Abstract
The spermatogonial stem cells (SSCs) are responsible for the transmission of genetic information from an individual to the next generation. SSCs play critical roles in understanding the basic reproductive biology of gametes and treatments of human infertility. SSCs not only maintain normal spermatogenesis, but also sustain fertility by critically balancing both SSC self-renewal and differentiation. This self-renewal and differentiation in turn is tightly regulated by a combination of intrinsic gene expression within the SSC as well as the extrinsic gene signals from the niche. Increased SSCs self-renewal at the expense of differentiation result in germ cell tumours, on the other hand, higher differentiation at the expense of self-renewal can result in male sterility. Testicular germ cell cancers are the most frequent cancers among young men in industrialized countries. However, understanding the pathogenesis of testis cancer has been difficult because it is formed during foetal development. Recent studies suggest that SSCs can be reprogrammed to become embryonic stem (ES)-like cells to acquire pluripotency. In the present review, we summarize the recent developments in SSCs biology and role of SSC in testicular cancer. We believe that studying the biology of SSCs will not only provide better understanding of stem cell regulation in the testis, but eventually will also be a novel target for male infertility and testicular cancers.
Keywords: spermatogonial stem cell, infertility, SSC transplantation, SSC culture, SSC plasticity, testicular cancer
Introduction
Spermatogenesis is a cyclic, well-organized and continuous process of male germ cell proliferation and differentiation that generates ∼100 million sperm each day in adult males [1–3]. It starts at puberty and is maintained until death. It takes place within the seminiferous tubules, which contains a mixture of germ cells and Sertoli cells. The Sertoli cells play a nursing role for germ cells as well as coordinate during events of spermatogenesis [4]. Spermatogenesis starts with a small number of spermatogonial stem cells (SSCs) that reside at the base of seminiferous tubules of the testes, which undergo self-renewal division, proliferation and differentiation to produce sperm. A single SSC by self-renewing division can produce two stem cells, by asymmetric division they can produce one stem and one differentiating, or undergo a symmetric/differentiating cell division that results in two differentiated cells (Fig. 1A). Most of the diploid germ cells and differentiating spermatogonia undergoes several rounds of mitotic divisions before they enter into meiotic phase and later differentiated to become haploid spermatids that subsequently transformed into mature spermatozoa in about 35 days in the mouse and 64 days in the human being (Fig. 1B) [5, 6]. It is well established that SSCs are responsible for the transmission of genetic information from an individual to the next generation. Besides transmitting the genetic information to the next generation, recent studies have demonstrated that both murine as well as human SSCs can be reprogrammed into embryonic stem (ES)-like cells that can further differentiate into derivatives of all three germ layers [7–14].
Fig 1.
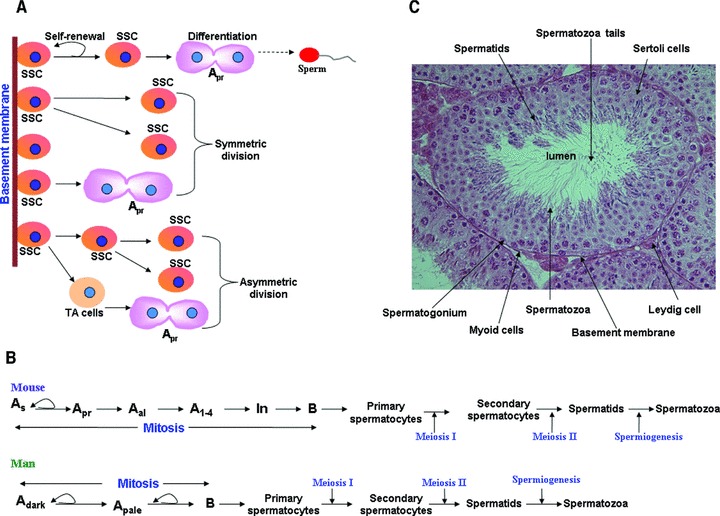
Cellular events in mouse and human spermatogenesis. (A) Spermatogenesis starts with a small number of SSCs, which reside at the base of seminiferous tubules of the testes, which undergo self-renewal, proliferation and differentiation to produce sperm. A single SSC by self-renewing division can produce two stem cells and by asymmetric division they can produce one stem and one differentiating cells or undergo a symmetric cell division to produce two differentiating cells. (B). Schematic diagram showing stages of spermatogenesis in mouse and men. (C). Haematoxylin and eosin staining of mouse testis illustrates the typical structure of the testis showing the seminiferous tubules containing the germ cells and Sertoli cells. SSC: spermatogonial stem cells; TA: trans-amplifying cells; As: Asingle; Apr: Apaired; Aal: Aaligned; In: intermediate spermatogonia.
Under normal conditions, the SSCs occasionally self-renew, however, in response to damage such as chemicals or radiation they can divide frequently [4, 15, 16]. The fate of SSCs is regulated by both paracrine and autocrine molecules [17, 18]. The study of SSC biology has been challenging due to two reasons: their small number in the testis (one in ∼2000 cells in the mouse) and the complexity of the niche microenvironment [19–21]. A detailed understanding of the cellular and molecular mechanisms that regulate self-renewal, differentiation and reprogramming of SSCs to pluripotent ES-like cells is crucial for the future use of stem cells in regenerative medicine as well as in the development of new therapeutic targets for male infertility and testicular cancers. In the present review, we focus our efforts in summarizing the characteristics and the potential of SSCs, as well as in understanding the various signalling pathways that regulate SSC fate decisions and differentiation, with special focus on infertility and testicular cancers.
Spermatogonial stem cells
SSCs are responsible for continual sperm production throughout the most of a male’s lifespan [9, 22–34]. SSCs are derived from more undifferentiated germ cells known as gonocytes, which in turn are derived from primordial germ cells (PGCs) [35]. The gonocytes proliferate to become spermatogonia. There are different types of spermatogonia in testes of all mammalian species. Some of these spermatogonia cycle regularly to maintain the spermatogonial population, and derive differentiating germ cells to maintain continuous spermatogenesis, whereas other spermatogonia remain quiescent under normal condition, but on gonadotoxic insult repopulate the empty seminiferous tubules [36]. Spermatogonia are heterogeneous subset of cells containing undifferentiated (stem cells) and differentiating cells [37]. It is estimated that ∼35,000 SSCs exist in each adult mouse testis and are located in the most peripheral region of the seminiferous tubule [2, 19] and total about 330,000 undifferentiated spermatogonia are present [19]. In rodent, the spermatogonia are divided into three types: type A, intermediate and type B spermatogonia (Fig. 1B, C). The most undifferentiated spermatogonia are called Type Asingle (As), Type Apaired (Apr) and Type Aaligned (Aal). Among these spermatogonia, the type As is the SSCs, which is similar to Adark spermatogonia in men [2, 38, 39]. It has also been shown that As is involved in maintenance of normal spermatogenesis as well as regeneration after tissue injury and transplantation [5, 40, 41]. However, in a recent study, Nakagawa et al.[41] used a live imaging to demonstrate that all three As Apr and Aal spermatogonia are acts as potential SSCs. Further, multiple other groups have shown that glial cell-derived neurotrophic factor (GDFN) receptor (GFR)α1+, promyelocytic leukaemia zinc finger (PLZF), E-Cadherin, neurogenin (NGN)3, early growth response gene (EGR)3, NOTCH1, SRY-related HMG-box (SOX), c-rearranged during transfection (RET), Oct4 and Stra8, are expressed in all three As Apr and Aal spermatogonia [7, 23, 40–42]. Most of the undifferentiated spermatogonia can revert to become SSCs when placed in right microenvironments [41]. Undifferentiated spermatogonia, including SSCs, are different from differentiating spermatogonia and are defined by the surface phenotype major histocompatibility complex (MHC) class I (MHC-I)−thymus cell antigen (THY)+ c-kit receptor tyrosine kinase (KIT)−. The As spermatogonia divide and produce two daughter cells and remain attached with an intercellular bridge. The first division of As results in a pair of Apr spermatogonia. The Apr spermatogonia then undergo a series of mitotic cell divisions and become Aal spermatogonia. The Apr and Aal spermatogonia of mice are similar to human undifferentiated Apale spermatogonia (Fig. 1B). The Aal spermatogonia later differentiated to become A1–A4 spermatogonia. The transition from SSC to differentiating spermatogonia is marked by the expression of c-KIT[43] and of the loss of expression of neurogenin3 (ngn3)[44]. The differentiating A4 spermatogonia can further differentiated into intermediate and type B spermatogonia (Fig. 1B), which enters meiosis to produce primary and secondary spermatocytes that undergo reduction of their DNA content, and become spermatids, which in turn are finally transformed into spermatozoa (Fig. 1B, C) [45].
