Summary
The contribution of Toll-like receptor (TLR) signaling to T cell-dependent (TD) antibody responses was assessed by using mice lacking the TLR signaling adaptor MyD88 in individual cell types. When a soluble TLR9 ligand was used as adjuvant for a protein antigen, MyD88 was required in dendritic cells but not in B cells to enhance the TD antibody response, regardless of the inherent immunogenicity of the antigen. In contrast, a TLR9 ligand contained within a virus-like particle substantially augmented the TD germinal center IgG antibody response, and this augmentation required B cell MyD88. The ability of B cells to discriminate between antigens based the physical form of a TLR ligand likely reflects an adaptation to facilitate strong anti-viral antibody responses.
Keywords: Toll-like receptor, MyD88, T cell-dependent antibody response, dendritic cells, B cells, virus-like particle, virus
INTRODUCTION
Activation of B cells to produce antibodies is of central importance in defense against infectious agents. B cell activation is initiated by antigen recognition through the B cell antigen receptor (BCR) and requires additional activating signals such as those coming from helper T cells and/or other immune cells stimulated via innate recognition. Recent attention has focused on the role of innate recognition by Toll-like receptors (TLRs) for antibody production (Pasare and Medzhitov, 2005) (Gavin et al., 2006). The ability of TLR ligands to boost antibody responses in mice is mainly dependent on expression of MyD88, a key adaptor for the signaling of most TLRs. MyD88-deficient mice exhibit reduced steady state amounts of IgG2 isotypes even under specific pathogen-free conditions (Gavin et al., 2006; Pasare and Medzhitov, 2005), and have been found to have attenuated antibody responses to some immunizations (Pasare and Medzhitov, 2005) and to a number of pathogens, including influenza virus and Streptococcus pneumoniae (Heer et al., 2007) (Khan et al., 2005). Although the importance of TLRs for antibody responses to protein antigens combined with commonly used adjuvants has not been strongly evident (Gavin et al., 2006), purified TLR agonists are known to act on their own as effective adjuvants for antibody responses both in mice and in humans (Bekeredjian-Ding and Jego, 2009) (Halperin et al., 2006) (Jennings and Bachmann, 2008).
TLR ligands may act on multiple cell types following immunization. Stimulation of TLRs on antigen presenting cells such as dendritic cells (DCs) is known to induce their maturation and secretion of cytokines (Reis e Sousa, 2004), which can promote the activation of helper T cells. B cells also express TLRs such as TLR4, TLR7 and TLR9, and can respond to TLR agonists in vitro by proliferating and differentiating into antibody-secreting cells (Bekeredjian-Ding and Jego, 2009). Indeed, B cell-intrinsic TLR-MyD88 signaling has been implicated in the anti-RNA and anti-DNA autoantibody responses in several spontaneous mouse models of systemic lupus erythematosus (SLE) (Shlomchik, 2009). However, in response to immunization that includes TLR agonists in the adjuvant, whereas MyD88 signaling in B cells is required for the production of IgG2b and IgG2c isotypes of T cell-dependent (TD) antibodies in some studies (Pasare and Medzhitov, 2005) (Jegerlehner et al., 2007), complete loss of MyD88 in mice or selectively in B cells has little effect on antibody responses in several other studies (Gavin et al., 2006) (Meyer-Bahlburg et al., 2007). Thus, although it is clear that TLRs and MyD88 signaling can promote antibody responses, there is little understanding of the rules governing how MyD88 and TLR signaling contribute to antibody responses in vivo.
It has been proposed that factors such as the nature or immunogenicity of the antigen used may explain the discrepancies seen in these studies (Lanzavecchia and Sallusto, 2007) (Palm and Medzhitov, 2009). However, another potentially important variable that has not been carefully investigated is the impact of the physical form of the TLR agonist. We recently reported that altering the physical form of a TLR ligand critically affects the ability of different immune cell types to respond in vivo (Hou et al., 2008). Here we have presented studies demonstrating that, depending on the physical context in which a TLR ligand is presented, MyD88 signaling in either B cells or in DCs can substantially augment antibody responses to protein antigens combined with TLR ligands. In particular, DC but not B cell TLRs enhanced the antibody response to soluble protein antigens mixed with or chemically linked to a TLR ligand. In contrast, B cell MyD88 greatly enhanced the TD antibody response to a virus-like particle antigen that incorporated TLR ligands or to chemically-inactivated influenza virus. Moreover, a dense array of antigenic epitopes on the viral particle promoted the ability of TLR signaling in the B cell to enhance the germinal center response. These results suggest that B cells are hard wired to respond vigorously to particles with the properties of many viruses, and that this may represent an evolutionary adaptation that contributes to immune defense against virus infection.
RESULTS
Generation of mice lacking MyD88 selectively in DCs and B cells
For these experiments, we took advantage of a recent mouse model in which specific ablation of the mouse Myd88 gene in certain cell types can be engineered using Cre-lox technology. Mice with specific deletion of MyD88 in DCs (DC-Myd88−/− mice) were generated by crossing mice with the Myd88fl allele to mice expressing the recombinase Cre under the control of the Cd11c promoter (Caton et al., 2007), as has been reported before (Hou et al., 2008). To generate mice lacking MyD88 specifically in B cells, mice with the Myd88fl allele were crossed to mice expressing Cre under the control of the murine Cd79a promoter (Cd79a-Cre, also known as mb1-Cre) (Hobeika et al., 2006). In these mice, deletion of the Myd88fl allele, measured by a quantitative polymerase chain reaction (PCR) assay (Hou et al., 2008), occurred in over 98% of follicular B cells and marginal zone B cells in the spleen, and of peritoneal B cells (data not shown). These mice had normal numbers of mature and immature B cells in the bone marrow and the spleen, except for a slight reduction in the number of recirculating mature B cells in the bone marrow (Figure S1A). Cell surface expression of IgM was also very similar to that of control mice for the various B cell subpopulations (Figure S1A). Moreover, the lymphoid organs (spleen and LNs) of these mice exhibited normal cellular composition and structure (data not shown). The functional and signaling responses to BCR stimulation were very similar to those of control mice (Figure S1B, 1C and data not shown). In contrast, B cells isolated from these mice showed selective defects in response to TLR9 stimulation, including upregulation of CD86 and proliferation (Figure S1D). These results demonstrated that the ablation of MyD88 signaling in B cells had little effect on the development of B cells or on BCR function in these mice. We refer to these mice here as B cell-Myd88−/− mice.
