Abstract
Objectives
To examine the association of meat consumption with diabetes risk in the Hawaii component of the Multiethnic Cohort and to assess effect modification by ethnicity.
Design
A prospective cohort study. Baseline data on diet and lifestyle was assessed by questionnaire and the cohort was followed up for incident cases of diabetes which were identified through self-reports, medication questionnaires, or health plan linkages. Cox regression was used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for diabetes associated with quintile of meat consumption.
Setting
Hawaii, USA.
Subjects
A total of 29,759 Caucasian, 35,244 Japanese American, and 10,509 Native Hawaiian men and women, aged 45-75 years at baseline.
Results
During a mean follow-up time of 14 years, 8587 incident diabetes cases were identified. Intake of red meat was positively associated with diabetes risk in men (HR [5th vs. 1st quintile] 1.43, 95% CI 1.29-1.59) and women (HR [5th vs. 1st quintile] 1.30, 95% CI 1.17-1.45) in adjusted models. The respective HR for processed red meat intake were 1.57 (95% CI 1.42-1.75) and 1.45 (95% CI 1.30-1.62). The association for processed poultry was weaker than for processed red meat, and fresh poultry intake was not associated with diabetes risk. For men only, we observed significant interactions of ethnicity with the red and processed red meat associations, with Caucasians experiencing slightly higher risks than Japanese.
Conclusion
Our findings support the growing evidence that red and processed meat intake are risk factors for diabetes, irrespective of ethnicity and level of BMI.
Keywords: type 2 diabetes, meat, ethnicity, prospective study
Introduction
Prevalence of type 2 diabetes mellitus is increasing worldwide; however, some ethnic groups, such as Asian Americans or Pacific Islanders, suffer from extremely high rates compared to Caucasians (1). In the Multiethnic Cohort (MEC), diabetes incidence rates of 15.5, 12.5, and 5.8 per 1000 person-years were found for Native Hawaiians, Japanese Americans, and Caucasians, respectively (2). Established risk factors for diabetes are overweight, obesity and physical inactivity (3); still, dietary factors might play an important role. A meta-analysis on meat intake and diabetes risk concluded that particularly red and processed meat increase diabetes risk (4). Thus far, no prospective study has examined whether this association is modified by ethnicity. We examined the association of meat consumption (red meat, processed red meat, fresh poultry, and processed poultry) with diabetes risk in men and women of Caucasian, Japanese American, and Native Hawaiian ancestry in the Hawaii component of the MEC.
Methods
Study population
The MEC was designed to investigate the association between diet and cancer among different ethnic groups in Hawaii and California (5). Between 1993 and 1996, more than 215,000 men and women, aged 45-75 years at recruitment, enrolled by completing a mailed questionnaire on diet, demographics, medical conditions, anthropometric measures, and lifestyle factors. The Hawaiian component of the MEC comprises 103,898 participants, primarily Caucasians, Japanese Americans, and Native Hawaiians. Response rates ranged from 28% to 51% in the different ethnic-sex groups, and comparison with US Census data indicated that the study population represented all levels of education. For the present analysis, subjects belonging to other ethnicities (n = 8797), prevalent diabetes cases (n = 10,028), and unconfirmed cases (n = 812) were excluded, as were subjects with missing covariate (n = 6202) or dietary information (n = 2537), and missing information on follow-up or diabetes at baseline (n = 10), leaving 36,256 men and 39,256 women. Study protocols were approved by the Committee on Human Studies at the University of Hawaii and by the Institutional Review Board of Kaiser Permanente.
Data assessment
Incident cases of diabetes mellitus were identified, either by self-report in a follow-up questionnaire mailed to the participants between 1999 and 2003 (response rate in Hawaii 88%), or via a medication questionnaire (including diabetes drugs) administered to 38% of the MEC participants who agreed to a blood draw between 2001 and 2007, or by a linkage in 2007 with the two major health plans in Hawaii, Kaiser Permanente and Blue Cross/Blue Shield (2). After excluding 812 self-reported cases not confirmed by a health plan, a total of 8587 incident cases were identified during a median follow-up time of 13.5 years: 2251 from the follow-up questionnaire, 996 from the medication questionnaire, and 5340 through the health plans. Information on vital status of all participants is updated annually by linkage with state and national death certificates.
