Soil actinomycetes produce a wide variety of secondary metabolites which may provide growth advantages in their microenvironments. While bacterial secondary metabolites are widely known for antibiotic activities, some metabolites may play roles beyond the existing paradigm. For example, it is possible that bacterial metabolites mediate interactions with plants and animals, bacterial signaling and quorum sensing.1 In fact, recent genome sequencing of Streptomyces griseus IFO 13350 revealed that this well-known producer of streptomycin possesses numerous as-yet uncharacterized biosynthetic gene clusters, such as terpenoid cyclases, nonribosomal peptide synthases (NRPS), polyketide synthases (PKS) and PKS-NRPS hybrids.2 In order to explore previously overlooked biosynthetic potential of soil bacteria, we examined soil samples from different parts of the State of New York. The study resulted in the identification of a Streptomyces strain that produces 17-hydroxycyclooctatin (1), a new member of the group of rare bacterial diterpenes with a fused 5-8-5 ring system. Here, we describe the isolation and somewhat unusual structural elucidation of 1.
Soil samples were collected from different places in New York State, including Lake Oakland, Phoenicia, and Bayside. Sixteen different strains were isolated. LC/MS-based screening of culture supernatants indicated the production of secondary metabolites by one strain, MTE4a, which was identified as a Streptomyces strain based on the sequence of 16S rRNA. The culture broth was extracted with ethyl acetate. The crude extract was fractionated by silica gel chromatography, followed by HPLC using C-18 column, to obtain 17-hydroxycyclooctatin (1) as a colorless solid.
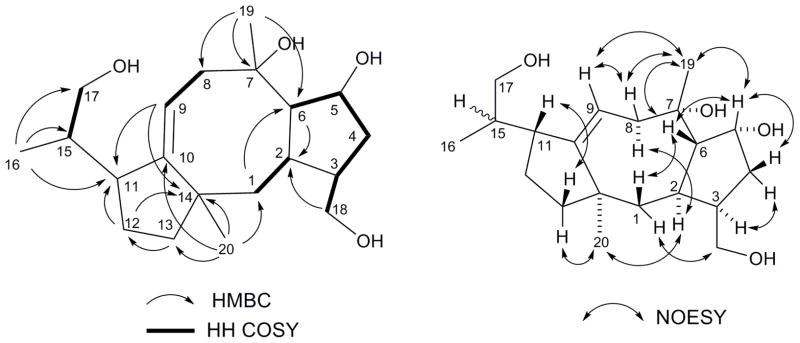
The molecular formula of the purified material was deduced to be C20H34O4 based on the molecular ions observed in the negative HRESIMS: namely, m/z 372.2155 [M+Cl]− (calcd 373.2151), m/z 383.2446 [M+HCOO]− (calcd 383.2439), and m/z 451.2319 [M+CF3COO]− (calcd 451.2313). The positive HRESIMS, on the other hand, showed only dehydrated ions at m/z 321.2425 [M+H-H2O]+ (calcd 321.2424) and m/z 303.2318 [M+H-2(H2O)]+ (calcd 303.2319), suggesting the presence of at least two hydroxyl groups. The 1H, 13C, DEPT, and HSQC spectra in chloroform-d were then used to identify twenty carbon pieces, which included two olefinic carbons (δC 119.6 and 154.0 ppm), four oxygen bearing carbons (δC 77.2, 75.3, 65.9 and 63.5 ppm), and three methyl carbons (δC 26.7, 25.1, and 17.1 ppm). HMBC (10 Hz) correlations from the three methyl groups, followed by COSY analysis led to the assembly of individual carbon pieces into larger fragments except for a methylene carbon at 24.8 ppm. Further analyses of HMBC unequivocally led to the elucidation of a diterpene framework with a fused 5-8-5 ring system (Figure 1 and Table 1).
Figure 1.
Structural elucidation of 1. Shown are key COSY, HMBC, and NOESY correlations. The relative stereochemistry of C-15 could not be conclusively determined due to the rotational flexibility around the C-11/C-15 bond.
Table 1.