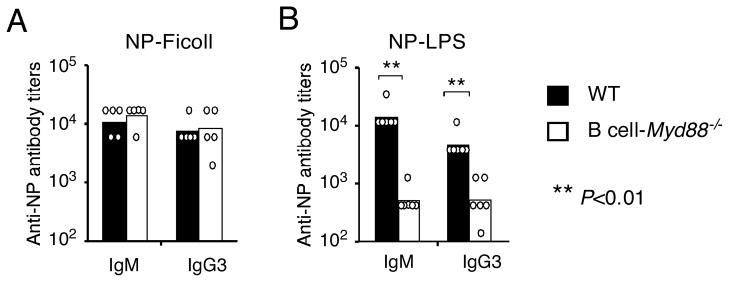
Antibody responses to T cell-independent antigens in the B cell-Myd88−/− mice
To see whether ablation of MyD88 in B cells affected their functional properties in vivo, we first examined the antibody responses to T cell-independent (TI) antigens. The prototypical TI type 2 antigen 4-Hydroxy-3-nitrophenylacetyl (NP)-Ficoll, which activates B cells by extensive crosslinking of multiple BCRs and hence generates very strong BCR signaling (Mond et al., 1995), induced comparable amounts of NP-specific IgM and IgG3 antibodies in the wild type and B cell-Myd88−/− mice (Figure 1A), consistent with normal BCR function in mice lacking Myd88 in B cells. In contrast, in response to NP-lipopolysaccharide (LPS), a TI type 1 antigen containing a TLR4 ligand as carrier, the mice lacking Myd88 selectively in B cells exhibited substantially attenuated NP-specific IgM and IgG3 responses compared to wild type mice (Figure 1B). This result agrees with the in vitro observation that dual recognition by the BCR and a TLR is required for an optimal antibody response to this type of antigen (Bekeredjian-Ding and Jego, 2009).
Figure 1. Antibody responses to TI antigens in the B cell-Myd88−/− mice.
Wild type and B cell-Myd88−/− mice were immunized i.p. with 20 μg of NP-Ficoll (A) or NP-LPS (B). The titers of anti-NP IgM and IgG3 were measured by ELISA. The titers of individual mice (circles on the graph) and geometrical mean of each mouse group (bar graph) on day 7 are shown. Similar results were obtained in one (A) and two (B) additional experiments. **, P<0.01
Antibody response to soluble protein antigens mixed with a soluble TLR ligand
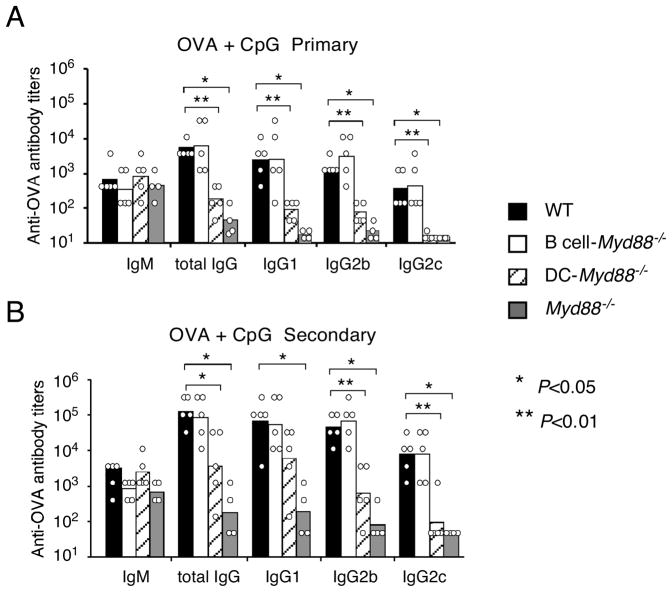
We next examined the role of TLR signaling in particular cell types for the antibody responses to T cell-dependent (TD) protein antigens in the presence of a soluble TLR ligand as adjuvant. Wild type, Myd88−/−, B cell-Myd88−/− and DC-Myd88−/− mice were immunized intraperitoneally (i.p.) with ovalbumin (OVA) mixed together with a TLR9 ligand, an oligodeoxynucleotide (ODN) containing an unmethylated CpG motif (hereby referred to as CpG). Whereas the early IgM response was not affected in the mice lacking MyD88 in all cells or just in DCs, the primary and/or secondary IgG1 and IgG2 responses were greatly reduced in these animals (Figure 2A and B). These results indicate a requirement for DC-intrinsic MyD88 signaling for soluble CpG to activate DCs, which presumably activate T cells to provide help to B cells. Surprisingly, however, the B cell-Myd88−/− mice exhibited no obvious defect in the production of OVA-specific IgM or IgG in either the primary response or in the secondary response after rechallenge with OVA (Figure 2A and B). Similar results were seen when using other soluble antigens including NP-haptenated chicken gamma globulin (data not shown). These results demonstrate that a TLR9 agonist can promote an antibody response to a protein antigen in a manner that requires DC MyD88 but not B cell MyD88.
Figure 2. Antibody response to OVA mixed with a soluble TLR9 ligand.
Wild type, DC-Myd88−/−, B cell-Myd88−/− or Myd88−/− mice were immunized i.p. with 100 μg of OVA mixed with 50 μg of CpG (ODN 1826), and were re-challenged i.p. with 10 μg of OVA four weeks later. Antibody titers of individual mice and the geometrical mean of each mouse group in the primary response (IgM on day 7 and all IgG isotypes on day14) (A) or secondary response (seven days after re-challenge) (B) are shown. Statistical comparison was between wild type mice and the other mouse groups, and was calculated with the Mann-Whitney U-test. Similar results with the same or different doses of OVA were obtained in four additional experiments. *, P<0.05, **, P<0.01.
Antibody response to soluble protein antigens physically-linked to a TLR ligand
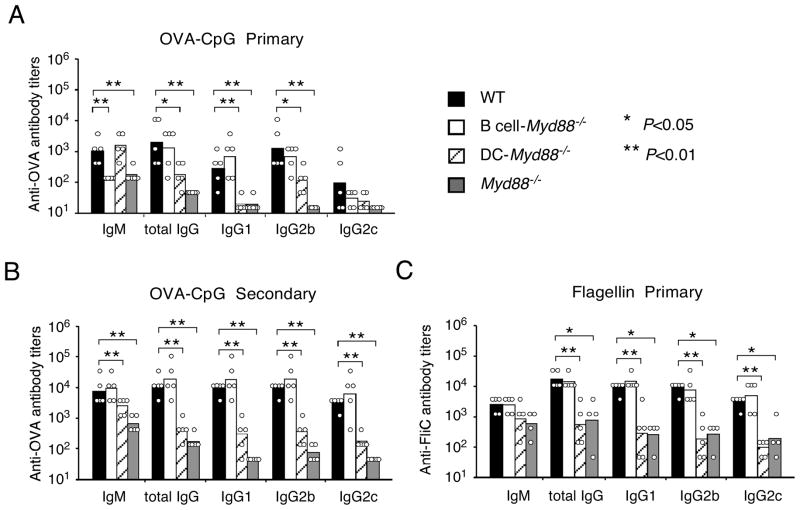
To test whether this lack of effect of TLR signaling in B cells may be due to poor availability of this soluble TLR ligand to the endocytically-localized TLRs of the antigen-specific B cells, we next examined the antibody responses to immunizations with a TLR agonist that was physically linked to the antigen, since it has been shown that BCR-mediated endocytosis can deliver TLR ligands to internal compartments where they can stimulate TLR9 (Chaturvedi et al., 2008; O’Neill et al., 2009) (Eckl-Dorna and Batista, 2009). To this end, CpG was covalently linked to OVA, and the resulting conjugate (OVA-CpG) contained on average 2–4 CpG ODNs per OVA molecule (data not shown). After immunization of mice with OVA-CpG, the early antigen-specific IgM response was reduced in the B cell-Myd88−/− mice to approximately 10% of the response in wild type mice (Figure 3A), a result that was similar to the IgM response to NP-LPS. These results indicate that efficient recognition of a soluble TLR ligand following BCR-mediated uptake promoted the IgM response. However, despite a reduction in the IgM response, the B cell-Myd88−/− mice made primary and secondary IgG responses that were very similar to those of wild type mice (Figure 3A, 2B). Again, the primary and secondary IgG response to the conjugate was reduced in the Myd88−/− mice and in the DC-Myd88−/− mice (Figure 3A, B), consistent with a requirement for TLR signaling in DCs to optimally promote the helper T cell response (Pasare and Medzhitov, 2004).
