Abstract
The chemistry of naturally-occurring compounds has long been pursued in the search for medicines, dyes, pesticides, flavors, and fragrances. In addition, the deeper aim of understanding life itself as a chemical phenomenon has motivated generations of scientists. One consequence of such studies has been the realization that natural products often serve central roles as biological signaling agents. We consider natural products from the viewpoint of the organisms that produce and/or respond to them, and suggest how a naturally-occurring compound may acquire its role in chemical communication.
Why explore Nature's chemistry? For at least two centuries, the desire to describe and understand living organisms at the molecular level, often closely coupled with the aim of advancing medical science, has driven the study of natural products chemistry. The basic disciplines of organic chemistry and biochemistry, as well as molecular biology and chemical biology have all sprung from this effort. It was the development of structural and stereochemical theory in the second half of the 19th century that provided the basic concepts essential for genuine progress in these endeavors. Subsequent spectacular theoretical and experimental advances in spectroscopy and mass spectrometry, along with X-ray crystallography, completely revolutionized the art of structure determination. Consequently, the beautiful but often convoluted logic of interpreting meticulously executed chemical transformations was replaced by a variety of powerful physical methods. The discovery and refinement of chromatographic techniques made possible the separation and characterization of individual components in even the most complex mixtures. As a result of all of these advances, the amounts of an organic compound needed for structure determination has been reduced from grams to milligrams, and then to micrograms. Finally, advances in the art of organic synthesis made chiefly in the 20th century, including the ability to control stereochemical outcomes, have enabled chemists to synthesize almost any desired natural product-related target molecules (typically referred to now as “small molecules”). This essay examines the frequently underappreciated role played by natural products in biological chemical communication, and suggests how some naturally occurring compounds may have acquired their signaling function.
While the chemical community occupied itself largely with the pursuit of naturally occurring drugs, flavors, fragrances, and colors, it showed remarkably little interest in exploring the reasons (if any) for the very existence of most natural products. The discovery, isolation, characterization, and production of compounds useful to mankind, such as indigo, penicillin, vancomycin, vinblastine, and artemisinin, has proven to be a full time, incredibly productive occupation. Consequently, it has been chiefly the biologists who asked and answered the question of the raison d'être of natural products, thereby laying the groundwork for our current understanding of the actual roles played by these intriguing compounds in the lives of the organisms that produce them.1 These small molecules, also referred to as “secondary metabolites,” are now recognized as performing a multitude of vital functions for their producers (or as it is now turning out, their producers' hosts), including serving as quorum sensing agents among bacteria, as algal and fungal gamete attractants, as sex attractants and alarm pheromones in many insect species, as attractants to plant pollinating organisms, as plant and animal defensive chemicals, etc., etc. It is now well established that organic chemistry lies at the heart of biotic interaction.2
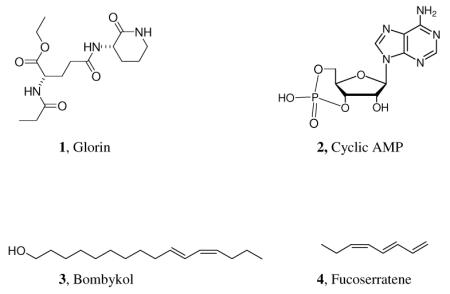
The structures of many biologically significant signal molecules, as well as their biosynthesis and the information that they transmit are now well known. It is therefore possible to ask how (or whether) the messages that are transmitted chemically are related to the structures of the messenger molecules. Surprisingly, this question does not seem to have been answered, or even explicitly asked. While we know that most organisms are “speaking” to one another using a “chemical language,” there has been little or no discussion of how the vocabulary of that language may have evolved. Why is it, for example, that the dipeptide glorin (1) induces underfed, free living cells of the slime mold Polysphondylium violaceum to aggregate into a slug, while the completely unrelated cyclic-AMP (2) serves the same role for another slime mold species, Dictyostelium discoideum?3,4 Why does bombykol (3) serve to inform and excite a male silkworm moth?5 How is it that fucoserratene (4) attracts sperm to swim towards eggs of the brown alga, Fucus serratus?6 In his searching study of nucleic acid chemistry, Albert Eschenmoser has been able to demonstrate why nucleic acid structures are particularly well suited to perform as bearers of genetic information.7 We have no comparable knowledge of how particular members of the most important classes of small signal molecules (peptides, isoprenoids, polyketides …) which function as pheromones or allelochemical agents have come to play the roles that they do. We would like to suggest here how the choice of some of the small molecules which transmit information to particular recipients may have come about, and, how the origins of biotic chemical communication may be understood.