Regulation of SSC maintenance and differentiation
To date, most of the works in SSC biology studies come from SSC transplantation and long-term cultures of SSCs as well as by mutant or knockout mice [25, 32, 34, 38, 46–49]. Several genes are known to regulate the self-renewal of SSCs (Fig. 2–4) (for references see [50]). It has been established that Plzf (promyelocyte leukaemia zinc-finger factor), a transcriptional repressor, is essential for SSCs self-renewal [24], as the loss of Plzf results in differentiation of SSCs at the cost of self-renewal. The Plzf protein is expressed in As, Apr and Aal spermatogonia. Further, it has been found that Plzf also interacts with other signalling pathways, specifically in response to Sertoli-derived signals such as glial cell-line-derived neurotrophic factor (GDNF) and stem cell factor (SCF) [51]. OCT4 (Octomer-4, also known as POU domain class 5 transcription factor 1, Pou5f1), localizes in proliferating gonocytes and after birth in Aundiff spermatogonia [52] and is used as a marker of SSCs. OCT4 has been shown to be important for SSC maintenance in knockdown experiments [53]. OCT4 knockdown SSCs could not colonize in both culture and transplanted testes. Further, it has been found that PLZF is not affected by knockdown of OCT4, which suggests that OCT4 and PLZF function in different pathways to maintain SSCs self-renewal. A recent study suggests that POU3F1, but not POU5F1, is an intrinsic regulator of GDNF-induced survival and self-renewal of mouse SSCs [54]. It has been found that a basic helix loop helix transcription factor, neurogenin3 (ngn3) is expressed only in early spermatogonia (between As and Aal) in testes [55] that are c-Kit– and is known to play an important role in the differentiation of spermatogonia. SRY-box containing gene 3 (Sox3), a family of the high mobility group of transcription factor, has been reported to express in As, Apr and Aal spermatogonia and plays a role in the progression of spermatogenesis [56]. Bcl6b, a transcriptional repressor, is the member of the same family as Plzf, and acts like Plzf to control SSC self-renewal and block SSCs from differentiation [for details see 27]. However, only Bcl6b is under the control of GDNF [27].
Fig 2.
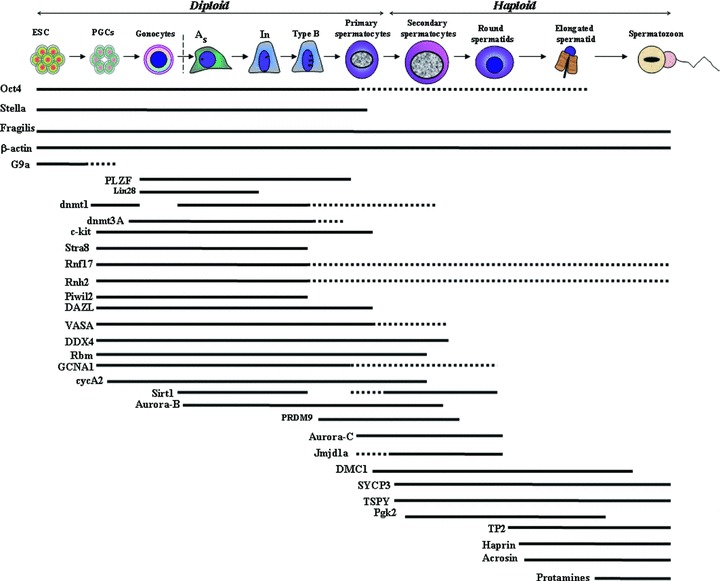
A schematic representation of the genes expressed during different stages of the mammalian spermatogenesis. As: Asingle; In: intermediate spermatogonia; ESC: embryonic stem cells; PGCs: primordial germ cells. Details of the genes are given in the text. Solid lines represent the high level of expression of particular genes at particular stage of spermatogenesis and broken lines represent the low level or no expression of the genes.
It has been reported that plzf promotes self-renewal of SSC by preventing expression of the c-kit in early spermatogonia (Fig. 2) [57]. Atchison et al. reported that Pin-1 (peptidyl-prolyl cis/trans isomerase NIMA-interacting-1) is required to regulate proliferation and cell fate of undifferentiated spermatogonia in the adult mouse testis. Pin-1 is ubiquitously expressed in adult testis, but is specifically expressed in spermatogonia and Sertoli cells [58]. TATA box-binding protein associated factor (TAF)-4b [Hogness box (TATA) box-binding protein associated factor 4b] is a germ cell specific component of the RNA polymerase complex [transcription Factor II D (TFIID)], known to be essential in SSC self-renewal and has been shown to express in gonocytes of post-natal testes and in spermatogonia and spermatids in the adult testes [59]. Zinc finger and broad complex/Tramtrack/bric-a-brac (ZBTB)16 is a transcription repressor, expressed only in Aundiff spermatogonia [23] and plays an essential role in SSC self-renewal. STRA8 is expressed in premeiotic germ cells and later found to be enriched in SSCs [60]. The protein kinase ATM (ataxia telangiectasia-mutated) plays critical role in self-renewal of SSC by suppressing DNA damage-induced cell-cycle arrest [61]. It is observed that E-cadherin (CDH1), which is expressed in undifferentiated type A spermatogonia, results in the production of clusters of one to eight cells and play an important role in the colonizing activity in recipient testes [42]. The transient receptor potential vanilloid receptor 1 (TRPV1), a receptor for capsaicin is expressed by SSCs in vitro and by premeiotic germ cells in situ. It has been demonstrated that capsaicin has adverse effects on spermatogonia in vitro by inducing apoptosis via the TRPV1 receptor [62]. Recently, ada et al. [63] demonstrated that Nanos2, a family of evolutionarily conserved zinc-finger motif-containing RNA-binding proteins is an intrinsic factor, which is both necessary and sufficient for SSC self-renewal by preventing differentiation. Nanos2 is exclusively expressed in As to Apr cells, however, Nanos3 is detectable in most undifferentiated spermatogonia (As to Aal and differentiating A1 spermatogonia) [64]. Accumulating evidence suggest that the piwi subfamily proteins, including murine P-element induced wimpy testis (MIWI), MIWI2 and MIWI-like (MILI), are required for stem cell self-renewal and the development of male germ cells in invertebrates [65]. Recently, it has been reported that ubiquitin carboxy-terminal hydrolase 1 (UCH-L1) is expressed only in undifferentiated spermatogonia located at the basement membrane of seminiferous tubules [66]. Luo et al. [66] further found that asymmetric segregation of UCH-L1 was associated with self-renewal versus differentiation divisions of SSCs, which they show by co-localization of UCH-L1 (high) and PLZF, versus co-localization of UCH-L1 (low or absent) with proteins expressed during SSC differentiation [deleted in azoospermia-like (DAZL), DEAD box protein (DDX)4 and c-KIT][66]. Luo et al. [66] are the first to provide direct evidence for existence of asymmetric division during SSCs self-renewal and differentiation in mammalian spermatogenesis.
Several genes are known to regulate the differentiation of SSCs (for references see [50]). There are three essential regulatory steps for spermatogonial differentiation: the conversion of As to Apr spermatogonia; transition of Aal to A1 spermatogonia and survival and progression of A1 to B spermatogonia [40]. It has been demonstrated that removal of GDNF/GDNF family receptor (GFRA)1 from the culture medium for SSCs up-regulate expression of Neurog3 and proposed that Neurog3 may be an initiation signal for SSCs differentiation [27]. Further, it is reported that Nanos3 (Drosophila nanos homolog 3), encodes for a zinc-finger protein and is expressed only in Aundiff spermatogonia. Up-regulation of Nanos3 results in the accumulation of the cells in the G1 phase and treatment of Aundiff spermatogonia with retinoic acid results in a dramatic reduction of Nanos3[67]. It has been reported that Sohlh1 (germ cells-specific helix loop helix) and Sohlh2 transcription factors are essential in SSC differentiation [68–70]. Sohlh1 is expressed in mouse A1-A4, intermediate and B spermatogonia [68] and Sohlh2 is only expressed in differentiating A spermatogonia [69]. Loss of any of these two genes blocks differentiation, resulting in infertility. Sox3 is known to expressed in Aundiff spermatogonia [56] and regulates spermatogonial differentiation together with Ngn3. It is reported that male mice with juvenile spermatogonial depletion (jsd) mutation in UTP14b gene causes cessation of spermatogonial differentiation and in adults, only spermatogonial cells are remain, which suggest that it is involved in spermatogonial differentiation [71]. A novel testis-specific protein Tsp57 has been identified in a yeast two-hybrid screening and results show that Tsp57 protein plays a role in the post-meiotic phase of germ cell differentiation [72]. Dazl, encodes a RNA-binding protein essential for essential for differentiation of Aal to A1 spermatogonia and is expressed exclusively in the primary spermatocytes and weakly in spermatogonia [73]. It has been shown that the gene encoding mouse cyclin A1, Ccna1, is highly expressed in late pachytene–diplotene spermatocytes and disruption of Ccna1 resulted in male infertility and complete spermatogenic arrest before first meiotic division (for references see [50]). Further, disrupted spermatogenesis in post-meiotic germ cells has been shown in mice lacking Msy2 (Y-box family of DNA-/RNA-binding proteins) and results in azoospermia [74]. It has been shown that PLZF may regulate genes related to differentiation via epigenetic regulations because of PLZF interaction with polycomb group proteins and histone deacetylases [75].
To maintain proper spermatogenesis, the communication between niche and SSCs is crucial and should be properly coordinated [76]. The specialized microenvironments known as stem cell niches regulate the behaviour of stem cells and its differentiating progeny [77–79]. The current understanding of germline niches comes from the study of Drosophila gonads, where the maintenance of the germline stem cells (GSCs) depends on signals from a somatic cell, called the hub, which ensures the asymmetric division of stem cells, producing one cell to maintain the stem cell populations and a daughter cell that will differentiates [80]. At the tip of the Drosophila testes, GSCs and cyst progenitor cells (CPCs) contact each other and share common niches (known as a hub) to maintain spermatogenesis. There are five to nine GSCs and twice as many CPCs anchored around the hub. The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is the major signalling pathway involved in stem cell maintenance in Drosophila testes. The hub cells, which function as a niche for stem cells, express a growth factor, Upd (unpaired), which acts as a short-range signal to activate the JAK/STAT pathway in GSCs [81]. The upd gene directs GSCs to undergo self-renewal through the Mom/Hop/Stat92E pathway. Stat92E then enters the nucleus to activate expression of genes that instruct the self-renewal of GSCs and CPCs [81]. In addition, centrosome positioning plays an important role in ensuring the correct mitotic spindle orientation [82] that is placed perpendicular to the interface with the hub during mitosis. Further, it has been demonstrated that changes in the GSC orientation with respect to the niche during ageing contribute to the decline in spermatogenesis and disoriented GSCs originate from dedifferentiation of spermatogonia [83].