Figure 3. Antibody response to protein antigens physically linked with TLR ligands.
Wild type, DC-Myd88−/−, B cell-Myd88−/− or Myd88−/− mice were immunized i.p. with OVA-CpG conjugate (containing 50 μg OVA and 32 μg CpG) and re-challenged i.p. with 10 μg of OVA four weeks later (A and B), or were immunized with 25 μg of flagellin (FliC) (C). Shown are the titers of anti-OVA or anti-flagellin antibody of individual mice and the geometrical mean of each mouse group in the primary response (A and C) or secondary response (B). Immunization with the same OVA-CpG in two additional experiments and with a different preparation resulted in similar findings to (A and B). Similar results to (C) were obtained in an additional experiment. *, P<0.05, **, P<0.01.
To test whether the same principles also applied to other protein antigens with TLR agonist activity, we next examined the antibody response to bacterial flagellin, a natural ligand for TLR5, which is strongly immunogenic compared to OVA. The primary IgM and IgG responses to flagellin were unaffected in mice lacking B cell-intrinsic MyD88 signaling (Figure 3C). In contrast, Myd88−/− mice had a strong reduction in both IgM and IgG responses and similar defects were seen in mice lacking MyD88 only in DCs (Figure 3C). Thus, the role of MyD88 signaling in augmenting the antibody response to flagellin was primarily in DCs. This is in contrast to what was observed in similar experiments utilizing adoptive transfer of Myd88−/− B cells into B cell-deficient mice (Pasare and Medzhitov, 2005). The results described above, in which mice were immunized with OVA, a weakly immunogenic antigen, or flagellin, a strongly immunogenic antigen, did not support the hypothesis that the inherent immunogenicity of an antigen affects the requirement for TLR signaling in B cells for promoting TD antibody responses, but instead demonstrated that inherent immunogenicity was poorly predictive of the importance of B cell-intrinsic MyD88 signaling for the magnitude of the antibody response.
Antibody response to a protein antigen mixed with aggregated TLR ligands
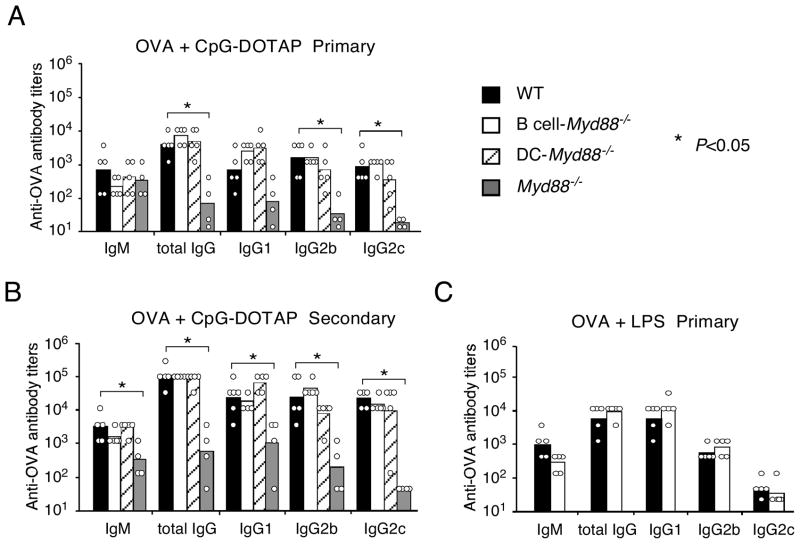
We previously found that CpG aggregated by the cationic lipid DOTAP stimulates a greater range of cell types in vivo than does soluble CpG (Hou et al., 2008). Consequently, immunization with OVA together with CpG-DOTAP induced substantially more antigen-specific IgG than immunization with OVA plus an equal amount of soluble CpG (Figure S2A). To examine whether MyD88 signaling in B cells is required for the optimal IgG response to immunization with this type of aggregated TLR ligand, B cell-Myd88−/− mice were immunized with OVA together with CpG-DOTAP. Again, B cell-Myd88−/− mice appeared to have no defect in their antibody responses, whereas Myd88−/− mice had a large defect (Figure 4A, B). Similar results were obtained when mice were immunized i.p. with OVA plus LPS, a naturally aggregated ligand of TLR4 (Figure 4C). These results indicated that MyD88 signaling in B cells is not essential for the TD antibody responses elicited by this type of TLR ligand.
Figure 4. Antibody response to a protein antigen mixed with aggregated TLR ligands.
Mice were immunized i.p. with 100 μg of OVA mixed with 25 μg of DOTAP-aggregated CpG (ODN1826) and re-challenged i.p. with 10 μg of OVA four weeks later (A and B), or were immunized with 100 μg of OVA mixed with 25 μg of LPS (C). Shown are the titers of antigen-specific antibody of individual mice and the geometrical mean of each mouse group in the primary response (A and C) or secondary response (B). Similar results were obtained in two (A and B) and one (C) additional experiments. *, P<0.05, **, P<0.01.
Whereas DC-Myd88−/− mice had a substantial defect in producing antibody following immunization with Ova + soluble CpG, these mice exhibited largely normal antibody responses to immunization with Ova plus CpG-DOTAP as we reported before. This response was lost, however, when the DC-specific deletion of Myd88 was coupled with a deficiency of the type 1 interferon receptor 1 (IFNAR1) (Figure S2B), indicating that the strong type 1 IFN response to CpG + DOTAP can provide an alternative to MyD88 signaling in DCs to promote the antibody response. Similarly, when DCs were acutely depleted via injection of diphtheria toxin into mice expressing the primate diphtheria toxin receptor selectively in DCs, the antibody response was strongly diminished (Figure S2C), proving that DCs were still required for the antibody response when CpG-DOTAP was used as adjuvant. These results suggested that the immune-enhancing effect of aggregating the TLR ligand, and/or any other adjuvant effects DOTAP may have, was mainly through augmentation of the DC-T cell component of the response.