In the most transparent examples of organisms gaining information from their chemical environment, the signal molecules are themselves compounds of intrinsic significance as potential nutrients or as repellants, serving to identify attractive or dangerous environments. It is instructive to begin our examination of the structural vocabulary, or chemical space, used in chemical sensing with a consideration of the phenomenon of bacterial chemotaxis, perhaps the best understood example of how an organism detects and responds to chemical stimuli.8 Escherichia coli possesses receptor proteins which, upon binding to any of a small group of carbohydrates, initiate a cascade of reactions which results in the bacterium continuing its “swimming” in a straight line. If the concentration of the stimulus increases during this swim, the action is prolonged (compared to an unstimulated swim). If, however, the stimulus concentration decreases, the swim is cut short, the bacterium “tumbles,” and a new swim is initiated in a randomly chosen direction. Overall, when paths rewarded by increasing stimulus concentration are lengthened, and those resulting in decreasing stimulus are shortened, this behavior, described as a “biased random walk,” guides the bacterium into a nutritionally favorable environment. (Interestingly, while D-galactose, for example, serves as a positive stimulus in this context, the chemotactic response does not depend on the bacterium's ability to take up D-galactose or to metabolize it.) This type of positive chemotactic response to simple sugars (and also to amino acids) provides a good example of adaptive behavior guided by a chemical signal. Potentially harmful substances, such as acetic acid or ethanol, when detected by E. coli, lead to a shortening of the swims responsible for increasing stimulus concentration. In summary, these molecular signals, which represent themselves, help bacterial cells to find rewarding environments and to stay out of harm's way. The ability to sense and profit from environmental chemical information seems to be a universal characteristic of living organisms.
It is useful to consider every individual organism as a chemical Sherlock Holmes, often exposed to a highly complex and ever-changing mixture of compounds derived from both biotic and abiotic sources, and constantly seeking clues from this chemical information. Most of these encountered compounds will have no special significance for our Holmesian subject, and there will have been no selective pressure for their detection. However, as we have already seen, certain compounds, such as nutrients and irritants, will have genuine importance. Consequently, the development of suitably specific receptors for such compounds would be highly beneficial. As an example, it has been shown recently that certain fish have receptors specifically tuned to detect (and thereby induce avoidance of) high toxic exudates of sponges that they are likely to encounter.9
For species dependent on sexual reproduction, mate location takes on great importance. Chemical cues that help with this function are frequently encountered, and have been extensively studied, especially in the world of insects. It is hard to imagine how male and female moths, non-social, nocturnal and quiet creatures that they are, would ever find potential mates were it not for the exquisitely effective sex pheromones emitted by virgin females. These chemically simple signals, composed largely of carefully regulated mixtures of twelve to twenty-carbon straight chain aliphatic compounds produced by female moths and characteristic of each species, serve to induce up-wind flight by their male counterparts. To reduce the likelihood that these signals would be detected and exploited by predators, it is valuable for a “calling” female to release only the minimum effective quantity of her pheromone. Consequently, for the male pheromone recipient, there would be a clear advantage to being able to detect the smallest possible amount of the calling female's signal. Obviously, there are certain physical and chemical characteristics that any component of a moth sex attractant would need to have: for example, it must be suitably volatile, it must have a certain degree of stability, and it must have a structure accessible to the moth's biosynthetic capability. But beyond this, it could be almost anything. So, why have female Bombyx mori ended up using bombykol as their sex attractant? Remarkably enough, the reasons why bombykol and its relatives have been selected to serve as moth female pheromones remain completely unknown, even though it is over a half century since bombykol was first characterized and synthesized. However, an unexpected insight into the choice of an entirely unrelated pheromone structure can be gained by considering what we have learned from the study of a different lepidopteran signaling system, one in which we can see females exercising Darwinian sexual selection on the basis of a male courtship pheromone.2
The example we want to examine concerns the response of the female arctiid moth Utetheisa ornatrix to its corresponding male courtship pheromone, the pyrrolizidine aldehyde hydroxydanaidal (5).2 This pyrrolizidine is applied to the female's antennae by a courting male, and it induces mating behavior in the female. Here, we are dealing with a signal compound which is neither a potential nutrient nor one known to be an irritant. We have shown that the courting male needs to have ingested and sequestered a plant-produced pyrrolizidine alkaloid, such as monocrotaline (6), in order to carry out the multi-step biosynthesis of this pheromone. How can we understand a female's reliance on the antennal signal induced by this heterocyclic hydroxyaldehyde in selecting a suitable male with which to mate?