The niches in the mammalian testis have been characterized directly when early germ cells were successfully transplanted into the seminiferous tubules of germ cell-depleted or-deficient recipient males [48, 84]. It has been shown that the transplanted germ cells from dogs, rabbits and large domestic species are able to repopulate the mouse testis, but they cannot differentiate [85]. The main components of the mammalian testis niche include the Sertoli cell, the basement membrane, peritubular myoid cells and stimuli from the vascular network between the seminiferous tubules. Spermatogenesis relies on interactions between supportive Sertoli cells and germ cells. Sertoli cells reside on the basal membrane of the tubules. However, their cytoplasmic ramifications reach from the basal membrane to tubule lumen. The interstitial tissue between the seminiferous tubules consists of blood and lymph vessels, macrophages and Leydig cells and produce testosterone. The Sertoli cells act as niche for SSC, and supply nutrient and growth factors. Sertoli cells form the blood–testis barrier, which sustain a selective substance flow between luminal fluid, blood plasma and interstitial fluid, and make the adluminal compartment an immune-privileged site. Sertoli cells provide growth factors and hormones that regulate testis development or feedback to regulate the hormonal signals that affect Sertoli cell. Recently, using time-lapse imaging of green fluorescent protein-labelled undifferentiated spermatogonia (Aundiff) and three-dimensional reconstitution, Yoshida et al. [55] found a biased localization of Aundiff to the vascular network and accompanying Leydig and other interstitial cells in the testis. They further found that differentiating spermatogonia left these niche regions and further spread everywhere in the basal compartment of the seminiferous epithelium. They proposed that mammalian germline niche is not fixed is one location as it has been observed in Drosophila or Caenorhabditis elegans, but established because of vasculature pattern formation. Further, a novel membrane skeletal protein, 4.1G, expressed in germ cells and interact with CADM1 (cell-adhesion molecule-1), to help in the attachment of both Sertoli-germ and germ-germ cells [86].
The process of spermatogenesis is regulated by a complex interaction of endocrine and paracrine signals (Fig. 3). The master control hormone is gonadotropin-releasing hormone (GnRH), which is secreted by specialized neurons, resides at hypothalamus. GnRH stimulates the synthesis and secretion of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) at the anterior pituitary. These hormones are known to act on the testis to regulate spermatogenesis. Further, LH binds to receptors on the surface of Leydig cells and accelerates the release of testosterone. Only Sertoli cells have the receptors for testosterone and FSH, so they are the major targets of hormonal signals, which in turn regulate spermatogenesis. Furthermore, the regulation of activin and inhibin secretion by Sertoli cells is directed by FSH. It has been shown in vivo and in vitro studies in both mouse and Rat that GDNF, an essential growth factor produced by Sertoli cells control SSCs self-renewal (Fig. 3) [51, 87, 88] by signalling through a heterodimer of the tyrosine kinase, RET and GFRα1 [17]. The transcription factor ERM (Ets-related molecule) expressed exclusively in Sertoli cells is required for self-renewal of SSCs. Loss of ERM results in spermatogonial depletion due to the failure of SSC self-renewal [89, 90]. It is shown that GDNF interacts with ERM and LHX1 (Lim homeobox1) in SSC maintenance [27]. The FGF2 (fibroblast growth factor 2) and epidermal growth factor, secreted by Sertoli cells, are important for SSC self-renewal in vitro[39]. The FGF family members (FGF2, FGF1 and FGF9) can induce the transcription of ERM and regulate the SSCs fate in vitro[89]. It has been demonstrated that GDNF production is also dependent on Fgf2, tumour necrosis factor α (Tnfα) and interleukin-1β (IL-1β) in vitro[91]. Sertoli cells also produce SCF, and its receptor c-kit, which is expressed in Aal spermatogonia and is considered as marker of differentiation as they involved in transition of Aal to A1 spermatogonia [92]. SCF stimulates proliferation of primary spermatogonia in culture and induces a spermatogonial cell line to differentiate into meiotic spermatocytes and haploid round spermatids without Sertoli cells [93]. Some of the genes are also required for male fertility and motility of sperm. Mutations in the USP26 (ubiquitin-specific protease 26) gene may play a role in male infertility and the Herc4 (E3 ubiquitin ligase), highly expressed in the testis, is also known to play a critical role in infertility by reducing motility of the spermatozoa [94].
Fig 3.
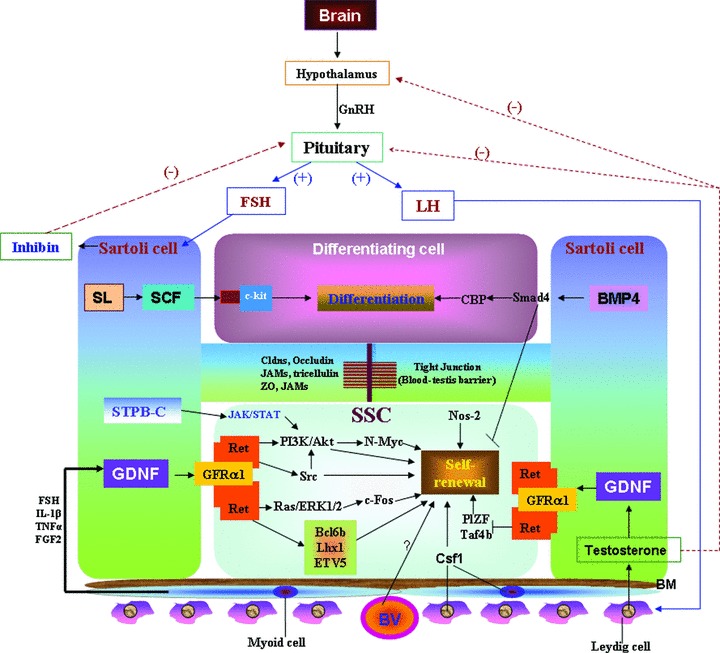
Proposed model in regulation of SSC self-renewal and differentiation. BV: blood vessel; BM: basement membrane; GnRH: gonadotropin-releasing hormone; GDNF: Glial cell-derived neurotrophic factor; FSH: follicle-stimulating hormone; LH: Luteinizing hormone; SL: steel; SCF: Stem cell factor; SSC: spermatogonial stem cells.
It has been reported that testicular gap junctional protein connexin43 (cx43) is located between neighbouring Sertoli cells and between Sertoli cells and germ cells and that cx43 expression in Sertoli cells is essential for normal testicular development and spermatogenesis [95]. It has been demonstrated that Csf1r (colony stimulating factor 1 receptor) acts as an extrinsic stimulator of SSC self-renewal [27]. Csf1r expression is enriched in Thy1+ germ cells and it has been demonstrated that addition of recombinant Csf1, the specific ligand for Csf1r, in culture medium significantly increased the self-renewal of SSCs in heterogeneous Thy1+ spermatogonial cultures, without showing any effect on expansion of the germ cells. Additionally, it has been found that Csf1 is localized in clusters of Leydig cells and peritubular myoid cells in both pre-pubertal and adult testes. Oatley et al. [27] findings have identified Csf1 to be an extrinsic stimulator of SSC self-renewal and have implicated Leydig and myoid cells as contributors of the SSC niche in mammals. It has been shown that integrin molecules, which are present on SSC surface, play a critical role in the migration and connection to the basement membrane [96]. CD9 is a membrane protein, associated with integrins, has been shown to be a common marker for SSC in mouse and rat [7], and are localized to cells on the basement membrane of the seminiferous tubules as well as in interstitial cells [7]. Additionally, neonate rat testes are Ep-CAM+, Thy-1+, β3-integrin+. Ep-CAM is required in homing of SSC to the basement membrane and it has been seen that once the cells are differentiated, they lose Ep-cellular adhesion molecules (CAM) and β3-integrin expression [96].
Besides the molecules in regulation of SSC fate, there are several signalling pathways also involved in the SSC self-renewal and differentiation (Fig. 3). Src family kinases, including Src, Yes, Lyn and Fyn, play important roles in GDNF-mediated proliferation of SSCs [27, 30]. GDNF activates Src family kinases, which further stimulate the phosphoinositide 3-kinase (PI3K)/Akt pathway [97] and eventually up-regulates N-Myc expression and promotes SSCs proliferation [98]. It has also been reported that the Erk1/2 pathway can be activated by SCF in c-KIT expressing spermatogonia to stimulate their proliferation [99]. Interestingly, it has been observed that Ras-cyclin D2 pathway regulates the balance between tissue maintenance and tumorigenesis in the SSC population [97]. Activin A and bone morphogenetic protein (BMP)4, when supplied in the culture of SSCs, can promote differentiation by blocking the SSC self-renewal [100]. Itman and Loveland [101] co-culture the immature mouse spermatogonia and Sertoli cells and found that BMP2 and BMP4 stimulate the expression of Smad1, Smad5 and Smad8 in the nucleus of spermatogonia. Recently, it has been demonstrated that Nodal regulates self-renewal of mouse SSCs through Smad2/3 activation via an autocrine signalling pathway [30]. He et al. [30] reported that both Nodal and its receptors are present in SSCs, but not in Sertoli cells or differentiated germ cells. It has been demonstrated that short-type PB-cadherin (STPB-C) promotes self-renewal of SSCs via activating JAK/STAT and phosphoinositide-3 kinase (PI3-K)/Akt and block TGF-β1 signalling [102]. It has been shown that STAT3 is expressed in the gonocytes of the neonatal mouse testes as well as the undifferentiated spermatogonial population that contains SSCs [103]. Oatley et al. [104] have demonstrated the role of STAT3 in SSC fate decision by in vitro and in vivo studies. Oatley et al. [104]in vitro studies showed that impairment of STAT3 signalling increased SSC concentration specifically without affecting overall spermatogonial proliferation. However, Oatley et al. [104]in vivo studies showed that SSCs deficient for STAT3 expression were incapable of re-establishing spermatogenesis after transplantation but could undergo initial colonization. Based on both in vitro and in vivo studies, Oatley et al. [104] suggest that STAT3 is required for spermatogonial differentiation and may block the ability of the few differentiating spermatogonia that remain from low level STAT3 to proceed to meiosis [104]. Recently, it has been reported that Wnt signalling promotes proliferation and stemness regulation of SSCs/ progenitors cells and that constitutively activated β-catenin signalling leads to continuous proliferation and compromised differentiation of Sertoli cells [105]. These findings suggest that Wnt/β-catenin pathways, is critical in the regulation of mouse and human spermatogonia [105].