The 13C-NMR dataa of 17-hydroxycyclooctatin (1) and cyclooctatin (2).
| 1 | 1 | 2 | |
|---|---|---|---|
| Position | δC (CDCl3) | δC (CD3OD) | δC (CD3OD) |
| 1 | 44.5 | 45.5 | 45.6 |
| 2 | 34.8 | 35.8 | 35.8 |
| 3 | 44.4 | 44.9 | 44.9 |
| 4 | 39.2 | 39.7 | 39.7 |
| 5 | 75.3 | 75.7 | 75.7 |
| 6 | 57.0 | 57.9 | 58.0 |
| 7 | 77.2 | 78.4 | 78.4 |
| 8 | 41.9 | 42.2 | 42.2 |
| 9 | 118.8 | 119.6 | 119.1 |
| 10 | 153.1 | 154.0 | 154.5 |
| 11 | 46.0 | 45.9 | 45.9 |
| 12 | 45.7 | 46.6 | 46.6 |
| 13 | 24.8 | 25.3 | 24.3 |
| 14 | 51.2 | 52.3 | 55.1 |
| 15 | 37.4 | 38.8 | 30.2 |
| 16 | 17.1 | 17.3 | 17.8 |
| 17 | 65.9 | 65.6 | 22.5 |
| 18 | 63.5 | 63.4 | 63.4 |
| 19 | 26.7 | 26.7 | 26.7 |
| 20 | 25.1 | 25.9 | 25.2 |
Recorded at 125 MHz.
Relative stereochemistry was then characterized based on NOESY (Figure 1). NOESY correlations on the β-face (H-1b/H-6; H-6/H3-19; H3-19/H-9) as well as those on the α-face (H-2/H-8a/H3-20) suggested a distinct U-shaped conformation of the central 8-membered ring as well as the ring junctions as shown in Figure 1. The relative stereochemistry at C-15, however, could not be conclusively determined due to the conformational flexibility between C-11 and C-15.
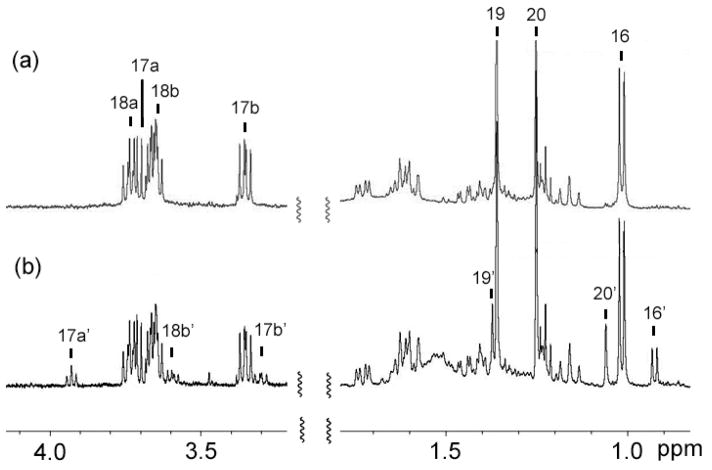
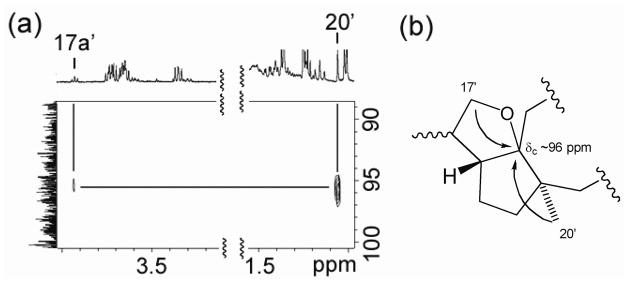
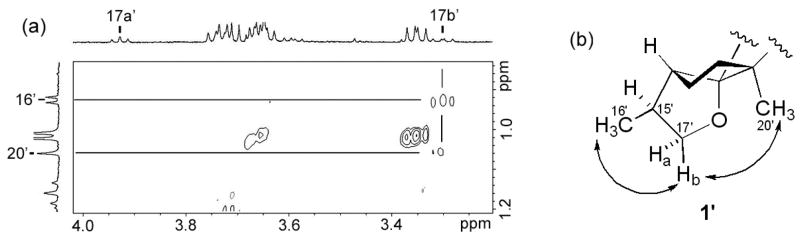
Assignment of the C-15 configuration, however, was accomplished in an unusual manner using a decomposition product of 1 in chloroform-d. While the NMR sample was stored at 4 °C for one week, a fraction of 1 turned into another compound (1′) probably because of the trace amount of acid in chloroform-d (Figure 2). Although many NMR signals of this decomposition product were buried under the signals of 1, it was possible to identify the 1H-signals of three methyl groups (H3-16′, H3-19′, and H3-20′) and two oxygenated methylene groups (H-17a′, H-17b′ and H-18b′) (Figure 2). A close examination of HMBC revealed that from H3-20′ and H-17a′ of 1′ showed correlations to a carbon at ~96 ppm (Figure 3a). These HMBC correlations were consistent with a cyclized structure, in which the 13C-NMR chemical shift of the oxygenated quaternary carbon is estimated to be ~100 ppm according to ChemBioDraw Ultra 11 (Figure 3b). NOESY spectrum was then re-analyzed to characterize the C-15′ stereochemistry of this cyclized compound. The analysis revealed that both H3-20′ and H3-16′ had NOESY correlations to H-17b′ but not to H-17a′ (Figure 4a). These results suggested the partial structure of 1′ as shown in Figure 4b, in which H3-16′ was positioned closer to H-17b′ than to H-17a′. Thus, the relative stereochemistry of C-15 in 1 was determined as shown in Figure 5. In order to confirm the reproducible production of 1 by MTE4a as well as its chemical structure. The isolation of 1 from the culture broth was repeated. NMR analyses of 1 in methanol-d4 (see Supplementary data), in which 1 does not decompose, led to the same structure as shown in Figure 5.