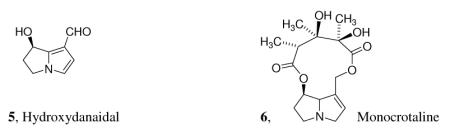
The evolution of this communication system is best understood by considering the interaction from the female's (the signal recipient's) viewpoint. To begin with, it is important to note that U. ornatrix caterpillars feed preferentially on the seeds of Crotalaria spectabilis. These seeds may contain up to 5% of the hepatotoxic (for mammals) alkaloid, monocrotaline (6). This alkaloid normally is sequestered by the moths of both sexes. It is passed along from caterpillars to adults, who gain protection from predators such as spiders, by virtue of their alkaloid content. Remarkably enough, chemically protected females can also protect their offspring from ladybird beetle predation by incorporating monocrotaline into their eggs, rendering them unpalatable. However, not all individuals are equally successful in acquiring this dietary alkaloid, and chemically unprotected females can no longer endow their eggs with the alkaloid that would protect them. In addition, females without alkaloid are themselves no longer distasteful to spiders. All is not lost, however, since a monocrotaline-containing male transfers an alkaloid-laden spermatophore to the female upon mating. Consequently, an alkaloid-deficient female who has not been successful in sequestering monocrotaline from her diet is still able to acquire this valuable chemical defensive agent with which to protect both herself and her offspring by mating appropriately. Of course, mating with a male which had not been successful in acquiring alkaloid would still leave an unprotected female vulnerable. The ability of a female to ascertain the defensive status of a courting male is, therefore, a very valuable trait.
In fact, it does turn out that U. ornatrix females mate preferentially with alkaloid-containing males. In spite of a courtship that may last for only about 10 seconds, she is able to identify and favor a chemically protected male over an unprotected male by virtue of the pheromonal signal presented to her by the former. The fact that the pheromone, hydroxydanaidal, cannot be produced unless the male has acquired a supply of its essential alkaloidal biosynthetic precursor provides the female with unambiguous evidence of the male's suitability as a mate. Interestingly, U. ornatrix females, given the opportunity, mate promiscuously with a number of males, thereby increasing their supply of defensive alkaloid, even though a single mating supplies more than enough sperm to fertilize all of their eggs. In summary, a female able to detect and respond to a male who can serve as a source of pyrrolizidine alkaloid enjoys a significant advantage over one who cannot.
Turning to the male side of the signaling system, what can be said about hydroxydanaidal biosynthesis? It is reasonable to assume that an herbivore living on a toxic plant needs to develop a mechanism to metabolize or excrete the toxin in order to avoid intoxication. This necessity has nothing at all to do with “intentional” synthesis of a chemical courtship signal. Nevertheless, if dietary monocrotaline (or any related pyrrolizidine alkaloid) is degraded to a metabolite such as hydroxydanaidal, which is released into the environment, this metabolite could then be encountered and responded to by any other organism in the environment. While hydroxydanaidal may not mean anything at all or have any relevance to individuals belonging to most taxa, it could serve as a clue to alert a U. ornatrix female to the presence of a chemically protected conspecific male. We have already seen that from a female's point of view, the reward for the recognition of and positive response to such a signal could be significant, so that the selective pressure favoring females with this capacity would be considerable. Although hydroxydanaidal in this example is neither intrinsically a potential nutrient nor a harmful agent, its exploitation by the female recipient as an indicator of a male's defensive status makes perfect sense.