Antibody response to Qβ Virus-like particles containing TLR ligands
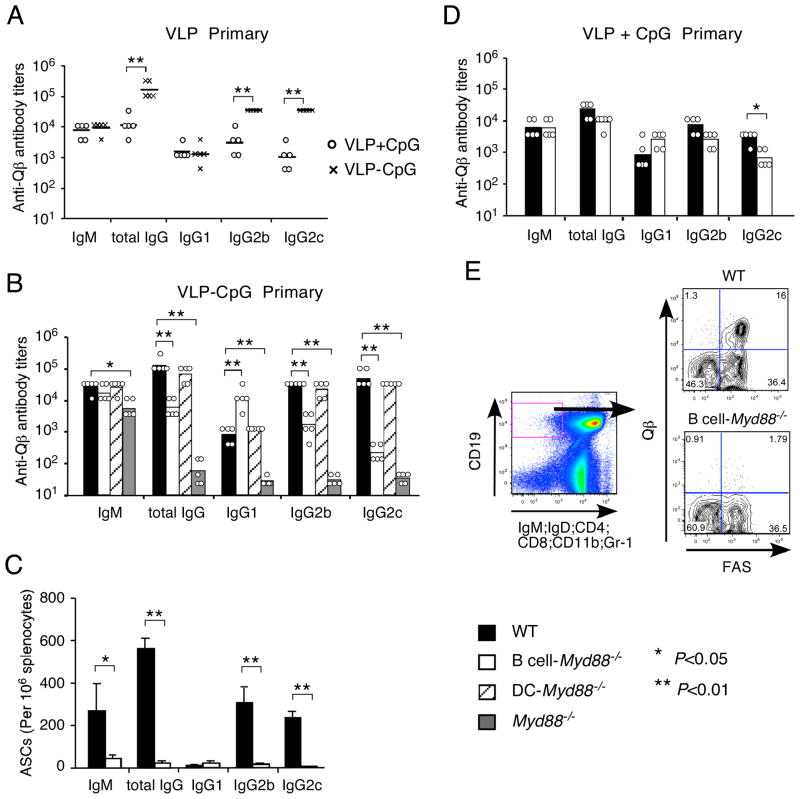
We next examined the role of TLR signaling in DCs and in B cells for response to a particulate antigen containing a TLR agonist as is typical of virus particles. For these experiments, we used the well characterized proteinaceous virus-like particles (VLPs) made by the bacteriophage Qβ capsid protein (Jegerlehner et al., 2007). The self-assembled VLPs contain single-stranded RNA (ssRNA) that can stimulate RNA-recognizing TLRs such as TLR7 (hereby referred to as VLP-ssRNA) (Jennings and Bachmann, 2008), but cannot directly infect mammalian cells. To make VLPs containing CpG, VLPs were disaggregated into Qβ dimers, which were then purified and assembled with G10 CpG ODNs. On average, each Qβ VLP with CpG incorporated (here referred to as VLP-CpG) contained 60–80 CpG ODNs together with its ~180 Qβ subunits (Senti et al., 2009). Immunization of mice with VLP-CpG induced potent Qβ-specific IgM and IgG antibody responses. The IgG response was largely dependent on αβ T cells, because it was greatly reduced (to ~1%) in mice lacking T cell receptor α chain (Figure S3A). This result is consistent with our previous report using mice largely deficient in CD4+ T cells due to genetic deficiency of major histocompatibility complex (MHC) class II molecules (Gatto et al., 2004). The amount of Qβ-specific IgM produced was similar following immunization with empty VLPs mixed with equal molar amounts of soluble CpG or immunization with VLPs that had physical inclusion of CpG in VLPs. In contrast, the IgG response was greatly enhanced by physical incorporation of CpG in the VLPs, and it was strongly directed to the IgG2c and IgG2b isotypes (Figure 5A). These and previous results (Jegerlehner et al., 2007) indicate that incorporation of a TLR9 agonist within the Qβ VLPs strongly promoted a TD antibody response.
Figure 5. Antibody response to Qβ VLP containing CpG ODNs.
(A) C57BL/6 mice were immunized i.p. with 50 μg of VLP-CpG or 37.5 μg empty VLP mixed with 12.5 μg CpG (ODN 1826). Shown are the titers of antigen-specific antibody of individual mice (circle for VLP mixed with CpG, and cross for VLP-CpG) and the geometrical mean of each mouse group (horizontal bar) in the primary responses. Similar results were obtained in one additional experiment. (B and C) Mice of specified genotype were immunized i.p. with 50 μg of VLP-CpG. (B) Shown are the titers of anti-Qβ antibody of individual mice and the geometrical mean of each mouse group in the primary responses. Similar results were obtained on day 21 and day 28. (C) Shown are the numbers of anti-Qβ antibody-secreting cells (ASCs) in the spleen on day 11 after immunization (mean+s.d. of 3 mice in each group). Statistical comparisons were calculated with Student’s t-test. Similar differences were also seen on day 21 after immunization. (D) Wild type and B cell-Myd88−/− mice were immunized i.p. with 37.5 μg of empty VLP mixed together with 12.5 μg of CpG (ODN1826). Shown are the titers of antigen-specific antibody of individual mice and the geometrical mean of each mouse group in the primary responses on day 14 after immunization. (E) Mice of the specified genotype were immunized i.p. with 50 μg of VLP-CpG. Representative flow cytometry plots of isotype-switched Qβ-specific B cells in the spleen on day 11 after immunization (3 mice in each group). Most Qβ-specific B cells also stained positive for GL-7 (data not shown). Similar results were obtained on day 21 after immunization. *, P<0.05, **, P<0.01.
The immune-stimulating effect of CpG incorporated inside VLPs depended on MyD88 signaling as expected, because the IgG response to VLP was almost completely ablated in Myd88−/− mice (Figure 5B). Mice lacking MyD88 in DCs exhibited as strong a VLP-specific IgG response as did wild type mice (Figure 5B), whereas depletion of DCs again completely ablated the response (Figure S3B), presumably reflecting a role for DCs in the activation of helper T cells specific for peptides derived from the VLPs. Whereas in the case of CpG + DOTAP immunization, type 1 interferons were apparently responsible for inducing DC maturation, that was not the case with VLPs, as deficiency of type 1 IFN signaling combined with deletion of Myd88 selectively in DCs did not diminish the IgG response to the VLP-CpG (data not shown).
Remarkably, B cell-Myd88−/− mice had a substantially attenuated IgG response, which manifested as dramatically decreased IgG2c and IgG2b titers combined with a relatively moderate elevation of IgG1 titers (Figure 5B). In agreement with the serum titers of anti-Qβ IgG, the B cell-Myd88−/− mice had substantially fewer anti-Qβ antibody-secreting cells in the spleen after immunization (Figure 5C). Immunization of Cd79a-Cre mice (without Myd88fl allele) induced similar amounts of IgG as in C57BL/6 mice, suggesting that the defect observed in the B cell-Myd88−/− mice is not caused by any non-specific Cre-mediated recombination or difference in genetic background (Figure S3C). Additionally, no dramatic defect in IgG response was observed in the B cell-Myd88−/− mice after immunization with empty VLPs (lacking internal ssRNA or CpG) mixed together with CpG (Figure 5D), indicating that incorporation of a TLR ligand in the VLP is required for a TLR signal in B cells to promote the TD antibody response. When the antibody responses to VLP-CpG and VLPs mixed with CpG in B cell-Myd88−/− mice were compared, similar titers of total IgG as well as of the different IgG isotypes were found (Figure 5B and 5D), consistent with the conclusion that activation of MyD88-dependent signaling in B cells was largely responsible for the boosting effect on the TD antibody response by CpG within VLPs.
