed to a confounding experimental design. Weight loss can be a symptom of major depressive disorder and the decreased weight gain seen after the CUS treatment may be how this symptom is phenotypically expressed. It is unlikely that the blunted weight gain is a result of anhedonia, because it has been reported that the measure of anhedonia (with palatable solutions) is independent of weight gain [16]. Additionally, it is also unlikely that the increased locomotor activity seen in the CUS treated animals could account for this decreased weight gain because other studies have shown an overall decrease in locomotor activity after CUS treatment is accompanied by a decrease in weight gain [8, 17, 19].
To observe insomnia-like behavior, locomotor activity was examined for one hour during the beginning of the light cycle (a period during which rodents normally begin their sleep cycle) one day after CUS. Interestingly, there was not a difference in total locomotor behavior or during the initial habituation phase. However, an increase in locomotor activity after CUS treatment was observed during resting phase when control animals were inactive and presumably asleep (figure 2). Typically, locomotor behavior has been reported to decrease after CUS treatment [18, 19], but to our knowledge this is the first study to report an increase in activity during the resting phase after CUS treatment. The difference in activity observed here may be attributed to the time of day (the light versus dark phase) or the length of time the test was performed. The decrease in locomotor activity after CUS treatment in previous studies was seen when measuring the activity for 24 h [18] or when an open field test was used to measure activity for 5 min during the light cycle [19]. The increase in locomotor behavior we observed in the CUS treated rats during the beginning of the light cycle may be attributed to insomnia or sleep disturbances, which are symptomatic of major depressive disorder [9].
Consistent with a previous report [30], we found no difference in anxiety-like behavior on the elevated plus maze 1 day after CUS; however, an increase in anxiety-like behavior has been reported 4 days after CUS treatment [10]. The differences between these studies suggest that anxiety-like behavioral changes may take longer than 1 day to emerge using this stress paradigm. However, an increase in plasma corticosterone levels was observed following chronic stress treatment, an effect similar to previous reports in humans [25, 35] and animals [20, 21]. The fact that increased levels of corticosterone were measured one day after treatment suggests that the observed behavioral and neurochemical effects were in fact due to the chronic stress treatment. Elevated corticosterone levels have been associated with stress activation of the HPA axis, specifically the activation of the paraventricular nucleus in the hypothalamus. This occurs through release of corticotrophin releasing factor from the hypothalamus, and subsequent release of adrenocorticotropic hormone from the anterior pituitary initiates the endocrine response to stress and the eventual release of glucocorticoids, including corticosterone from the adrenal gland into the blood [36].
One day following CUS, we found an increase in 5-HIAA, a metabolite of 5-HT, but no increase in 5-HT in the hypothalamus. This resulted in an increased ratio of 5-HIAA / 5-HT, suggesting increased turnover of 5-HT which is reflective of increased serotonergic activity. Increase in 5-HT turnover in the hypothalamus has been previously reported to occur in response to chronic social stress [23]. Serotonin has been found to be very important in the regulation of stress response in the hypothalamus; specifically the 5-HT1A and 5-HT2A receptors, located on neurons in the paraventricular nucleus in the medial hypothalamus, have been found to mediate the release of stress hormones [36, 37]. These observations agree with our correlation analysis showing similar increases in hypothalamic serotonergic activity and plasma corticosterone levels. The density of 5-HT2A receptors were also found to be altered in the cerebral cortex after chronic stress treatment [38], suggesting further involvement of the serotonin system in response to stress. The subsequent change of a single monoamine metabolite in the hypothalamus and the lack of change in any other brain region (supplemental table 1) can most likely be attributed to the relatively short duration of stress and the mild intensity of the stressors. Using a similar paradigm as used here (i.e. ~10 days CUS) but a longer time after stress treatment (i.e. 6 days versus 1 day), levels of 5-HT or dopamine were not different in the frontal cortex, striatum, or hippocampus [39, 40]. The studies that report monoamine or metabolite changes after stress in these brain regions use stress paradigms with a longer duration [22, 41]. Stress-induced plasticity in the 5-HT system may be associated with the depressive-like behaviors and may indicate the delayed emergence of anxiety-like behaviors.