Figure 2.
Decomposition of 1 in chloroform-d. (a) Initial 1H-NMR. (b) After storage for 1 week at 4 °C.
Figure 3.
Structural analysis of 1′. (a) HMBC correlations to a carbon at δC ~96 ppm were observed from H3-20′ and H-17a′. (b) The cyclized structure consistent with the observed HMBC signals. ChemBioDraw Ultra 11 estimated δC ~100 ppm for the oxygenated quaternary carbon of this cyclized structure.
Figure 4.
Characterization of C-15′ relative stereochemistry. (a) NOESY correlations were observed to H-17b′ from H3-16′ and H3-20′. (b) The partial structure of 1′ consistent with the NOESY data.
Figure 5.
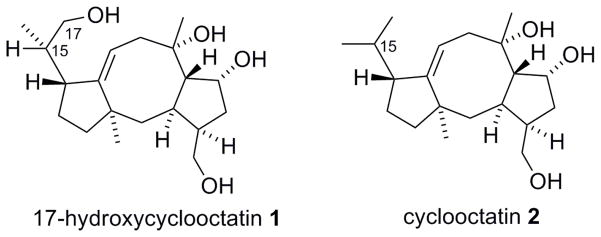
Structure of 1 from Streptomyces sp. MTE4a, and its known congener, cyclooctatin (2) from S. melanosporofaciens MI614-43F2.
It is noted that bacterial production of terpenoids with fused 5-8-5 ring framework is rare. Such framework is more commonly produced by fungi and insects.3 The only other example of the bacterial production of a member of this class of compounds is cyclooctatin (2) (Figure 5), a lysophospholipase inhibitor from Streptomyces melanosporofaciens MI614-43F2.4, 5 The elucidated structure of 1 closely resembles cyclooctatin. In fact, the 13C-NMR signals of the two compounds are almost identical except for the 17-position (Table 1). The close structural similarity suggests that the two compounds are produced through the same biosynthetic pathway in the respective producing strains,6 but strain MTE4a introduces an hydroxyl at the C-17 position.
If we consider the typical soil environment, which can turn acidic (pH 5-6), the production of an acid sensitive compound by soil bacteria is a peculiar finding. Although our preliminary studies suggest that 1 has weak antibacterial activity against Staphylococcus aureus (Figure S1), its true biological activities may be revealed after acid catalyzed transformation.
In conclusion, we isolated and determined the structure of 17-hydroxycyclooctatin (1) from Streptomyces sp. MTE4a. It is interesting to note that due to its chemical structure and reactivity it undergoes facile transformation under mildly acidic environment.