SSC culture, transplantation and plasticity
The establishment of culture system that provides self-renewal and proliferation of SSCs is greatly valuable for experimental research and potential treatment for male infertility. It had been thought that germ cells survive only a short time in culture. However, an exact knowledge of the presence of SSCs in any cell population has only recently become possible with development of the spermatogonial transplantation technique. Using this technique, it has been demonstrated that mouse SSCs can be maintained in culture for about 4 months and can generate sperm after transplantation in the seminiferous tubules of an suitable recipient [100] and suggested that GDNF was important for short-term SSC maintenance in vitro, without losing their proliferation and differentiation potential [100]. A long-term culture system for SSCs was reported [7]. Cultured SSCs, designated as SSCs, continued to proliferate for more than 2 years while maintaining stable genetic and epigenetic properties. The most used methods for SSCs culture are based on supplementation of the culture medium with GDNF [88]. It has been found that in the presence of GDNF, SSCs grow on feeder cells as islands or clumps of cells. When the GDNF is removed, these clump cells, as in vivo, begin to grow in chains resembling the initial stages of SSC differentiation [88]. Recently, Kanatsu-Shinohara et al. [26] reported long-term culture of hamster SSCs similar to mouse SSC culture. There are other growth factors (such as FGF2 and LIF), vitamins and hormones have also been used [10, 39]. Long-term maintenance of SSCs without GDNF has been reported and it has been found that leukemia inhibitory factor (LIF) is an important factor for SSC self-renewal in adult testes [10]. Both serum and serum-free methods have been used [8, 39]. SSCs can be maintained in co-culture with sertoli cells without losing the capacity to replicate their DNA. SSCs cultures contain a heterogeneous group of cells as the SSCs also undergo partial differentiation in vitro[8]. It has been estimated that only about 1% of cultured cells represent stem cells [26]. Further, it is found that SSCs can expand in complete absence of serum or somatic feeder cells in vitro[8]. Moreover, SSCs can undergo anchorage-independent self-renewal division in vitro[8]. Later, the immortalized mouse SSCs lines were generated and based on expression of phenotypic and molecular markers such as GFRα1 and Oct3/4, it has been shown that these cells were SSCs [106].
Transplantation of SSCs has been done first time in 1994 [107]. The SSC transplantation technique has been used for multiple applications. These include to determine the presence of SSCs in a cell population after positive or negative selection, the production of transgenic mice, evaluation of in vitro approaches to selectively culture SSCs, and importantly to determine if a gene mutation results in inadequate spermatogenesis and infertility [5–8, 23, 39]. SSCs transplantation, in which injection of a donor testis cell suspension into the seminiferous tubules of recipient male lacking germ cells has been shown [39]. Further, it has been found that SSCs present in the injected cell suspension are able to colonize the recipient seminiferous tubules and insure the normal spermatogenesis [5–8].
In addition of using SSC transplantation with mice, with modifications it has been used in several other species, including rats, pigs and cattle [108]. There is a report of successful spermatogenesis obtained following human-to-rat and mouse transplantation [109]. Reis et al.[110] observed the human to immunodeficient mouse testicular tissue transplantation, with no evidence of donor tissue survival. It has been found that human spermatogonia in mouse survived up to 6 months, but no meiotic activity was seen in donor tissues [100]. Mouse seminiferous tubules provide a favourable environment for germ cells from distant species to interact with its niche cells. Donor-derived spermatogenesis was observed when germ cells from hamster transplanted to mouse testes (for references see [38]). Izadyar et al. [111] reported the successful transplantation of bovine type A spermatogonia in recipient bulls that resulted in full spermatogenesis following autologus transplantation. SSC can be successfully cryopreserved for long periods with common techniques used for somatic cells [5]. There was a report in which successful transplantation after freezing the donor tissue for 156 days was observed (for references see [38]). Experimental data suggest that frozen thawed bovine SSCs can survive during cryopreservation and be maintained during co-culture with a feeder cell line, and influenced by GDNF [27]. Cryopreserved testis cells of dogs and rabbits can colonize the recipient mouse testis [85]. Recently, Kanatsu-Shinohara et al. [112] have developed a novel transplantation assay for SSCs, and demonstrated that p21 and p27 cyclin-dependent kinase inhibitors are key players in SSC self-renewal and differentiation. Further, they suggested that it could be useful in examining minor defects in spermatogenesis, which is not seen by classical spermatogonial transplantation [112]. In addition, it has been shown that purified mouse spermatogonial progenitors committed to differentiation can generate functional SSCs, which in turn repopulate germ-cell-deficient testes after transplanted into adult mice [113]. Barroca et al. [113] further found that GDNF and FGF2 are responsible for above dedifferentiation process and suggest that stemness is not confined in adults to a limited pool of self-renewing cells, but it can be obtained by differentiating progenitors following tissue injury and throughout life.
Until recently, it has been believed that SSCs are unipotent and they can only differentiate to become sperm cells. It has been shown that somatic cells are able to convert into pluripotent state by induction of four transcription factors [114, 115]. Recently, it has been demonstrated that SSCs can be converted into a pluripotent state in culture in vitro[7, 10, 116, 117]. These reprogrammed SSCs cells were named, multipotent adult GSCs. Interestingly, SSCs show expression of all the genes (Oct4, Sox2, Klf4, Myc and Lin28) that are required for induction of pluripotency except for Nanog[9]. However, it has been shown that Nanog is important for PGC maturation during embryonic development [118]. It has been demonstrated that similar to ESCs, SSCs can grow in vitro on feeder cells in islands or clumps, and are positive for Oct 3/4 and alkaline phosphatase [10]. Further, it has been found that these ES-like SSC cells are positive for GPR125, which shows that the ES-like cells are of germ cell origin [10, 12]. Recently it has been demonstrated that ES-like cells can also be generated under culture conditions, which is known to support long-term proliferation of SSCs [119]. Further, it is found that these ES-like cells were able to generate teratomas on transplantation in recipient mice, and differentiate in vitro into derivatives of all three germ layers including neural, epithelial, osteogenic, myogenic, adipocyte, cardiomyocytes and pancreatic lineages [11, 12, 117, 119, 120]. Recently, it has been reported that knockdown of Trp53 and Pten independently result in significantly higher expression levels of the pluripotency-associated gene Nanog[121]. Further, Kuijk et al. [121] proposed that Trp53- and Pten-mediated repression is critical for the insulation of male germ cells from pluripotency [121]. It has also been found that similar to ESCs, ES-like cells of testis can generate TE when transplanted into immunodeficient mice raising the question of their potential clinical application. Gonzalez et al. [122] recently isolated a novel renewable stem cell population from the adult testes that has characteristics of MSCs, termed gonadal stem cells. They demonstrated that gonadal stem cells could be easily isolated and have similar growth kinetics, expansion rates, clonogenic capacity and differentiation potential as MSCs [122]. These studies suggest that SSCs represent a novel alternative source of pluripotent cells for use in human regenerative medicine.
SSC in infertility
It is estimated that about 80 million people worldwide are infertile and growing evidence from clinical and epidemiological studies suggest an increasing incidence of male reproductive problems [123]. Infertility has affected as many as 15% of couples worldwide and in about half of these infertile cases, male infertility is a factor [124]. Causes of male infertility includes failure in germ cell proliferation and differentiation, abnormal sperm production or function, impaired delivery of sperm, general health and lifestyle issues, as well as genetic and environmental factors (Fig. 5) [125]. Therefore, progress in reproductive biology is critical for future diagnosis and treatment of infertility [126]. In recent years, tremendous work has been done to understand the infertility problem. Around 500 mutant mouse models with specific reproductive abnormalities have been produced and many human association studies have been done [32]. However, with few exceptions, the translation of these basic findings to clinical practice remains to be seen. In men, oligozoospermia, asthenozoospermia, teratozoospermia and azoospermia are the main causes of infertility, and these account for 20–25% of cases [32]. Spermatogenesis is mainly regulated by extrinsic and intrinsic signalling factors. It is known that abnormal expression of signalling molecules and the disruption of signalling pathways results in impaired spermatogenesis. Recent genetic studies show that abnormality of GDNF expression blocks sperm development and results in germ cell depletion [87]. It has been demonstrated that loss of BMP4 in mice results in degeneration of germ cells, reduced sperm counts, decreased sperm motility, and hence results in infertility [127]. Disruption of fibroblast growth factor receptors (FGFR)1 signalling, which is important in normal spermiogenesis and male fertility, resulted in depletion of sperm production and incapacitated sperm [128]. It has been shown that c-KIT-induced activation of the PI3K pathway is critical in male fertility because mutant males with loss of PI3K bind to c-KIT are sterile, which is due to complete disruption of spermatogonial proliferation and early differentiation [129].