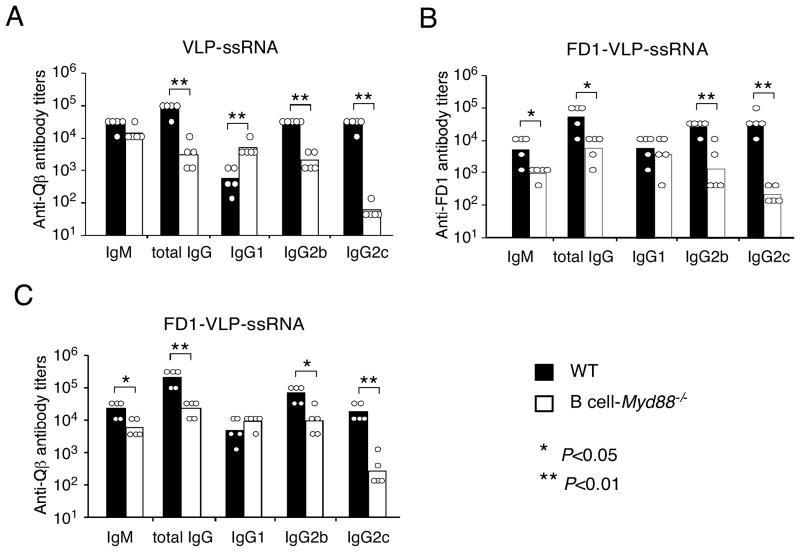
Immunization with VLP-CpG has been shown to induce a strong and prolonged germinal center (GC) response (Gatto et al., 2004). To examine whether the GC response was affected in mice lacking MyD88 signaling in B cells, the phenotypes of isotype-switched Qβ-specific B cells in the spleen were analyzed by flow cytometry after immunization. Eleven days after immunization, isotype-switched Qβ-specific B cells had expanded dramatically in wild type mice, and the majority of these cells exhibited a GC phenotype (Figure 5E), whereas these responses were severely defective in the B cell-Myd88−/− mice. These results demonstrate that intrinsic MyD88 signaling is required for B cells to make an optimal GC response following immunization with a virus-like particulate antigen containing a TLR9 ligand. When we used the Qβ VLPs that contained ssRNA, we found that the IgG anti-Qβ response was also greatly diminished in the B cell-Myd88−/− mice (Figure 6A), indicating that a TLR7 ligand can also engage B cell TLRs and promote the TD antibody response to VLPs.
Figure 6. Antibody response to a protein antigen coupled to VLPs.
Wild type and B cell-Myd88−/− mice were immunized i.p. with 50 μg of VLP-ssRNA (A) or FD1-VLP-ssRNA conjugates (B-C). Shown are the primary response titers of anti-Qβ antibody (A and C) and anti-Fel d1 (FD) antibody (B) of individual mice and the geometrical mean of each mouse group. Similar results were obtained in one additional experiment. *, P<0.05, **, P<0.01.
Antibody response to a protein antigen coupled to VLPs
BCR-mediated delivery of nucleic acid TLR ligands to an endocytic compartment, where TLR7 and TLR9 signal (Barton and Kagan, 2009), is likely essential for VLPs containing these ligands to induce a strong TD anti-VLP antibody response. Other properties of VLPs, however, may also be required for them to synergize with B cell TLR signaling to enhance the TD antibody response, since CpG conjugated to a soluble protein antigen did not exhibit a requirement for B cell MyD88 (Figure 3A). The antigen of the VLPs exhibits a repeating array of epitopes, which are likely to induce especially strong BCR signaling, and could be of different immunogenicity from the other soluble antigens due to its bacteriophage origin. To test how these factors might influence the antibody response and the B cell TLR contribution to it, we examined the antibody responses to a covalent conjugate of the Qβ ssRNA VLP and a soluble protein antigen, Fel d1, a cat allergen (the conjugate is hereby referred to as FD1-VLP-ssRNA) (Schmitz et al., 2009). The Fel d1 epitopes did not appear to have any unique properties compared to other soluble antigens we have tested, because immunization with soluble Fel d1 mixed with CpG induced a TD antibody response that was not dependent on MyD88 signaling in B cells (data not shown). The Fel d1-conjugated particles exhibited new epitopes supplied by the Fel d1 protein (~50 Fel d1 per VLP containing 180 Qβ monomers), and likely had a reduced density of the Qβ protein epitopes exposed for recognition by the BCR.
Immunization with FD1-VLP-ssRNA induced antibody responses to both Fel d1 and Qβ epitopes. Remarkably, the IgG response to Fel d1 epitopes was substantially enhanced (~10-fold) in wild type mice compared to B cell-Myd88−/− mice (Figure 6B), indicating that the ability of B cell TLR signaling to enhance the TD antibody response was not limited to Qβ epitopes. The Fel d1-modified VLPs retained a strong IgG response to Qβ epitopes that was highly dependent on B cell MyD88 (Figure 6C). However, the difference between the anti-Qβ IgG response of the wild type and B cell-Myd88−/− mice appeared to be somewhat decreased compared with immunization with underivatized VLP-ssRNA (Figure 6A and 6C), suggesting that the degree of multimerization of the VLP epitope determines the extent to which B cell TLR signaling boosts the TD antibody response. Consistent with this hypothesis, when mice were immunized with a CpG VLP conjugate that contained a lower density of Fel d1 (9–18 Fel d1 per CpG-containing VLP), the Fel d1 specific IgG antibody response of wild type mice was only ~3-fold greater than that of B cell-Myd88−/− mice, whereas the Qβ specific response ranged from 20- to 30-fold more (Figure S4 and Figure 5B).
Antibody response to virons of H1N1 influenza virus
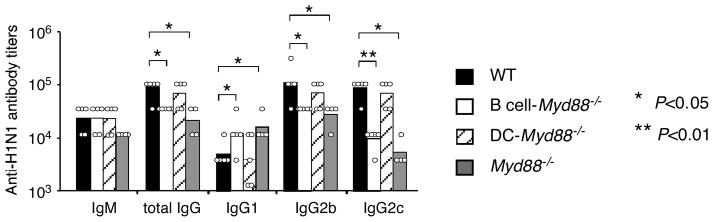
Lastly, to examine whether B cell-intrinsic MyD88 signaling is also required for antibody responses to a native virus particle, we challenged mice with inactivated influenza virus, which contains single-stranded RNA genomic segments that can activate TLR7 (Diebold et al., 2004), but unlike Qβ VLPs, is an enveloped-virus. After immunization, a strong anti-influenza IgG response was rapidly induced in the wild type mice. Similar to what was seen in response to Qβ VLP, the anti-influenza total IgG and IgG2 responses were greater (3–10 fold) in wild type mice than in Myd88−/− mice and there was no reduction in DC-Myd88−/− mice (Figure 7). Strikingly, B cell-Myd88−/− mice exhibited a defective response similar to that in the Myd88−/− mice (Figure 7), indicating that B cell-intrinsic MyD88 signaling contributes importantly to the magnitude of the anti-influenza virus humoral response
Figure 7. Antibody responses to immunization with inactivated H1N1 influenza virus.