Experimental Section
General Experimental Procedures
NMR spectra were recorded on Brüker Avance 500MHz Spectrometer equipped with a dual [13C, 1H] CryoProbe. Data were acquired and processed with the Brüker XWIN-NMR software package. LC/MS analysis was carried out with an Agilent Technologies 6210 Time-of-Flight mass spectrometer equipped with an Agilent Technologies 1200 capillary HPLC system. Chromatography was performed on a Zorbax 0.5×150 mm SB-C18 column (#5064-8256) using water containing 0.1% formic acid and 50 μM ammonium formate (Solvent A) and methanol containing 0.1% formic acid and 50μM ammonium formate (Solvent B) at a flow rate of 12 μl/min. The gradient program was as follows: 10% B (0–2min), 10–100% B (2–20 min), 100% B (20–50 min). The temperature of the column was held at 40°C for the entire run. Sample ionization was accomplished using an Agilent Technologies Electrospray source with data collection in both positive and negative ion modes. Ionization source parameters were the following: nebulizer pressure of 20 psi, drying gas temperature of 300°C, drying gas flow rate of 8.0 L/min, and capillary voltage was set to 3500V. The mass spectrometer was set to acquire data with a fragmentor voltage of 165V. A mass range of 100 to 3200 m/z was scanned using 10,000 transients per scan. All solvents for purification were in the HPLC grade and were purchased from VWR and Fisher Scientific. Unless specified otherwise, all other chemicals and reagents were obtained through Fisher Scientific and used without further purification.
MTE4a collection and culture
MTE4a was isolated from the soil sample obtained from Phoenicia, NY. Glycerol-yeast extract agar supplemented with cyclohexamide (50 mg/L) was used for the isolation of actinomycete strains. Purification of the isolated culture was performed using soy flour mannitol (SFM) agar (soy flour 20 g/L; mannitol 20 g/L; agar 20 g/L). 50 ml of Difco nutrient broth (3g of beef extract, 5g of peptone per liter) were then inoculated with spores and shaken at room temperature at 200 rpm for 3 days. On the third day 10 ml of the inoculum were added to 125 ml of sterile MTF1 (250 mL flasks). MTF1 medium was prepared by mixing yeast extract (1 g), tryptone (10 g), K2HPO4 (0.5 g), glycerol (8 g), dextrose (2 g) in 1 liter of distilled water (adjusted to pH 7.1-7.3). The cultures were grown at room temperature and 200 rpm for seven days and the supernatant was collected.
Purification of 1
7 L of MTE4a culture was centrifuged for 30 minutes at 6,000 rpm at 4 °C. The supernatant (~6 L) was then extracted with the 6 L of EtOAc. The extract was separated by silica gel chromatography using step gradient from CH2Cl2 to 10% MeOH in CH2Cl2. The fraction containing 1 was eluted with 10% MeOH in CH2Cl2. The fraction was further purified with RP-HPLC (C18, 4.6×150 mm, 1 ml/min, 20–80% MeOH aq. over 30 min). HPLC eluent was monitored with photodiode array (PDA) detector and evaporative light scattering detector (ELSD). The major ELSD peak at 22.5 min was collected and dried to give a colorless and clear solid of 1 (2 mg).
Supplementary Material
Acknowledgments
We thank CUNY Community College Collaborative Research Incentive Grant. RR-03037 from NCRR/NIH, which supports the research infrastructure at Hunter College, is also acknowledged.
Footnotes
“Dedicated to Dr. Koji Nakanishi of Columbia University for his pioneering work on bioactive natural products.”
Supporting Information Available. NMR spectra and a preliminary characterization of antimicrobial activity of 1 are available free of charge at http://pubs.acs.org.
References and Notes
- 1.Waters CM, Bassler BL. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, Yamashita A, Hattori M, Horinouchi S. J Bacteriol. 2008;190:4050–4060. doi: 10.1128/JB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au TK, Chick WS, Leung PC. Life Sci. 2000;67:733–742. doi: 10.1016/s0024-3205(00)00668-8. [DOI] [PubMed] [Google Scholar]; Ballio A, Brufani M, Casinovi CG, Cerrini S, Fedeli W, Pellicciari R, Santurbano B, Vaciago A. Experientia. 1968;24:631–635. doi: 10.1007/BF02153818. [DOI] [PubMed] [Google Scholar]; Rios T, Quijano L. Tetrahedron Lett. 1969;17:1317–1318. doi: 10.1016/s0040-4039(01)87873-4. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama T, Naganawa H, Muraoka Y, Aoyagi T, Takeuchi T. J Antibiot (Tokyo) 1992;45:1703–1704. doi: 10.7164/antibiotics.45.1703. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi T, Aoyama T, Kojima F, Hattori S, Honma Y, Hamada M, Takeuchi T. J Antibiot (Tokyo) 1992;45:1587–1591. doi: 10.7164/antibiotics.45.1587. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Zhao P, Igarashi M, Sawa R, Tomita T, Nishiyama M, Kuzuyama T. Chem Biol. 2009;16:736–743. doi: 10.1016/j.chembiol.2009.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.