Fig 5.
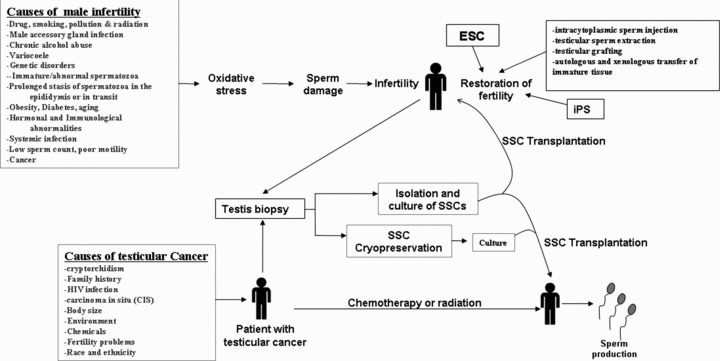
Proposed model for role of SSC in restoration of fertility in patients with testes cancer and infertility. ESC: embryonic stem cells; iPS: induced pluripotent stem cells; SSC: spermatogonial stem cells.
Thousands of genes are involved in spermatogenesis, and so far, only a small number of them have been identified and screened in infertile men. There is a report that lack of expression of Steel (Sl) factor on Sertoli cells in infertile Sl mutant mice prevent the differentiation of spermatogonia, which express c-KIT receptor that result in azoospermia [130]. A large number of genes have been shown to be up-regulated in mouse models of male infertility. It is reported that genetic ablation of SIRT1 (Sirtuins class-III NAD-dependent histone deacetylases) in the mouse leads to male infertility [131]. Further, loss of Dmc1, a Meiotic recombination protein, in mice result in infertility because of a defect in meiotic homologous chromosome pairing and an arrest in zygotene spermatocytes [132]. In the mouse, Jmjd1a (jumonji domain-containing histone demethylases) is expressed in a stage-dependent manner in meiotic and post-meiotic cells with the enrich expression in round spermatids. It has been shown that Jmjd1a null mice are infertile because of several post-meiotic defects, such as incomplete chromatin condensation, failure of acrosome formation and defects in heterochromatin distribution [133]. Further, loss of Tex11 (testis expressed gene 11) function in mice results in chromosomal asynapsis, reduced crossover, elimination of spermatocytes and infertility [134]. There is an arrest of spermatocytes, which is evident with degeneration of synaptonemal complexes. It has been found that defects in Sertoli cells compromise spermatogenesis and results in male infertility.
There are several reports of SSCs/germ cell transplantation to restore fertility (for references see [135, 136]) and can be valuable ways to study preservation, and manipulation of male fertility together with tissue regeneration in animals. The regenerative potential of SSCs and development of SSCs transplantation technique have been developed, in which testis cells from a fertile donor is transplanted into the testes of infertile recipients [46, 47]. Kanatsu-Shinohara et al.[137] demonstrated that Sertoli cell transplantation can rescue SSCs in the defective host microenvironment and restore spermatogenesis and the production of offspring from infertile animals. Ogawa et al. [138] used the spermatogonial transplantation techniques in two infertile mouse strains, Sl and dominant white spotting (W), to verify if stem cells from an infertile male have the capacity to generate spermatogenesis. They found that transplantation of germ cells from infertile Sl/Sld mutant male mice to infertile W/Wv or Wv/W54 mutant male mice can revive the fertility to the recipient mice. It has been shown that loss-of-function mutation in the KIT gene results in a severe spermatogenesis defect, which is turn led to infertility because of inability of its ligand, KITL, to stimulate spermatogonial proliferation and differentiation [39]. Long-term culture has been evaluated of rat SSCs following cryo-storage for their capacity to restore fertility to rats deficient in the DAZ-like (DAZL) gene, which show intact SSC compartment, but fail to produce mature sperm, resulted in infertility [139]. A novel testis-expressed gene, aurora kinase C has been shown to play a role in meiotic division in mice is responsible for male infertility (for references see [32]). Further, the spermatogenesis specific gene SPATA16 has been shown cause globozoospermia in three related male infertile patients (for references see [32, 123]). Germ cell line has been generated from the pluripotent teratocarcinoma cells by a novel promoter-based sequential selection strategy, which shows that germ cell line form mature seminiferous tubule can support normal spermatogenesis after transplantation into recipient testes (for references see [14]). In addition to SSC transplantation, the male fertility can be restored by testicular grafting, autologous and xenologous transfer of immature tissue, cryopreservation of sperm and several other ways (Fig. 5) [136, 137]. Many markers have been identified that can help in the outcome of SSC transplantation in restoration of male fertility [140]. Based on the above studies, clearly SSCs transplantation has the great clinical potential in regeneration of spermatogenesis and restoration of male fertility [140].
SSC in testicular germ cell tumours
Testicular germ cell tumours (TGCTs) are the most diagnosed solid cancers among men aged 15–40 years in industrialized countries. It has become even more prevalent during the last 30 to 50 years. In the United States, about 9000 new cases of TGCTs are diagnosed with testicular cancer each year. The risk to get the testicular cancer of a man over his lifetime is about one in 300. Most testicular cancers originate from germ cells, whereas Sertoli and Leydig cell tumours account for only 5% of testicular cancer. If it has not metastasized, testicular cancer has the one of the highest cure rates among all cancers. TGCTs consist of a heterogeneous group of neoplasm, establish at different anatomical locations in the testis. There are three groups of TGCTs: TE, yolk sac (YS) and choriocarcinomas tumours of infants; seminomas (SE) and non-seminomas (NS) of adolescents and adults; and spermatocytic seminomas (SS) of the adults (Fig. 4). SE seems like undifferentiated primitive germ cells, and NS show a mix of embryonic and extra-embryonic patterns of differentiation. Among TGCTs, about 60% is SE and 30% NS. The SE is the transformed germ cell homogeneous tumour, which is similar to PGC and early gonocytes, and usually confined to the testis. However, the NS are highly heterogeneous tumours that display various stages of differentiation ranging from undifferentiated cells to highly differentiated cells of somatic tissue types in teratomas and of extra-embryonic tissue types in YS tumours and chorio-carcinomas [141]. The rest 10% show mixed SE and NS [141]. Further, embryonal carcinomas (EC) are the undifferentiated stem cell component. TEs are the somatic differentiation. The YS tumours and choriocarcinomas are the components, which show extra-embryonal differentiation. It has also been reported that SE can switch fate to become NS via reprogramming or activation of pluripotency genes [142].
Fig 4.
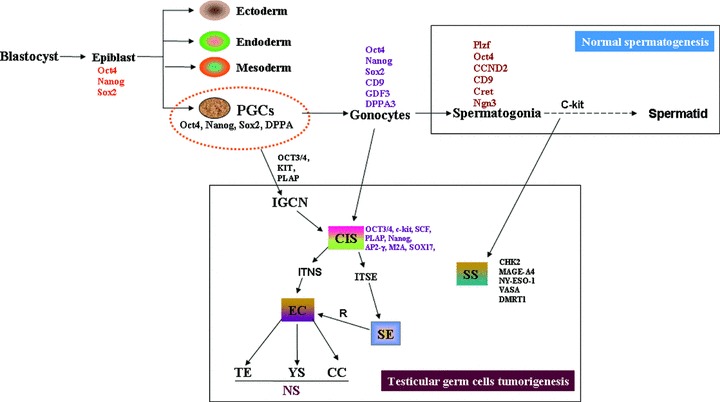
A schematic representation of the development of normal spermatogenesis (top) and testicular germ cell tumorigeneis (bottom). CIS: carcinoma in situ; EC: embryonal carcinoma; TE: teratoma; YS: yolk sac tumour; TGCT: testicular germ cell tumours; NS: non-seminoma; SE: seminoma; SS: spermatocytic seminoma; ITNS: intratubular non-seminoma; ITSE: intratubular seminoma.
All TGCTs originate from carcinoma in situ (CIS), or intratubular germ-cell neoplasia (IGCN). The standard chemotherapy regimen for first-line treatment of TGCTs is a combination of bleomycin, etoposide and cisplatin. Further, cisplatin-based chemotherapy together with surgery has an overall cure rate of 90% for patients with TGCTs. The complications associated with treatment of TGCTs can include retrograde ejaculation, decreased fertility and long-term impairment of metabolic, renal and neurological functions, contralateral TGCTs, second primary cancers and blood disorders [143]. Various risk factors are associated with testicular tumours, such as cryptorchidism, prior testicular cancer, genetics, family history, infertility and environmental exposure, but the specific aetiology is not clear [144]. Walsh et al. [144] have reported that men with male factor infertility are nearly three times more likely to develop subsequent testicular cancer [144]. In addition, there is an association of infertility and testicular cancer at or beyond 2 years after therapy [145]. Other risks associated with survivors of TGCT include the risk of cardiovascular disease, reduced sexual function and a risk of a second malignancy following radiotherapy and chemotherapy. Recent developments in the cancer treatment, including high-dose chemotherapy and stem cell rescue, have significantly improved the prognosis of metastatic testicular cancer. In fact, testicular cancer is a masterpiece in the oncological landscape and can be used as a model for the treatment of solid tumours.