Mice were immunized i.p. with 10 μg of formalin-inactivated H1N1 PR/8 influenza virus. Shown are the titers of antigen-specific antibody of individual mice and the geometrical mean of each mouse group in the primary responses. Similar results were obtained in one additional experiment. *, P<0.05, **, P<0.01.
DISCUSSION
In this study, we obtained evidence that TLR ligands can engage TLR signaling in both B cells and DCs resulting in augmentation of TD antibody responses, and that a dominant determinant of the cellular pathway engaged and the extent of augmentation is the physical context in which the TLR ligand is presented to the immune system. In particular, TLR-MyD88 signaling in DCs but not in B cells was required to enhance the IgG response to a soluble protein antigen either mixed with a soluble TLR9 ligand or chemically conjugated to it. In contrast, VLPs that contained a TLR9 or TLR7 ligand or inactivated influenza virus were able to engage TLR and MyD88 signaling within the antigen-specific B cells to strongly enhance a primary germinal center response.
The selective utilization of B cell TLR signaling by TLR7 or TLR9 ligands contained within VLPs but not by soluble TLR ligand or by TLR ligand chemically coupled to soluble antigen for the enhancement of T cell-dependent IgG production is the most remarkable finding of this study. TLR ligands are potent activators of murine B cells in vitro (Bekeredjian-Ding and Jego, 2009) (Shlomchik, 2009), and thus, it has been presumed that TLRs in B cells play an important role in promoting antibody responses in vivo. Indeed, Pasare and Medzhitov provided evidence that B cell TLRs were responsible for enhancing the magnitude of the TD antibody response. Subsequent studies have either provided evidence for a role for TLRs in promoting antibody responses or failed to see a role, depending on the circumstance examined, illustrating that much needs to be learn about the role of TLRs in antibody response. We examined antibody responses to several soluble protein antigens with different apparent immunogenicity mixed a TLR ligand as adjuvant, and found that deletion of Myd88 selectively in B cells did not decrease the magnitude of the IgG responses. In addition, when we used a TLR ligand that had been physically-linked to an antigen to increase delivery of the TLR ligand to B cell TLRs, we observed a contribution of TLR signaling in B cells only to the early IgM response but not to the TD IgG response. Instead, in all these circumstances, we found that MyD88 signaling in DCs accounted for most of the effect of TLR stimulation, suggesting that signals from helper T cells are sufficient to promote a strong B cell response, provided the CD4 T cells are activated by TLR-stimulated DCs. Similarly, MyD88 signaling in DCs was recently shown to be important for the IgA response to intranasal immunization (Bessa et al., 2009). Our results argue against the hypothesis that proteins of low immunogenicity enable TLRs to boost the response, whereas highly immunogenic proteins, such as haptenated proteins, do not (Palm and Medzhitov, 2009). Rather, regardless of the strength of the TD antibody response to a soluble protein antigen, it was MyD88 in DC, not B cell that boosted the response.
In contrast to what was seen with soluble protein antigens and soluble TLR ligands, we found that similar TLR ligands contained within VLPs had the unique ability to engage TLR signaling in B cells to strongly enhance TD IgG production. The magnitude of the IgG response to VLPs was typically approximately 30-fold greater in wild type mice compared to mice with deletion of Myd88 selectively in B cells. The low IgG response in B cell-Myd88−/− mice was similar to what was seen upon immunization of wild type mice with VLPs depleted of nucleic acid and mixed with free CpG ODNs, and this latter response was not affected by deletion of Myd88 in B cells. Thus, incorporation of the TLR ligand within the VLP was necessary for enabling B cell TLR signaling to boost the IgG response. This ability of VLPs to induce a robust IgG response by engaging B cell TLR signaling was a property of the physical form of the VLPs, not the inherent immunogenicity of the protein epitopes exposed on the particle. For example, a weakly immunogenic protein, the cat allegen Fel d1, when used as a soluble antigen mixed with CpG ODN behaved like the other soluble protein antigens tested in that the IgG response was decreased by deletion of Myd88 in DCs but was unaffected by deletion of Myd88 in B cells. In contrast, when Fel d1 was conjugated to the VLPs containing nucleic acid ligands for TLRs, these VLPs induced a robust anti-Fel d1 IgG response that benefited substantially from Myd88 expression in B cells.
Earlier studies designed to distinguish the contribution of B cell MyD88 from the contribution of MyD88 in other cell types (Pasare and Medzhitov, 2005) used adoptive transfer of MyD88-deficient B cells into μMT mice, which have deletion of exon encoding the transmembrane domain of IgM and therefore are genetically defective in B cell development. Such mice are known to have substantial alterations in the structure of their secondary lymphoid organs due to the role of B cells in expressing lymphotoxin-β (Chaplin, 2002). These mice may also have elevated amounts of the cytokine B cell activating factor belonging to the TNF family (BAFF) due to B cell lymphopenia, and either of these changes may alter the timing or other aspects of the B cell response. Thus, it is possible that the activation of CD4 helper T cells is suboptimal in this experimental system. In this respect, the use of Cre-lox technology for deletion of MyD88 selectively in B cells in situ, as was done in the experiments presented here, has obvious advantages.
The mechanism by which virus particles engage B cell TLR7 or TLR9 signaling to enhance the TD IgG response deserves further investigation, but the results presented here provide some insights. The major component of the TD response that was stimulated was the germinal center response, which is responsible for producing high affinity antibody, long-lived plasma cells, and memory B cells (King et al., 2008). Moreover, the magnitude of the enhancement seen in wild type mice compared to B cell-Myd88−/− mice was consistently affected by the epitope density on the VLPs: unmanipulated VLP-ssRNA or VLP-CpG exhibited the strongest enhancement by B cell MyD88, whereas low density Fel d1 conjugation resulted in a lower enhancement of the anti-Fel d1 IgG response compared to the anti-Qβ IgG response. When Fel d1 was conjugated onto the VLPs at a higher density, then a larger enhancement of the anti-Fel d1 IgG response was seen. Thus, there was a consistent trend toward greater enhancement by B cell MyD88 when the epitope density on the surface of the VLP was higher, indicating that greater engagement of BCRs and presumably stronger BCR signaling enabled B cell TLR enhancement of the response. Another feature of VLPs that may enable B cell MyD88 to enhance the antibody response may be their likely ability to induce strong TLR signaling by virtue of the amount of TLR7 or TLR9 ligand contained within a single VLP. For example, the VLP-CpG contained an estimated 60–80 ODNs per virus particle. Additionally, it is possible that soluble and particulate antigens are processed differently in B cells after being taken-up by BCRs; the latter may be more efficiently trafficked to the location where nucleic acid-recognizing TLRs encounter their cognate ligands. Similar cell biological contributions to the nature of TLR responses have been described in plasmacytoid DCs (Honda et al., 2005).