Dietary data were collected at baseline by a validated quantitative food frequency questionnaire (FFQ) specifically designed for use in this multiethnic population (5). Nutrient intake was determined by linking food intake to an ethnic-specific food composition database developed and maintained at the Cancer Research Center of Hawaii. In a validation and calibration sub study average correlation coefficients ranged from 0.26 to 0.57 for nutrients and from 0.57 to 0.75 for nutrient densities for the different sex-ethnic groups, indicating good validity.
Food group intake was calculated as grams per day of the basic food commodities and covered single food items as well as mixed dishes. Intakes were converted to energy densities (g/4184 kJ*d). Food groups examined for the current analysis were red meat (beef, pork, and lamb), fresh poultry, processed red meat, and processed poultry.
Statistical analysis
We applied Cox proportional hazard regression with follow-up time as the underlying time metric and stratified by age at cohort entry to estimate hazard ratios (HR) and 95% confidence intervals (CI) for sex-specific quintiles of meat consumption. Linear trend tests were performed using an ordinal variable representing the median of each quintile. Follow-up time was calculated as the difference between date of cohort entry and date of diabetes diagnosis, date of death or last date when data on diabetes status were available, whichever came first (2). The final models were adjusted for ethnicity, BMI, physical activity, education, and energy intake (logarithm). We tested for interaction of meat consumption and ethnicity and additionally calculated ethnic specific HR of diabetes for meat consumption. No major violations of the proportional hazards assumption were observed when examined with time-dependent explanatory variables. All statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
The median intake of beef or fresh poultry did not differ by ethnicity, but higher amounts of pork, red meat, and processed red meat were consumed by Native Hawaiians, while Caucasians tended to consume least of these meat intake groups (Table 1).
Table 1.
Baseline characteristics of participants in the Hawaii component of the Multiethnic Cohort Study by ethnicity and sex, 1993-2007
| Men |
Women |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caucasian (n= 15,116) | Native Hawaiian (n= 4568) | Japanese American (n= 16,572) | Caucasian (n= 14,643) | Native Hawaiian (n= 5941) | Japanese American (n= 18,672) | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Age | ||||||||||||
| 45-54 years | 6766 | 44.76 | 2305 | 50.5 | 5437 | 32.8 | 6901 | 47.1 | 3158 | 53.2 | 6058 | 32.4 |
| 55-64 years | 4194 | 27.75 | 1334 | 29.2 | 4645 | 28.0 | 3909 | 26.7 | 1678 | 28.2 | 5686 | 30.5 |
| ≥65 years | 4156 | 27.49 | 929 | 20.3 | 6490 | 39.2 | 3833 | 26.2 | 1105 | 18.6 | 6928 | 37.1 |
| Diabetes status | ||||||||||||
| Non-case | 14,036 | 92.9 | 3770 | 82.5 | 13,895 | 83.9 | 13,928 | 95.1 | 4998 | 84.1 | 16,298 | 87.3 |