The important lesson to be learned from this example, is that these individual insects are capable of using a chemical cue (in this example, an alkaloid metabolite) which is neither in itself of direct nutritional nor of harmful significance, to guide their behavior in an adaptive way. As all organisms pursue their lives, they encounter environmental information which presents itself to their senses of smell, taste, sight, hearing, and touch. Their behavior and/or development may be influenced by any of these potential stimuli. In this context, each individual has the possibility of responding adaptively to any outside stimulus it is able to detect. We may regard these responses simply as “doing what comes naturally.” In many cases, the greater the sensitivity, dynamic range, and specificity of a detection system, the greater the advantage to the individual will be. It is also important to note that the ability to detect specific mixtures of chemical components adds greatly to the number of characteristic messages that can be composed from a given number of chemical components.
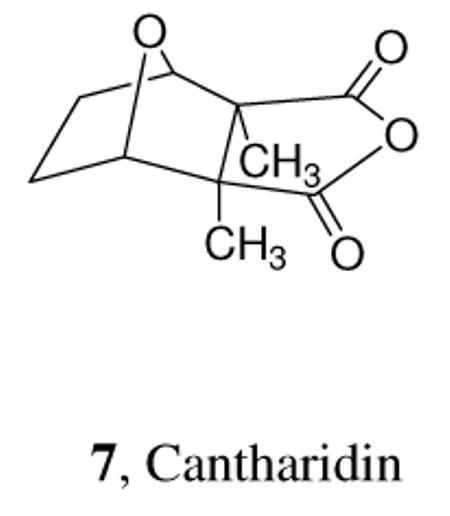
In the bacterial example, positive chemotactic signals are themselves simply potential nutrients. In a similar way, noxious compounds such as formic acid or hydrogen cyanide serve as negative chemotactic signals and find use as defensive agents. Surprisingly, such noxious compounds may also serve as pheromonal signals, as in the case of cantharidin and the pyrochroid beetle Neopyrochroa flabellata, which uses the dietarily acquired, dangerously vesicant isoprenoid cantharidin (7) first as a male courtship pheromone, then as a protective agent passed from male to female during mating, and ultimately for protection of the beetles' eggs.2
There are undoubtedly very many other instances of what we may think of as “inadvertent” or “unintentional” biotic signaling, in which a recipient makes use of a chemical cue released as a consequence of another individual's activity. The response may or may not be to the advantage of the originator of the cue. An intriguing case of what may be considered as unintended signaling by humans was examined from a legal viewpoint in 1988 by the U.S. Supreme Court.10 The court was concerned with the legality of using evidence of clandestine drug-related activities discovered by the San Francisco police in the garbage discarded by a suspect. The defense argued that the examination of the contents of discarded garbage by police constituted an illegal, unwarranted violation of the defendants'privacy. However, the court ruled that there can be no expectation of privacy with respect to the garbage which we throw away; anyone interested in examining what we discard may do so, and may use the information gained freely. In this decision, the court recognized the plain truth that materials released by an organism into the environment may provide information to any other organism that encounters them. Chemical privacy does not exist.