It is striking that particles with two of the characteristic features of most virus particles, and lacking in many other types of particles, namely high density of particular epitopes and internal ligands for TLR7 or TLR9, uniquely engage this mechanism to strongly enhance the germinal center IgG response. Given the well-established function of neutralizing antibodies for defense against many viruses (Zinkernagel et al., 2001), this property of B cells is likely to be an evolutionary advantageous regulatory mechanism to promote rapid and robust production of high affinity antibodies to protect against virus infection. This phenomenon may also explain the common observations that immunization with attenuated or inactivated complete pathogens typically induces stronger antibody responses than immunization with the components of pathogen mixed with a TLR ligand or with other commonly used adjuvants. For example, Geeraedts et al have reported that vaccination with inactivated whole H5N1 influenza virus induced better protective antibody responses than vaccination with split virus or a viral subunit vaccine (Geeraedts et al., 2008). Our results indicate that activation of MyD88 signaling in B cells by a TLR ligand presented in the physical context of a virus particle may be an important mechanism to account for the superior immunogenicity of this type of antigen.
The ability of VLPs to promote vigorous IgG responses by TLR signaling in B cells is intriguing in light of previous work implicating B cell TLR signaling in spontaneous production of anti-nuclear antibodies and anti-RNA antibodies, the autoantibodies characteristic of the human autoimmune disease systemic lupus erythematosus (Marshak-Rothstein and Rifkin, 2007). Based on our results, we propose that the autoantigens in these cases may be exposed to B cells in the form of apoptotic blebs, since many antibodies of this type will bind to those structures (Cline and Radic, 2004). Apoptotic blebs resemble VLPs in their particulate nature and in their inclusion of a substantial amount of ligands for TLR7 and/or TLR9. Interestingly, the role of MyD88, TLR7 and TLR9 in augumenting spontaneous or induced autoantibody production in a few mouse models has been attributed to promoting extrafollicular antibody responses (Shlomchik, 2009), whereas we found that it was the germinal center response that was promoted by VLPs and TLR signaling in B cells. Therefore, it would be worth examining the contribution of the germinal center B cell response to spontaneous anti-DNA or anti-ribonucleoparticle autoantibody production in other mouse models of SLE.
In summary, our experiments clearly demonstrate that TLRs of DCs and B cells can both augment antibody responses, depending on the physical nature of the antigen. In particular, immunizations with soluble protein antigens mixed with or conjugated to TLR ligands as the major adjuvant demonstrated a requirement for TLR signaling in DCs but not B cells for a high titer IgG response to soluble protein antigens, whereas immunization with VLPs containing TLR ligands within them had a requirement for B cell MyD88 for a robust IgG response to the repeating epitopes of the VLP. Pathogens such as viruses contain nucleic acid that serves as ligands for TLRs such as TLR7 and TLR9, but it was not previously appreciated that such particles are especially able to engage B cell TLRs to enhance TD antibody responses. Thus, the results presented here suggest that B cells are hard-wired to respond strongly to virus particles and use their TLRs for this purpose, a property that likely has considerable advantage for defense against viral infection. These insights may be useful in the development of more effective vaccines.
MATERIALS AND METHODS
Mice
B6 (000664; C57BL/6J) and BoyJ (002014; B6.SJL-Ptprca Pepcb/BoyJ) mice were from Jackson Laboratory. The Myd88fl mice (B6.129P2-Myd88tm1Defr) and the Myd88fl/fl CD11c-Cre (DC-Myd88−/−) mice have previously been described (Hou et al., 2008), and were backcrossed to a B6 background for at least nine generations for this study. Cd79a-Cre transgenic mice on a C57BL/6 background (eight generations) were originally from M. Reth (Hobeika et al., 2006), and were crossed to mice with the Myd88fl allele to generate Myd88fl/fl Cd79a-Cre mice. Myd88−/− mice were originally from S. Akira (Osaka University, Osaka, Japan) (Adachi et al., 1998), and were backcrossed to B6 for 10 generations in our colony. Tcra−/− mice (Mombaerts et al., 1992) were obtained from M. Hermiston (UCSF). CD11c-Diphtheria Toxin Receptor (DTR) (004509; B6.FVB-Tg (Itgax-DTR/EGFP) 57Lan/J) mice were originally purchased from Jackson Laboratory (Jung et al., 2002), and were backcrossed to a B6 background for ten generations in our colony.
All experimental mice were used at 8–16 weeks of age and were sex-matched and age-matched (within 2 weeks) within experiments. All animals were housed in a specific pathogen-free animal facility at UCSF under conditions that meet institutional animal care and use committee (IACUC) and NIH guidelines.
Reagents
Chicken ovalbumin fraction VI (OVA) was purchased from Sigma-Aldrich, and endotoxin-free OVA was obtained by Triton X-114 treatment as described (Aida and Pabst, 1990). NP-Ficoll (NP-AECM-FICOLL) and NP-LPS were purchased from Biosearch Technologies. NP conjugated bovine serum albumin (NP10-BSA) was conjugated as described (Hannum et al., 2000). Salmonella typhimurium flagellin (FliC) was purified from a fljB− fliC+ strain (TH4778) kindly provided by K. Hughes (University of Utah, UT), following a protocol (Ibrahim et al., 1985) modified by K.D. Smith (University of Washington, WA). Diphtheria toxin was purchased from Biomol research laboratories. Influenza A/PR/8/34 (H1N1) virus was purchased from Charles River, and inactivated with 1/4000 of stock formaldehyde solution (37% w/w containing 10–15% methanol) following a protocol reported before (Katz and Webster, 1989).
Type B CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from Integrated DNA Technologies (IDT), and type B CpG ODN 1018 (5′-TGACTGTGAACGTTCGAGATGA-3′) was provided by Dynavax, Inc. Type A CpG G10 (5′-GGGGGGGGGGGACGATCGTCGGGGGGGGGG-3′) with a phosphodiester backbone used for incorporation into VLPs was provided by BioSpring GmbH. All other ODNs contained a phosphorothioate backbone. Dioleoyloxy-trimethylammonium-propane-methylsulfate (DOTAP) was obtained from Roche, and was complexed with CpG as described previously (Hou et al., 2008). Ultra pure LPS (E.coli 0111:B4) were purchased from Invivogen.
Conjugation of OVA with 1018 CpG followed a modified protocol as previously described for conjugation of ragweed allergen Amb a 1 (Tighe et al., 2000). Briefly, OVA was activated by reaction with sulfosuccinimidyl 4-N-maleimidomethyl cyclohexane-1-carboxylate (sulfo-SMCC) in 100 mM NaPO4, 150 mM NaCl, 1 mM EDTA pH 7.5 buffer. The activated OVA then reacted with a sulfhydryl derivative of the ODN 1018 to give a mixture of conjugates with 1–7 CpG ODNs attached. Free OVA and CpG ODN were removed from the conjugate by pooling gel filtration (Superdex 200 10/300 GL (GE Healthcare Life Sciences)) fractions, based on analysis by SDS-PAGE.