| Incident case | 1080 | 7.1 | 798 | 17.5 | 2677 | 16.2 | 715 | 4.9 | 943 | 15.9 | 2374 | 12.7 |
| Education | ||||||||||||
| ≤ 12 years | 2870 | 19.0 | 2172 | 47.6 | 6472 | 39.1 | 3398 | 23.2 | 3115 | 52.4 | 7691 | 41.2 |
| 13 - 15 years | 4377 | 29.0 | 1456 | 31.9 | 4777 | 28.8 | 5023 | 34.3 | 1789 | 30.1 | 5255 | 28.1 |
| > 15 years | 7869 | 52.1 | 940 | 20.6 | 5323 | 32.1 | 6222 | 42.5 | 1037 | 17.5 | 5726 | 30.7 |
| Body mass index | ||||||||||||
| < 22 (kg/m2) | 2047 | 13.5 | 318 | 7.0 | 3107 | 18.8 | 4965 | 33.9 | 969 | 16.3 | 8040 | 43.1 |
| 22 – <25 (kg/m2) | 5080 | 33.6 | 900 | 19.7 | 6439 | 38.9 | 4180 | 28.6 | 1321 | 22.2 | 5765 | 30.9 |
| 25 – <30 (kg/m2) | 6135 | 40.6 | 2019 | 44.2 | 6084 | 36.7 | 3670 | 25.1 | 1985 | 33.4 | 4002 | 21.4 |
| ≥ 30 (kg/m2) | 1854 | 12.3 | 1331 | 29.1 | 942 | 5.7 | 1828 | 12.5 | 1666 | 28.0 | 865 | 4.6 |
| Total energy (kJ/day)* | 9251 (7167, 11899) | 10724 (7853, 14523) | 9155 (7155, 11611) | 7339 (5720, 9422) | 8786 (6406, 12083) | 7289 (5699, 9318) | ||||||
| Red meat* | 16.5 (9.3, 24.7) | 19.9 (13.2, 27.8) | 17.4 (10.5, 25.0) | 12.8 (6.5, 20.7) | 18.1 (11.5, 26.2) | 14.7 (8.7, 22.0) | ||||||
| Processed red meat* | 6.4 (3.2, 10.7) | 9.3 (5.6, 13.8) | 8.2 (4.7, 12.3) | 4.1 (1.9, 7.6) | 7.5 (4.1, 11.8) | 6.2 (3.2, 9.9) | ||||||
| Fresh poultry* | 14.4 (8.7, 22.2) | 14.3 (9.1, 21.6) | 15.0 (9.7, 22.0) | 15.2 (8.9, 24.3) | 15.1 (9.7, 22.7) | 15.5 (10.0, 22.8) | ||||||
| Processed poultry* | 0.5 (0.1, 1.6) | 0.7 (0.1, 1.6) | 0.6 (0.1, 1.3) | 0.3 (0.1, 1.2) | 0.6 (0.1, 1.3) | 0.4 (0.1, 1.1) | ||||||
| Beef* | 12.2 (6.9, 18.4) | 12.9 (8.1, 18.4) | 11.7 (7.0, 17.2) | 9.5 (4.8, 15.4) | 11.9 (7.2, 17.3) | 9.8 (5.6, 14.8) | ||||||
| Pork* | 3.0 (1.1, 5.7) | 6.1 (3.6, 8.8) | 4.8 (2.5, 7.6) | 2.3 (0.7, 4.8) | 5.4 (3.0, 8.1) | 4.2 (2.2, 6.8) | ||||||
| Dietary fiber* | 10.3 (8.0, 13.1) | 8.1 (6.3, 10.6) | 8.7 (6.7, 11.4) | 12.0 (9.5, 15.2) | 10.1 (7.8, 13.2) | 11.3 (8.8, 14.2) | ||||||
| Physical activity (METS) | 1.6 (1.4, 1.8) | 1.7 (1.5, 1.9) | 1.6 (1.5, 1.8) | 1.6 (1.4, 1.8) | 1.6 (1.4, 1.8) | 1.6 (1.4, 1.7) | ||||||
METS, metabolic equivalent of tasks (given as median and interquartile range)
Total energy and nutrient intakes (g/(4184 kcal*d)) are given as median and interquartile range
Red meat and processed red meat were positively associated with diabetes risk in men (Table 2). The HR comparing extreme quintiles were 1.43 (95% CI 1.29-1.59) for red meat and 1.57 (95% CI 1.42-1.75) for processed red meat in multivariate adjusted models. Further adjustment for fiber intake, which was recently shown to be associated with diabetes in this cohort, attenuated this association slightly with HR of 1.38 (95% CI 1.24-1.53) for red meat and 1.53 (95% CI 1.37-1.71) for processed red meat comparing highest versus lowest quintile (data not shown). Intake of fresh poultry was not associated with diabetes risk, while intake of processed poultry increased risk by 30% for the highest intake quintile compared to the lowest.
Table 2.