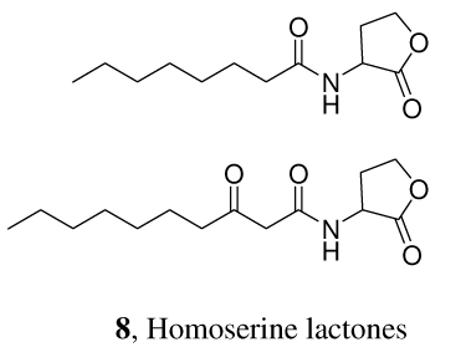
Not enough is known about the biology and chemistry of most species to be able to say to what extent the semiochemicals to which they respond may be seen simply as indicators of normal characteristic activities of their conspecifics or of other species of interest. The courtship of U. ornatrix certainly may be understood in this way. However, we do not know, for example, why the crucially important N-acyl homoserine lactones (8) have been recruited for use in Gram-negative bacterial quorum sensing.11 Might the production and release of aggregation pheromones 1 and 2 be direct metabolic consequences of slime mold under-nutrition? Might the sex attractants 3 and 4 be related to chemical by-products of moth and algal egg production? Questions of this sort suggest countless new lines of research which may possibly illuminate the basis of many chemical signaling mechanisms. The origin of most of the structural vocabulary of biological chemical communication is currently obscure, and it is likely that there are a variety of mechanisms that have led to the evolution of the semiochemical vocabulary. But we can hope that significant progress in understanding nature's chemical language will flow from future research into the possible intimate relationship between an organism's performing of some essential function, or its attainment of a particular physiological state, and the associated production and release of telltale “indicator” compounds into the environment. “Meaning” (with respect to the recipient of any signal) is then bestowed upon these indicator compounds by the responding individuals. It is important to note that different recipients may read quite different meanings into the message carried by the same chemical cue. As an example, a bark beetle aggregation pheromone may be exploited as a kairomone leading to a source of food by predators of bark beetles.
Francis Crick has commented that
“The visual system has evolved to detect those many aspects of the real world that, in evolution, have been important for survival, such as the recognition of food, predators, and possible mates. Evolution will latch onto any features that will give useful information.”12
Chemical sensing systems perform exactly these functions, and many more, as well. They differ from the visual system only in that they derive their input information from molecules rather than from light. The potential impact of natural products research on our understanding of chemistry, ecology, and evolution, as well as on the practice of medicine, agriculture, forestry, and environmental science can hardly be overestimated. In this context, the elucidation of Nature's chemistry, especially carried out in conjunction with the study of relevant receptor and transducer systems, clearly constitutes one of mankind's great intellectual pursuits.
Acknowledgment
Our research on the subject of chemical signaling has been supported for many years by grant no. 5R01GM5380 from the National Institutes of Health.
Footnotes
This paper is dedicated to Koji Nakanishi, a friend and colleague for well over a half-century, whose immense contribution to the field of Natural Products Chemistry has been unique.
References and Notes
- 1.Hartman T. Proc. Natl. Acad. Sci. USA. 2008;105:4541–4546. doi: 10.1073/pnas.0709231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meinwald J. J. Org. Chem. 2009;74:1813–1825. doi: 10.1021/jo802606t. [DOI] [PubMed] [Google Scholar]
- 3.Shimomura O, Suthers HLB, Bonner JT. Proc. Natl. Acad. Sci. USA. 1982;79:7376–7379. doi: 10.1073/pnas.79.23.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner JT, Barkley DS, Hall EM, Konijn TM, Mason JW, O'Keefe G, III, Wolfe PB. Dev. Biol. 1969;20:72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- 5.Butenandt A, Beckmann R, Stamm D, Hecker E. Z. Naturforsch. 1959;14B:283–284. [Google Scholar]
- 6.Müller DG, Jaenicke L. FEBS Letters. 1973;30:137–139. doi: 10.1016/0014-5793(73)80636-2. [DOI] [PubMed] [Google Scholar]
- 7.Eschenmoser A. Science. 1999;284:2118–2124. doi: 10.1126/science.284.5423.2118. [DOI] [PubMed] [Google Scholar]
- 8.Koshland DE., Jr. Bacterial Chemotaxis As A Model Behavioral System. Raven Press; New York: 1980. [Google Scholar]
- 9.Cohen SP, Haack KKV, Halstead-Nusslock GE, Bernard KF, Hatt H, Kubanek J, McCarty NA. Proc. Natl. Acad. Sci. USA. 2010;107:12339–12344. doi: 10.1073/pnas.1000343107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.California vs. Greenwood, Supreme Court of the United States, 486 U.S. 35. 1988 [Google Scholar]
- 11.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. FEMS Microbiol. Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 12.Crick F. What Mad Pursuit. Basic Books, Inc.; New York: 1988. p. 155. [Google Scholar]