VLPs derived from the bacteriophage Qβ were expressed in E. coli strain JM109 harboring the expression plasmid pQ10 and purified as previously described (Cielens et al., 2000). Self-assembled VLPs contained ~300 μg of E. coli-derived ssRNA per milligram of Qβ VLPs. ssRNA-free VLPs (VLP) were obtained by digestion with RNase A, and VLPs containing CpG were obtained by in vitro packaging VLPs with CpG ODN G10, both as described in detail elsewhere (Jegerlehner et al., 2007). Briefly for the later procedure, Qβ VLPs were disassembled in vitro and Qβ coat proteins purified. Purified coat proteins were then mixed with aggregated G10 ODN and allowed to assemble to VLPs. Free G10 ODN was removed from the assembled VLPs containing G10 ODN inside the particles. One milligram of packaged Qβ contained 250–300 μg of CpG ODN. Recombinant Fel d1 was generated by expression of plasmid pET-42T x Fel d1-15as-HC in E. coli strain BL21 (DE3), and FD1-VLP conjugate was obtained by coupling of Fel d1 to VLP, as previously described (Schmitz et al., 2009). For staining Qβ VLP-specific B cells, Qβ VLPs were labeled with fluorescent dye Alexa 647® using a kit from Invitrogen.
Immunization and serum collection
Mice were immunized by i.p. injection of antigen and TLR ligand in PBS. For primary antibody responses, sera were collected on day 7 for the measurement of IgM or IgG3, and on day 14, and in some experiments also on days 21 and 28 for the measurement of total IgG, IgG1, IgG2b, and IgG2c. For secondary responses to OVA immunization, mice were injected i.p with 10 μg of endotoxin-free OVA on day 28 after the primary immunization, and sera were collected 7 days later.
Enzyme-linked immunosorbent assay (ELISA) and ELISPOTs
To determine the amount of antigen-specific immunoglobulin isotypes in serum, 96-well ELISA plates (BD Falcon) were coated overnight with antigens using the following conditions: 5 μg/ml NP10-BSA, 10 μg/ml OVA, or 0.5 μg/ml inactivated H1N1 virus in PBS, 1 μg/ml Flagellin, 2 μg/ml VLP-ssRNA, or 5 μg/ml recombinant Fel d1 in 0.1M carbonate buffer (pH9.5). Pre-diluted sera were loaded onto the plate and then were serial diluted. Antigen-specific IgM, total IgG and IgG isotypes were detected with horseradish peroxidase (HRP)-conjugated anti-mouse IgM, total IgG (Bethyl Laboratories), IgG1, IgG2b, IgG2c or IgG3 reagents (Southern Biotech). The HRP substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was purchased from Vector Laboratories. Antibody titers were determined as the reciprocal of the dilution that gave an optical density value (450–570 nm wavelength) that was more than ten times of the standard deviation above the mean value of the negative control wells. When no signal was detected, a value of one third of the reciprocal of the initial dilution factor was assigned.
To detect anti-Qβ antibody-secreting cells (ASCs) in the spleen, 96-well filter plates (Millipore) were coated overnight with 2 μg/ml of VLP-ssRNA in carbonate buffer. Three-fold dilution of splenocytes from immunized mice were added in the filter plates starting at 1×106/well in RPMI1640 medium with 10% FBS, and incubated at 37°C for 5 hours. The plates were then washed and incubated with HRP-conjugated anti-mouse IgM, total IgG, IgG1, IgG2b or IgG2c reagents before addition of the substrate 3-amino-9-ethyl carbazole (AEC). The substrate reaction was stopped by rinsing plates with tap water. Plates were dried overnight and ELISPOTs were counted in each well.
Flow cytometry
For detection of Qβ-specific B cells, single cell suspensions of splenocytes from immunized mice were blocked with antibody against CD16 and CD32 (BD PharMingen), and then stained with fluorescent antibodies in ice-cold flow cytometry buffer (PBS supplemented with 2mM EDTA, 1% heat-inactivated FBS, and 0.02% sodium azide). The antibodies included FITC-labeled anti-IgM, anti- IgD, anti-CD4, anti-CD8, anti-CD11b, anti-Gr-1 antibody, and PE-labeled anti-CD19 antibody, PE-Cy7-labeled anti-CD95 antibody, and Alexa Fluro® 647-labeled Qβ VLPs. All fluorochrome-conjugated monoclonal antibodies were purchased from BD PharMingen or eBioscience. All data were collected on a LSRII flow cytometer (Becton Dickinson) and were analyzed with FlowJo software (TreeStar).
Statistical analysis
Statistical significance was calculated with the unpaired Student’s t-test or the Mann-Whitney U-test. All P values of 0.05 or less were considered significant.
Supplementary Material
Acknowledgments
We thank D.K. Hong (Stanford University) for providing protocols for inactivating influenza virus; S. Jiang (UCSF) for assistance with cell purification by flow cytometry, and S. Anderson (UCSF) for advice. This research was supported by NIH R01 AI072058 (ALD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J, Jegerlehner A, Hinton HJ, Pumpens P, Saudan P, Schneider P, Bachmann MF. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol. 2009;183:3788–3799. doi: 10.4049/jimmunol.0804004. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD. Regulation of spleen white pulp structure and function by lymphotoxin. Adv Exp Med Biol. 2002;512:49–56. doi: 10.1007/978-1-4615-0757-4_7. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cielens I, Ose V, Petrovskis I, Strelnikova A, Renhofa R, Kozlovska T, Pumpens P. Mutilation of RNA phage Qbeta virus-like particles: from icosahedrons to rods. FEBS Lett. 2000;482:261–264. doi: 10.1016/s0014-5793(00)02061-5. [DOI] [PubMed] [Google Scholar]
- Cline AM, Radic MZ. Apoptosis, subcellular particles, and autoimmunity. Clin Immunol. 2004;112:175–182. doi: 10.1016/j.clim.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–3977. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173:4308–4316. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraedts F, Goutagny N, Hornung V, Severa M, de Haan A, Pool J, Wilschut J, Fitzgerald KA, Huckriede A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 2008;4:e1000138. doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine. 2006;24:20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim GF, Fleet GH, Lyons MJ, Walker RA. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JM, Webster RG. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J Infect Dis. 1989;160:191–198. doi: 10.1093/infdis/160.2.191. [DOI] [PubMed] [Google Scholar]
- Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- O’Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, Uccellini M, Alegre ML, Cambier JC, Clark MR. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci U S A. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Immunostimulatory activity of haptenated proteins. Proc Natl Acad Sci U S A. 2009;106:4782–4787. doi: 10.1073/pnas.0809403105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, Saudan P, Bachmann MF. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–1955. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Muller P, Pfister T, Maurer P, Bachmann MF, Graf N, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39:562–570. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol. 2000;106:124–134. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, LaMarre A, Ciurea A, Hunziker L, Ochsenbein AF, McCoy KD, Fehr T, Bachmann MF, Kalinke U, Hengartner H. Neutralizing antiviral antibody responses. Adv Immunol. 2001;79:1–53. doi: 10.1016/S0065-2776(01)79001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.