There are many genes and signalling pathways associated with testicular cancer [146]. Several genes express on 12p region and are known to overexpress in TGCT, including genes associated with stem cells [147]. Further, Korkola et al. [148] identified target genes on 12p and related to TGCT as well identified genes that are associated with the pluripotent phenotype of EC. They examined expression profiles of 17 SE, 84 NS TGCTs and five normal testis samples. They found that 73 genes on 12p are significantly overexpressed, including glucose transporter 3(GLUT3), repressor of estrogen activation (REA), cyclin D2 (CCND2) and filaggrin 22028 (FLJ22028). Further, they characterized a 200 kb gene cluster at 12p13.31, which shows overexpression in EC and SE, which included the known stem cell genes NANOG, STELLA and growth differentiation factor (GDF)3. Furthermore, they compared the EC with SE that resulted in relative overexpression of other stem cell-associated genes in EC, such as endometrial bleeding-associated factor (EBAF), teratocarcinoma-derived growth factor 1 (TDGF1) and SOX2 and several downstream targets of wingless (WNT), NODAL and FGF signalling. Their results show that 12p gain is resulted in activation of proliferation and maintenance of stem cell function via activation of key stem cell genes [148]. The cytogenetic analysis have shown that 12p gain as the most common change in TGCTs and involved in the early stages of germ cell tumorigenesis [149]. Gashaw et al. [150] identified novel germ cell markers such as BOB1 and prominin-1 that are significantly up-regulated in SE compared to normal testes. Further, it has been found that Aurora B expression is restricted to spermatogonia; however, it is highly expressed in TGCTs [151] Furthermore, two Sertoli cell markers, stem cell growth factor (SCGF) [stem cell factor (SCF)] and neuronal SCF, multiple coagulation factor deficiency (MCFD)2 [stem cell-derived neural stem/progenitor cell supporting factor (SDNSF)] are expressed in SE cells [150]. It has been found that Krüppel-like factor 4 (KLF4) plays an important role in both iPS and ES cells. However, it has been shown that KLF4 does not express in normal spermatogonia [152]. In contrast, IGCN and SE cells strongly express KLF4. Godmann et al. [152] concluded that KLF4 may be a critical factor for the maintenance of the developmental and the tumorigenic potential of IGCN and for the malignancy of SE. Further, it has been reported that placental alkaline phosphatase and c-KIT are expressed in IGCN and SE, but not in other germ cell tumours [151]. Several studies suggest that CIS, SE and NS are characterized by abnormal gene expression patterns, in which genes associated in proliferation, pluripotency and epigenetic mechanisms are differentially regulated in testicular cancer subtypes [153–156].
There are several reports where alternation in expression of signalling molecules or pathways may result in an increased risk of testicular cancer. It has been shown that mice overexpressing GDNF develop malignant testicular cancer, which is similar to classic SE of human beings [157] and PI3K/Akt signalling pathway is associated to TGCT [158]. Further, SCF has been shown to be a diagnostic marker for TGCT. Currently, it is believed that CIS is developed because during development environmental factors inhibit or delay the maturation of some gonocytes. These CIS later become invasive in adult and develop into SE or NS, which may be due to accumulation of genetic and epigenetic aberrations [152]. The SS in older male develops from the differentiated cells. The epigenetic mechanism that is involved in all cancers including TGCT is aberrant DNA methylation at gene promoters leading to silencing of tumour suppressor genes [159]. It has been shown that miRNAs also play major roles in carcinogenesis of human testicular cancer. There are reports that Mirn-322 and Mirn-323, potential novel oncogenes, participate in the development of human TGCTs (for references see 160]). It has been demonstrated that the loss of Dnd1 (dead end) results in loss of germ cells and development of TGCTs [161]. Further, it has been found that Dnd1 interacts with APOBEC3 (Apolipoprotein B editing complex 3) and binds to mRNA to inhibit miRNA-mediated repression of mRNA and may take part in the prevention of TGCT development [162]. Cao et al. [163] found that SALL4 (sal-like 4), a transcription factor, is a novel, sensitive and highly specific marker for metastatic germ cell tumours, and is specifically valuable in detection of metastatic YS tumours.
It has been proposed in several organ systems that cancer originates from cancer stem cells (CSCs) and CIS may originate from CSCs, because of the expression of embryonic pluripotency genes [149]. Further, CIS cells have a close phenotypic similarity to human foetal PGC and gonocytes [164] and it has been shown that NANOG, OCT-3/4, c-KIT and AP-2c are expressed in PGC and gonocytes, but are down-regulated later in development and germ cell differentiation in the adult. The above studies clearly show that CIS cells originate from PGC or gonocytes that have failed to differentiate into spermatogonia [reviewed in 164]. To determine whether CSCs can be identified in a transplantation model of TGCTs, Conway et al. [165] transplanted mouse embryonic germ cells (EGCs) into the testis of adult severe combined immunodeficient mice. Their results show that transplantation resulted in a locally invasive solid tumour, with a cellular component that generated secondary tumours upon serial transplantation. They suggested that transplantation of EGCs or ESCs into the adult testis will provide an in vivo model for adult-type TE formation and the generation of CSCs capable of recapitulating tumour growth after transplantation [165]. Further, they emphasize that their results will be helpful in the identification of molecular pathways that are deregulated in CSCs formation in both TGCTs and tumours originated from other pluripotent cells and may provide insight into pathways that are deregulated in the generation of somatic cancers with an ESC signature [165].
Conclusions
SSCs are a sub-population of unspecialized stem cells that reside along the basement membrane of the seminiferous tubules of the testes and are critical in spermatogenesis in the male. To restore fertility, SSC can be transplanted into the seminiferous tubules of infertile men. Further, transplantation studies are important in studying niche development and spermatogenesis as well as its underlying defects. Understanding the molecular mechanisms that regulate SSCs behaviour is of critical importance. The knowledge acquired from SSCs research, using the culture and transplantation techniques will enhance our understanding of male fertility and general stem cell biology in human and other model organisms [166, 167]. Testicular cancer represents for 1% of all cancers in men, and is the most common malignancies to affect males aged 15–40 years. The worldwide incidence of the TGCT is rising every year without any obvious reasons. Unravelling the biology of CIS is essential for the understanding of the cause of TGCT. Understanding the biology of TGCT will help in improving the treatment of other cancers. A detailed analysis of tumour formation and transformation of normal stem cells to CSCs may provide a new tool to understand the tumorigenic potential and the risk of tumour formation [168]. CSCs may be used as novel therapeutic targets for the treatment of TGCT. Recent studies have shown that SSCs from the adult mouse and human show features of pluripotency as they can generate all three germ layer derivatives. The above findings suggest that SSC may represent a novel alternative source of pluripotent cells that can play a pivotal role in regenerative and reproductive medicine.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. We apologize to scientific colleagues whose work could not be cited or was cited indirectly, because of space limitations.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 1363–34. [Google Scholar]
- 2.de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;9:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dadoune JP. New insights into male gametogenesis: what about the spermatogonial stem cell niche. Folia Histochem Cytobiol. 2007;45:141–7. [PubMed] [Google Scholar]
- 4.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98. [PubMed] [Google Scholar]
- 5.Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–6. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–5. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–12. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Kanatsu-Shinohara M, Ikawa M, Takehashi M, et al. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci USA. 2006;103:8018–23. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanatsu-Shinohara M, Lee J, Inoue K, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–87. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 10.Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 11.Guan K, Wagner S, Unsöld B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- 12.Seandel M, James D, Shmelkov, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–50. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad S, Renninger M, Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–9. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 14.Mardanpour P, Guan K, Nolte J, et al. Potency of germ cells and its relevance for regenerative medicine. J Anat. 2008;213:26–9. doi: 10.1111/j.1469-7580.2008.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dym M, Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat. 1970;128:265–82. doi: 10.1002/aja.1001280302. [DOI] [PubMed] [Google Scholar]
- 16.Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci USA. 1994;91:11287–9. doi: 10.1073/pnas.91.24.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naughton CK, Jain S, Strickland AM, et al. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 18.Godmann M, Lambrot R, Kimmins S. The dynamic epigenetic program in male germ cells: its role in spermatogenesis, testis cancer, and its response to the environment. Microsc Res Tech. 2009;72:603–19. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- 19.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara T, Orwig KE, Avarbock MR, et al. Restoration of spermatogenesis in infertile mice by Sertoli cell transplantation. Biol Reprod. 2003;68:1064–71. doi: 10.1095/biolreprod.102.009977. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Meachem S, von Schönfeldt, V Schlatt S. Spermatogonia: stem cells with a great perspective. Reproduction. 2001;121:825–34. doi: 10.1530/rep.0.1210825. [DOI] [PubMed] [Google Scholar]
- 23.Buaas FW, Kirsh AL, Sharma M, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–52. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 24.Brehm R, Steger K. Regulation of Sertoli cell and germ cell differentation. Adv Anat Embryol Cell Biol. 2005;181:1–93. [PubMed] [Google Scholar]
- 25.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–86. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanatsu-Shinohara M, Ogonuki N, Miki H, et al. Genetic influences in mouse spermatogonial stem cell self-renewal. J Reprod Dev. 2010;56:145–53. doi: 10.1262/jrd.09-153n. [DOI] [PubMed] [Google Scholar]