Diabetes risk (hazard ratio and 95% confidence intervals) associated with quintiles of meat consumption in men, Hawaii component of the Multiethnic Cohort Study, 1993-2007
| Quintile of Intake |
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ptrend | ||
| Red meat intake* | < 9.40 | 9.40 – 15.62 | 15.63 – 21.60 | 21.61 – 29.76 | ≥ 29.77 | ||
| N of cases | 690 | 880 | 1034 | 1077 | 874 | ||
| Adjusted HR† | 1.00 | 1.17 (1.06, 1.29) | 1.31 (1.19, 1.44) | 1.42 (1.29, 1.56) | 1.43 (1.29, 1.59) | <0.0001 | |
| Processed red meat intake* | < 3.22 | 3.22 – 6.01 | 6.02 – 9.01 | 9.02 – 13.39 | ≥ 13.40 | ||
| N of cases | 537 | 746 | 930 | 1161 | 1181 | ||
| Adjusted HR† | 1.00 | 1.22 (1.09, 1.37) | 1.30 (1.17, 1.45) | 1.45 (1.31, 1.61) | 1.57 (1.42, 1.75) | <0.0001 | |
| Fresh poultry intake* | < 9.09 | 9.09 – 14.22 | 14.23 – 19.98 | 19.99 – 29.50 | ≥ 29.51 | ||
| N of cases | 986 | 1087 | 1029 | 867 | 586 | ||
| Adjusted HR† | 1.00 | 1.05 (0.96, 1.15) | 1.10 (1.01, 1.20) | 1.11 (1.01, 1.21) | 1.06 (0.96, 1.18) | 0.19 | |
| Processed poultry intake* | < 0.04 | 0.04 – 0.24 | 0.25 – 0.82 | 0.83 – 1.80 | ≥ 1.81 | ||
| N of cases | 543 | 987 | 968 | 1100 | 957 | ||
| Adjusted HR† | 1.00 | 1.19 (1.07, 1.32) | 1.19 (1.07, 1.32) | 1.27 (1.14, 1.40) | 1.30 (1.17, 1.44) | 0.0001 | |
Intake in g/(4184 kJ*d)
Hazard ratio adjusted for ethnicity, education, BMI, physical activity and total calorie intake (log transformed) as well as stratified by age at cohort entry
Similar associations between meat consumption and diabetes risk were found in women (Table 3), although the risk estimates tended to be lower than in men. HR for diabetes comparing the highest versus lowest intake quintile were 1.30 (95% CI 1.17-1.45) for red meat and 1.45 (95% CI 1.30-1.62) for processed red meat. Additional adjustment for fiber intake did not alter these estimates (HR 1.29 (95% CI 1.15-1.45) for red meat and HR 1.45 (95% CI 1.30-1.65) for processed red meat) (data not shown). Fresh poultry intake was not associated with diabetes, but women in the 5th quintile of processed poultry intake had a 23% increased diabetes risk compared to the lowest quintile.
Table 3.
Diabetes Risk (hazard ratio and 95% confidence intervals) associated with quintiles of meat consumption in women, Hawaii component of the Multiethnic Cohort Study, 1993-2007
| Quintile of Intake |
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ptrend | ||
| Red meat intake* | < 7.12 | 7.12 - 12.59 | 12.60 - 18.26 | 18.27 - 25.97 | ≥ 25.98 | ||
| N of cases | 570 | 745 | 865 | 974 | 878 | ||