There have been relatively few studies that use a shortened stress paradigm to look at anxiety-like behavior. The majority of the literature involves longer treatments (greater than 3 weeks) using the chronic mild stress paradigm and have shown conflicting results including no effect [30], anxiolytic [27–29] and anxiogenic [10, 26] behavioral changes. While both long and short stress paradigms have consistently produced depressive phenotypes, the anxiety-like behavior reported has been rather inconsistent. With the longer stress paradigms reporting many conflicting results in anxiety-like behavior, further studies are needed to determine whether anxiety-like behavior accompanies the depressive phenotypes using this shortened stress paradigm days after the cessation of the stress treatment. These data suggest that in the immediate period after cessation of stress behavioral phenotypes associated with depression, but not anxiety, emerge and 5-HT activity in the hypothalamus is increased.
Supplementary Material
Acknowledgments
This research was supported by funding from the National Institute on Drug Abuse to Shane A. Perrine (K01-DA024760), and fellowships to Fares Alsawah and Peter C. McNeill for the Summer Research Experience Program were provided by the Wayne State University School of Medicine Basic Medical Science Program and the Department of Psychiatry and Behavioral Neurosciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant BF, Hasin DS, Stinson FS, Dawson DA, June Ruan W, Goldstein RB, et al. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2005;35:1747–59. doi: 10.1017/S0033291705006069. [DOI] [PubMed] [Google Scholar]
- 2.Blazer D, Bachar JR, Hughes DC. Major depression with melancholia: a comparison of middle-aged and elderly adults. J Am Geriatr Soc. 1987;35:927–32. doi: 10.1111/j.1532-5415.1987.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186:661–9. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 5.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16:965–8. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 6.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 7.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 8.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association., American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- 10.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 11.Monleon S, D'Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–7. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 12.Stamford JA, Muscat R, O'Connor JJ, Patel J, Trout SJ, Wieczorek WJ, et al. Voltammetric evidence that subsensitivity to reward following chronic mild stress is associated with increased release of mesolimbic dopamine. Psychopharmacology (Berl) 1991;105:275–82. doi: 10.1007/BF02244322. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–52. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 14.Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124:637–46. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Bielajew C, Konkle AT, Merali Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behav Brain Res. 2002;136:583–92. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- 16.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–34. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 17.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacology Biochemistry and Behavior. 1996;54:229–34. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 18.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav. 1996;54:229–34. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 19.Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia W, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1417–24. doi: 10.1016/j.pnpbp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005;179:769–80. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- 21.Strausbaugh HJ, Dallman MF, Levine JD. Repeated, but not acute, stress suppresses inflammatory plasma extravasation. Proc Natl Acad Sci U S A. 1999;96:14629–34. doi: 10.1073/pnas.96.25.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 2002;27:519–25. doi: 10.1023/a:1019856821430. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer RP, Jr, Rowlett JK, et al. Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res. 1991;568:61–6. doi: 10.1016/0006-8993(91)91379-f. [DOI] [PubMed] [Google Scholar]
- 24.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 25.Romer B, Lewicka S, Kopf D, Lederbogen F, Hamann B, Gilles M, et al. Cortisol metabolism in depressed patients and healthy controls. Neuroendocrinology. 2009;90:301–6. doi: 10.1159/000235904. [DOI] [PubMed] [Google Scholar]
- 26.Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–61. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Zheng X, Liang J, Peng Y. Coexistence of anhedonia and anxiety-independent increased novelty-seeking behavior in the chronic mild stress model of depression. Behav Processes. 2010;83:331–9. doi: 10.1016/j.beproc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193:311–4. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 29.D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–7. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 30.Matuszewich L, Karney JJ, Carter SR, Janasik SP, O'Brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav. 2007;90:674–81. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. 7. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 32.Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–72. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam ; Boston: Elsevier; 2007. [Google Scholar]
- 34.Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1995;56:179–86. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- 35.Deuschle M, Schweiger U, Standhardt H, Weber B, Heuser I. Corticosteroid-binding globulin is not decreased in depressed patients. Psychoneuroendocrinology. 1996;21:645–9. doi: 10.1016/s0306-4530(96)00033-9. [DOI] [PubMed] [Google Scholar]
- 36.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–77. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–9. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- 39.Johnson BN, Yamamoto BK. Chronic unpredictable stress augments +3,4-methylenedioxymethamphetamine-induced monoamine depletions: the role of corticosterone. Neuroscience. 2009;159:1233–43. doi: 10.1016/j.neuroscience.2009.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson BN, Yamamoto BK. Chronic stress enhances the corticosterone response and neurotoxicity to +3,4-methylenedioxymethamphetamine (MDMA): the role of ambient temperature. J Pharmacol Exp Ther. 2010;335:180–9. doi: 10.1124/jpet.110.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem. 2001;79:1113–21. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.