- 27.Oatley JM, Oatley MJ, Avarbock MR, et al. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–99. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi BP, Morrison SJ. Stem cells use distinct self-renewal programs at different ages. Cold Spring Harb Symp Quant Biol. 2008;73:539–53. doi: 10.1101/sqb.2008.73.049. [DOI] [PubMed] [Google Scholar]
- 29.Maki CB, Pacchiarotti J, Ramos T, et al. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum Reprod. 2009;24:1480–91. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- 30.He Z, Kokkinaki M, Jiang J, et al. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–72. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caires K, Broady J McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol. 2010;205:133–45. doi: 10.1677/JOE-09-0275. [DOI] [PubMed] [Google Scholar]
- 32.Yatsenko AN, Iwamori N, Iwamori T, et al. The power of mouse genetics to study spermatogenesis. J Androl. 2010;31:34–44. doi: 10.2164/jandrol.109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermo L, Pelletier RM, Cyr DG, et al. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–78. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 34.von Kopylow K, Kirchhoff C, Jezek D, et al. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–12. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- 35.McLaren A. Primordial germ cells in the mouse. Dev Biol. 262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 36.Ehmcke J, Schlatt S. Identification and characterization of spermatogonial subtypes and their expansion in whole mounts and tissue sections from primate testes. Methods Mol Biol. 2008;450:109–18. doi: 10.1007/978-1-60327-214-8_7. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto H, Kanatsu-Shinohara M, Takashima S, et al. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS ONE. 2009;4:e7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaira H, McLean D, Ohl DA, et al. Spermatogonial stem cell isolation, storage, and transplantation. J Androl. 2005;26:442–50. doi: 10.2164/jandrol.05062. [DOI] [PubMed] [Google Scholar]
- 39.Kubota H, Brinster RL. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida S. Spermatogenic stem cell system in the mouse testis. Cold Spring Harb Symp Quant Biol. 2008;73:25–32. doi: 10.1101/sqb.2008.73.046. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, Sharma M, Nabeshima Y, et al. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–7. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokuda M, Kadokawa Y, Kurahashi H, et al. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–41. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 43.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, et al. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 2009;140:5894–900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida S, Takakura A, Ohbo K, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–58. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 45.Russell LD, Griswold MD. Spermatogonial transplantation – an update for the millennium. Mol Cell Endocrinol. 2000;161:117–20. doi: 10.1016/s0303-7207(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 46.Brinster RL, Avarbock MR. Germ line transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinster RL, Zimmerman JW. Spermatogenesis following male germ cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82:381–8. doi: 10.1532/IJH97.05088. [DOI] [PubMed] [Google Scholar]
- 49.Kokkinaki M, Lee TL, He Z, et al. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol Reprod. 2009;80:707–17. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Q, Griswold MD. Regulation of spermatogonia, StemBook, ed. The Stem Cell Research Community. StemBook. 2008 10.3824/stembook.1.7.1. [PubMed] [Google Scholar]
- 51.Kotaja N, De Cesare D, Macho B. Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. Proc Natl Acad Sci USA. 2004;101:10620–5. doi: 10.1073/pnas.0401947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pesce M, Wang X, Wolgemuth DJ, et al. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 53.Dann CT, Alvarado AL, Molyneux LA, et al. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 54.Wu X, Oatley JM, Oatley MJ, et al. The POU domain transcription factor POU3F1 is an important intrinsic regulator of GDNF-Induced survival and self-renewal of mouse spermatogonial stem cells. Biol Reprod. 2010;82:1103–11. doi: 10.1095/biolreprod.109.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 56.Raverot G, Weiss J, Park SY, et al. Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev Biol. 2005;283:215–25. doi: 10.1016/j.ydbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Filipponi D, Hobbs RM, Ottolenghi S, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–81. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atchison FW, Means AR. Spermatogonial depletion in adult Pin1-deficient mice. Biol Reprod. 2003;69:1989–97. doi: 10.1095/biolreprod.103.020859. [DOI] [PubMed] [Google Scholar]
- 59.Falender AE, Shimada M, Lo YK, et al. TAF4b, a TBP associated factor, is required for oocyte development and function. Dev Biol. 2005;288:405–19. doi: 10.1016/j.ydbio.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 60.Giuili G, Tomljenovic A, Labrecque N, et al. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–59. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takubo K, Ohmura M, Azuma M, et al. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–82. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 62.Mizrak SC, Gadella BM, Erdost H, et al. Spermatogonial stem cell sensitivity to capsaicin: an in vitro study. Reprod Biol Endocrinol. 2008;6:52. doi: 10.1186/1477-7827-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sada A, Suzuki A, Suzuki H, et al. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–8. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki H, Sada A, Yoshida S, et al. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–31. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 66.Luo J, Megee S, Dobrinski I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J Cell Physiol. 2009;220:460–8. doi: 10.1002/jcp.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lolicato F, Marino R, Paronetto MP, et al. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–38. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 68.Ballow D, Meistrich ML, Matzuk M, et al. Sohlh1 is essential for spermatogonial differentiation. Dev Biol. 2006;294:161–67. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 69.Ballow DJ, Xin Y, Choi Y, et al. Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns. 2006;6:1014–18. doi: 10.1016/j.modgep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Toyoda S, Miyazaki T, Miyazaki S, et al. Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol. 2009;325:238–48. doi: 10.1016/j.ydbio.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 71.Bolden-Tiller OU, Chiarini-Garcia H, Poirier C, et al. Genetic factors contributing to defective spermatogonial differentiation in juvenile spermatogonial depletion (Utp14bjsd) mice. Biol Reprod. 2007;77:237–46. doi: 10.1095/biolreprod.107.060087. [DOI] [PubMed] [Google Scholar]
- 72.Kim ST, Gye MC. Expression of p57 in mouse and human testes. Dev Growth Differ. 2004;46:495–502. doi: 10.1111/j.1440-169x.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 73.Schrans-Stassen BH, Saunders PT, Cooke HJ, et al. Nature of the spermatogenic arrest in Dazl−/- mice. Biol Reprod. 2001;65:771–6. doi: 10.1095/biolreprod65.3.771. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Medvedev S, Reddi PP, et al. The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc Natl Acad Sci USA. 2005;102:1513–8. doi: 10.1073/pnas.0404685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barna M, Merghoub T, Costoya JA, et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 76.Singh SR, Zheng Z, Wang H, et al. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–10. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–30. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 78.Wang H, Singh SR, Zheng Z, et al. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell. 2006;10:117–26. doi: 10.1016/j.devcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Singh SR, Zhen W, Zheng Z, et al. The Drosophila homolog of the human tumor suppressor gene BHD interacts with the JAK-STAT and Dpp signaling pathways in regulating male germline stem cell maintenance. Oncogene. 2006;25:5933–41. doi: 10.1038/sj.onc.1209593. [DOI] [PubMed] [Google Scholar]
- 80.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–4. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 81.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–49. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita YM, Mahowald AP, Perlin JR, et al. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng J, Türkel N, Hemati N, et al. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–4. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hess RA, Cooke PS, Hofmann MC, et al. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle. 2006;5:1164–70. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57:270–9. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 86.Terada N, Ohno N, Saitoh S, et al. Involvement of a membrane skeletal protein, 4.1G, for Sertoli/germ cell interaction. Reproduction. 2010;139:883–92. doi: 10.1530/REP-10-0005. [DOI] [PubMed] [Google Scholar]
- 87.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 88.Hamra FK, Chapman KM, Nguyen DM, et al. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci USA. 2005;102:17430–5. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Ouyang W, Grigura V, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyagi G, Carnes K, Morrow C, et al. Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol Reprod. 2009;81:258–66. doi: 10.1095/biolreprod.108.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simon L, Ekman GC, Tyagi G, et al. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313:3090–9. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Zhao GQ, Garbers DL. Male germ cell specification and differentiation. Dev Cell. 2002;2:537–47. doi: 10.1016/s1534-5807(02)00173-9. [DOI] [PubMed] [Google Scholar]
- 93.Feng LX, Chen Y, Dettin L, et al. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–5. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- 94.Zheng J, Xia X, Ding H, et al. Erasure of the paternal transcription program during spermiogenesis: the first step in the reprogramming of sperm chromatin for zygotic development. Dev Dyn. 2008;237:1463–76. doi: 10.1002/dvdy.21499. [DOI] [PubMed] [Google Scholar]
- 95.Brehm R, Zeiler M, Rüttinger C, et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryu BY, Orwig KE, Kubota H, et al. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274:158–70. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Lee J, Kanatsu-Shinohara M, Inoue K, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–59. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 98.Braydich-Stolle L, Kostereva N, Dym M, et al. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dolci S, Pellegrini M, Di Agostino S, et al. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem. 2001;276:40225–33. doi: 10.1074/jbc.M105143200. [DOI] [PubMed] [Google Scholar]
- 100.Nagano M, Ryu BY, Brinster CJ, et al. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68:2207–14. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 101.Itman C, Loveland KL. SMAD expression in the testis: an insight into BMP regulation of spermatogenesis. Dev Dyn. 2008;237:97–111. doi: 10.1002/dvdy.21401. [DOI] [PubMed] [Google Scholar]