| Adjusted HR† | 1.00 | 1.06 (0.95, 1.18) | 1.17 (1.06, 1.31) | 1.25 (1.13, 1.39) | 1.30 (1.17, 1.45) | <0.0001 | |
| Processed red meat intake* | < 2.07 | 2.07 - 4.22 | 4.23 - 6.78 | 6.79 - 10.60 | ≥ 10.61 | ||
| N of cases | 465 | 635 | 792 | 1027 | 1113 | ||
| Adjusted HR† | 1.00 | 1.20 (1.07, 1.35) | 1.24 (1.10, 1.39) | 1.37 (1.22, 1.53) | 1.45 (1.30, 1.62) | <0.0001 | |
| Fresh poultry intake* | < 9.91 | 9.91 - 15.56 | 15.57 - 22.14 | 22.15 - 33.65 | ≥ 33.66 | ||
| N of cases | 912 | 1050 | 977 | 697 | 396 | ||
| Adjusted HR† | 1.00 | 1.12 (1.02, 1.22) | 1.18 (1.07, 1.29) | 1.09 (0.98, 1.20) | 1.01 (0.90, 1.14) | 0.95 | |
| Processed poultry intake* | < 0.03 | 0.03 - 0.20 | 0.21 - 0.71 | 0.72 - 1.61 | ≥ 1.62 | ||
| N of cases | 496 | 884 | 892 | 990 | 770 | ||
| Adjusted HR† | 1.00 | 1.19 (1.06, 1.32) | 1.18 (1.06, 1.32) | 1.20 (1.08, 1.34) | 1.23 (1.10, 1.38) | 0.03 | |
Intake in g/(4184 kJ*d)
Hazard ratio adjusted for ethnicity, education, BMI, physical activity and total calorie intake (log transformed) as well as stratified by age at cohort entry
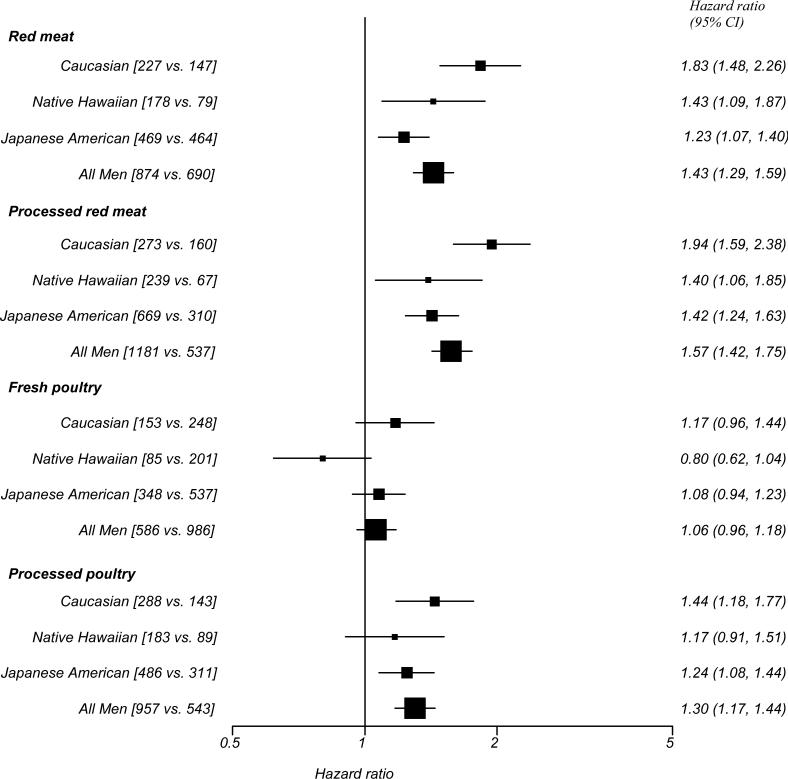
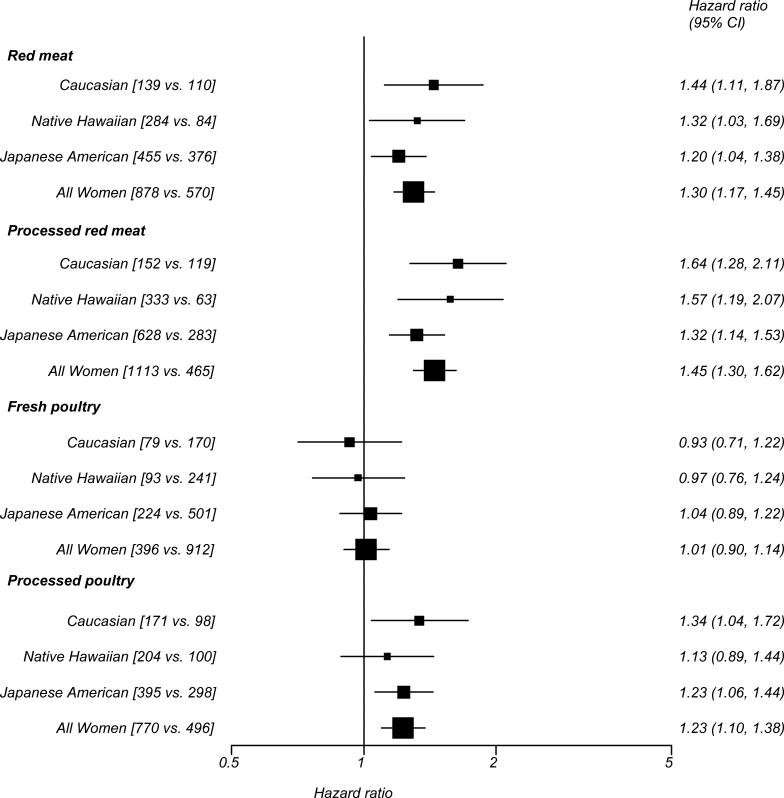
Associations of the 5th versus the 1st meat intake quintile with diabetes risk stratified by ethnicity are shown in Figure 1 for men and Figure 2 for women. In men, the tests for interaction between ethnicity and red meat intake (pinteraction = 0.006) and processed red meat intake (pinteraction = 0.002) were significant, with slightly higher risk for Caucasians and lower risk for Japanese Americans. We did not find a significant interaction for fresh poultry (pinteraction = 0.47) or processed poultry (pinteraction = 0.46) in men or for any meat type in women (pinteraction = 0.47 for processed red meat, 0.32 for fresh poultry and 0.24 for processed poultry), except for consumption of red meat with a borderline significant interaction (pinteraction = 0.05).
Figure 1.
Diabetes risk (hazard ratio and 95% confidence interval) comparing highest vs. lowest quintile of meat consumption by ethnicity in men, Hawaii component of the Multiethnic Cohort Study, 1993-2007
Hazard ratio adjusted for education, BMI, physical activity and total calorie intake (log transformed) as well as stratified by age at cohort entry, square brackets represent number of diabetes cases in the highest versus lowest quintile of meat consumption
Figure 2.
Diabetes risk (hazard ratio and 95% confidence interval) comparing highest versus lowest quintile of meat consumption by ethnicity in women, Hawaii component of the Multiethnic Cohort Study, 1993-2007