- 102.Wu J, Zhang Y, Tian GG, et al. Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal. 2008;20:1052–60. doi: 10.1016/j.cellsig.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Murphy K, Carvajal L, Medico L, et al. Expression of Stat3 in germ cells of developing and adult mouse ovaries and testes. Gene Expr Patterns. 2005;5:475–82. doi: 10.1016/j.modgep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Oatley JM, Kaucher AV, Avarbock MR, et al. Regulation of mouse spermatogonial Stem Cell Differentiation by STAT3 Signaling. Biol Reprod. 2010;83:427–33. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanwar PS, Kaneko-Tarui T, Zhang L, et al. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–32. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–24. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–2. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrid M, Vignarajan S, Davey R, et al. Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction. 2006;132:617–24. doi: 10.1530/rep.1.01125. [DOI] [PubMed] [Google Scholar]
- 109.Sofikitis N, Ono K, Yamamoto Y, et al. Influence of the male reproductive tract on the reproductive potential of round spermatids abnormally released from the seminiferous epithelium. Hum Reprod. 1999;14:1998–6. doi: 10.1093/humrep/14.8.1998. [DOI] [PubMed] [Google Scholar]
- 110.Reis MM, Tsai MC, Schlegel PN, et al. Xenogeneic transplantation of human spermatogonia. Zygote. 2000;8:97–105. doi: 10.1017/s0967199400000873. [DOI] [PubMed] [Google Scholar]
- 111.Izadyar F, Den Ouden K, Creemers LB, et al. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–81. doi: 10.1095/biolreprod.102.004986. [DOI] [PubMed] [Google Scholar]
- 112.Kanatsu-Shinohara M, Takashima S, Shinohar T, et al. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc Natl Acad Sci USA. 2010;107:6210–5. doi: 10.1073/pnas.0914448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barroca V, Lassalle B, Coureuil M, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–6. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 115.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 116.Golestaneh N, Beauchamp E, Fallen S, et al. Wnt signaling promotes proliferation and stemness regulation of spermatogonial stem/progenitor cells. Reproduction. 2009;138:151–62. doi: 10.1530/REP-08-0510. [DOI] [PubMed] [Google Scholar]
- 117.Kossack N, Meneses J, Shefi S, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–49. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chambers I, Silva J, Colby D, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–4. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 119.Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, et al. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–67. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- 120.Streckfuss-Bömeke K, Vlasov A, Hülsmann S, et al. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res. 2009;2:139–54. doi: 10.1016/j.scr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Kuijk EW, van Mil A, Brinkhof B, et al. PTEN and TRP53 independently suppress Nanog expression in spermatogonial stem cells. Stem Cells Dev. 2010;19:979–88. doi: 10.1089/scd.2009.0276. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez R, Griparic L, Vargas V, et al. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun. 2009;385:570–5. doi: 10.1016/j.bbrc.2009.05.103. [DOI] [PubMed] [Google Scholar]
- 123.Borg CL, Wolski KM, Gibbs GM, et al. Phenotyping male infertility in the mouse: how to get the most out of a ‘non-performer’. Hum Reprod Update. 2010;16:205–24. doi: 10.1093/humupd/dmp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poongothai J, Gopenath TS, Manonayaki S. Genetics of human male infertility. Singapore Med J. 2009;50:336–47. [PubMed] [Google Scholar]
- 125.O’Flynn O’Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 126.Yatsenko AN, Iwamori N, Iwamori T, et al. The power of mouse genetics to study spermatogenesis. J Androl. 2010;31:34–44. doi: 10.2164/jandrol.109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu J, Chen YX, Wang D, et al. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–71. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 128.Cotton L, Gibbs GM, Sanchez-Partida LG, et al. FGFR-1 signaling is involved in spermiogenesis and sperm capacitation. J Cell Sci. 2006;119:75–84. doi: 10.1242/jcs.02704. [DOI] [PubMed] [Google Scholar]
- 129.Kissel H, Timokhina I, Hardy MP, et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312–26. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–35. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 131.Coussens M, Maresh JG, Yanagimachi R, et al. Sirt1 deficiency attenuates spermatogenesis and germ cell function. PLoS ONE. 2008;3:e1571. doi: 10.1371/journal.pone.0001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bannister LA, Pezza RJ, Donaldson JR, et al. A dominant, recombination-defective allele of Dmc1 causing male-specific sterility. PLoS Biol. 2007;5:e105. doi: 10.1371/journal.pbio.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Z, Zhou S, Liao L, et al. Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis. J Biol Chem. 2010;285:2758–70. doi: 10.1074/jbc.M109.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adelman CA, Petrini JH. ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. PLoS Genet. 2008;4:e1000042. doi: 10.1371/journal.pgen.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen F, Zhang C, Zheng H, et al. Long-term culture and transplantation of spermatogonial stem cells from BALB/c mice. Cells Tissues Organs. 2010;191:372–81. doi: 10.1159/000276586. [DOI] [PubMed] [Google Scholar]
- 136.Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53:274–80. doi: 10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- 137.Kanatsu-Shinohara M, Miki H, Inoue K, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 138.Ogawa T, Dobrinski I, Avarbock MR, et al. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Richardson TE, Chapman KM, Tenenhaus Dann C, et al. Sterile testis complementation with spermatogonial lines restores fertility to DAZL-deficient rats and maximizes donor germline transmission. PLoS ONE. 2009;4:e6308. doi: 10.1371/journal.pone.0006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yeh JR, Nagano MC. Spermatogonial stem cell biomarkers: improved outcomes of spermatogonial transplantation in male fertility restoration. Expert Rev Mol Diagn. 2009;9:109–14. doi: 10.1586/14737159.9.2.109. [DOI] [PubMed] [Google Scholar]
- 141.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–65. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 142.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–22. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 143.Fléchon A, Rivoire M, Droz JP. Management of advanced germ-cell tumors of the testis. Nat Clin Pract Urol. 2008;5:262–76. doi: 10.1038/ncpuro1101. [DOI] [PubMed] [Google Scholar]
- 144.Walsh TJ, Croughan MS, Schembri M, et al. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Almstrup K, Nielsen JE, Hansen MA, et al. Analysis of cell-type-specific gene expression during mouse spermatogenesis. Biol Reprod. 2004;70:1751–61. doi: 10.1095/biolreprod.103.026575. [DOI] [PubMed] [Google Scholar]
- 147.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–4. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 148.Korkola JE, Houldsworth J, Chadalavada RS, et al. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006;66:820–7. doi: 10.1158/0008-5472.CAN-05-2445. [DOI] [PubMed] [Google Scholar]
- 149.Clark AT, Rodriguez RT, Bodnar MS, et al. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells. 2004;22:169–79. doi: 10.1634/stemcells.22-2-169. [DOI] [PubMed] [Google Scholar]
- 150.Gashaw I, Dushaj O, Behr R, et al. Novel germ cell markers characterize testicular seminoma and fetal testis. Mol Hum Reprod. 2007;13:721–7. doi: 10.1093/molehr/gam059. [DOI] [PubMed] [Google Scholar]
- 151.Esposito F, Libertini S, Franco R, et al. Aurora B expression in post-puberal testicular germ cell tumours. J Cell Physiol. 2009;221:435–9. doi: 10.1002/jcp.21875. [DOI] [PubMed] [Google Scholar]
- 152.Godmann M, Gashaw I, Eildermann K, et al. The pluripotency transcription factor Krüppel-like factor 4 is strongly expressed in intratubular germ cell neoplasia unclassified and seminoma. Mol Hum Reprod. 2009;15:479–88. doi: 10.1093/molehr/gap040. [DOI] [PubMed] [Google Scholar]
- 153.Leroy X, Augusto D, Leteurtre E, et al. CD30 and CD117 (c-kit) used in combination are useful for distinguishing embryonal carcinoma from seminoma. J Histochem Cytochem. 2002;50:283–5. doi: 10.1177/002215540205000216. [DOI] [PubMed] [Google Scholar]
- 154.Juric D, Sale S, Hromas RA, et al. Gene expression profiling differentiates germ cell tumors from other cancers and defines subtype-specific signatures. Proc Natl Acad Sci USA. 2005;102:17763–8. doi: 10.1073/pnas.0509082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okada K, Katagiri T, Tsunoda T, et al. Analysis of gene-expression profiles in testicular seminomas using a genome-wide cDNA microarray. Int J Oncol. 2003;23:1615–35. [PubMed] [Google Scholar]
- 156.Skotheim RI, Lind GE, Monni O, et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65:5588–98. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 157.Meng X, de Rooij DG, Westerdahl K, et al. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–71. [PubMed] [Google Scholar]
- 158.McIntyre A, Gilbert D, Goddard N, et al. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromo.Cancer. 2008;47:547–57. doi: 10.1002/gcc.20562. [DOI] [PubMed] [Google Scholar]
- 159.Lind GE, Skotheim RI, Fraga MF, et al. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1) J Pathol. 2006;210:441–9. doi: 10.1002/path.2064. [DOI] [PubMed] [Google Scholar]
- 160.Voorhoeve PM, le Sage C, Schrier M, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv Exp Med Biol. 2007;604:17–46. doi: 10.1007/978-0-387-69116-9_2. [DOI] [PubMed] [Google Scholar]
- 161.Youngren KK, Coveney D, Peng X, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–4. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bhattacharya C, Aggarwal S, Kumar M, et al. Mouse apolipoprotein B editing complex 3 (APOBEC3) is expressed in germ cells and interacts with dead-end (DND1) PLoS ONE. 2008;3:e2315. doi: 10.1371/journal.pone.0002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cao D, Humphrey PA, Allan RW. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer. 2009;115:2640–51. doi: 10.1002/cncr.24308. [DOI] [PubMed] [Google Scholar]
- 164.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–23. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 165.Conway AE, Lindgren A, Galic Z, et al. A self-renewal program controls the expansion of genetically unstable cancer stem cells in pluripotent stem cell-derived tumors. Stem Cells. 2009;27:18–28. doi: 10.1634/stemcells.2008-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–78. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yoshida S. Stem cells in mammalian spermatogenesis. Dev Growth Differ. 2010;52:311–7. doi: 10.1111/j.1440-169X.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 168.Cheng X, O’Neill HC. Oncogenesis and cancer stem cells: current opinions and future directions. J Cell Mol Med. 2009;13:4377–84. doi: 10.1111/j.1582-4934.2008.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