Hazard ratio adjusted for education, BMI, physical activity and total calorie intake (log transformed) as well as stratified by age at cohort entry, square brackets represent number of diabetes cases in the highest versus lowest quintile of meat consumption
Discussion
In this analysis of the Hawaii component of the MEC, we found a positive association between intakes of red meat, processed red meat and processed poultry with risk of diabetes in men and women, independent of BMI status. Fresh poultry consumption was not associated with diabetes risk.
Strengths of this study are the large sample size, the prospective design with long follow-up time, and the extensive data collection allowing adjustment for a variety of known confounders, such as BMI. However, the possibility of residual confounding cannot be excluded. The study FFQ was specifically designed for use in this multiethnic cohort, and reproducibility and validity of nutrient intake densities were found to be satisfactory and comparable to those of other similar studies (6). Moreover, mixed dishes containing meat were disaggregated into their component ingredients and considered in the estimation of total meat intake. However, misreporting of certain foods might have biased our results, although due to the prospective design, disease status could not have influenced reporting of meat intake. Since we did not have repeat measurements of diet, changes in diet over time could not be considered in analysis. Diabetes status was ascertained by medication questionnaires and linkage with health plans. Information on type of diabetes was not available; however, given the median age of 59 years of the participants at baseline, more than 90% of cases were likely to have type 2 diabetes.
Our results agree with several prospective studies on meat intake and diabetes risk. In a recent meta-analysis (4), the summary risks comparing high vs. low intake were 1.21 (95% CI 1.07-1.38) for red meat and 1.41 (95% CI 1.25-1.60) for processed meat. The magnitude of these estimates corresponds well with those from our study; although caution is needed for such comparisons due to different units in exposure measurement. Furthermore, the type of red meat consumed (i.e., beef or pork) and the proportion of poultry in comparison to red meat intake likely differs among countries. For example, in a Finnish study, intake of red meat (mean intake in non-cases: 79.6 g/d) and processed meat (52.0 g/d) were considerably higher than poultry intake (2.6 g/d), while intake of poultry and red meat were nearly equal in our study (7).
Few studies examined the association between intake of fresh poultry and diabetes risk, with one reporting no association (8), while several others observed inverse associations (7, 9, 10). To our knowledge, no other study examined the association between intake of processed poultry and diabetes.
In an earlier analysis of the MEC, we found an inverse association between dietary fiber intake and diabetes risk in men but not in women (11). As red meat and processed red meat were negatively correlated with fiber intake, we additionally adjusted the present analysis for fiber intake to exclude the possibility of confounding. The HR for red and processed meat in men decreased slightly but remained significant, indicating an effect of meat irrespective of fiber intake. Nevertheless, one has to consider that the positive association of meat consumption and diabetes risk might not be attributable to meat intake per se, but rather to a dietary pattern like the so called “Western” pattern, which combines high meat intake, especially processed red meat and processed poultry, with refined grains and sweets (12).
We found no strong indication for effect modification by ethnicity. Tests for interaction were statistical significant only for red meat and processed red meat consumption in men, which might be explained by ethnically different meat preparation practices or differences in the choice of red meat types. However, the HR for the three ethnic groups did not differ meaningfully, and thus, the statistical significance might be driven more by the large sample size than an underlying biological difference.
One hypothesis for a role of meat intake in diabetes etiology is that meat consumption increases fat intake, especially saturated fat intake, and thus might act indirectly by increasing body weight, an established risk factor for diabetes (3). However, we adjusted for BMI, and still found an association, indicating that other mechanisms might be important. For example, heating foods such as meat, can lead to high levels of advanced glycation end-products, which have been associated with inflammatory responses in humans (13). Red meat is a source of heme-iron; higher body iron stores might impair insulin sensitivity (14) and increase the risk of diabetes (15) by promoting oxidative stress causing tissue damage (16). Processed meat might contain preservatives, additives, or other chemicals, such as nitrates, nitrites, and heterocyclic amines, formed during food preparation. Nitrites, for example, might be converted to nitrosamines, which exert pancreatic β-cell toxicity (17).
In conclusion, our findings add to the growing evidence for a positive association of red meat and processed meat intake with diabetes risk. We found this association to be consistent over the different ethnic strata of the MEC, despite the higher incidence rates of diabetes in Native Hawaiians and Japanese Americans compared to Caucasians. Besides the known role of body weight, these results highlight the importance of diet and food choices in diabetes etiology.
Reference List
- 1.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27:66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 2.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58:1732–1738. doi: 10.2337/db08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 4.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 5.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stram DO, Hankin JH, Wilkens LR, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montonen J, Jarvinen R, Heliovaara M, et al. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59:441–448. doi: 10.1038/sj.ejcn.1602094. [DOI] [PubMed] [Google Scholar]
- 8.van Dam RM, Willett WC, Rimm EB, et al. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 9.Villegas R, Shu XO, Gao YT, et al. The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci. 2006;3:152–159. doi: 10.7150/ijms.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulze MB, Manson JE, Willett WC, et al. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia. 2003;46:1465–1473. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- 11.Hopping BN, Erber E, Grandinetti A, et al. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in hawaii. J Nutr. 2010;140:68–74. doi: 10.3945/jn.109.112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erber E, Hopping BN, Grandinetti A, et al. Dietary Patterns and Risk for Diabetes: The Multiethnic Cohort. Diabetes Care. 2010;33:532–538. doi: 10.2337/dc09-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uribarri J, Cai W, Sandu O, et al. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 2005;1043:461–466. doi: 10.1196/annals.1333.052. [DOI] [PubMed] [Google Scholar]
- 14.Hua NW, Stoohs RA, Facchini FS. Low iron status and enhanced insulin sensitivity in lactoovo vegetarians. Br J Nutr. 2001;86:515–519. doi: 10.1079/bjn2001421. [DOI] [PubMed] [Google Scholar]
- 15.Salonen JT, Tuomainen TP, Nyyssonen K, et al. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ. 1998;317:727–730. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 17.LeDoux SP, Woodley SE, Patton NJ, et al. Mechanisms of nitrosourea-induced beta-cell damage. Alterations in DNA. Diabetes. 1986;35:866–872. doi: 10.2337/diab.35.8.866. [DOI] [PubMed] [Google Scholar]