Abstract
Hallucinogenic drugs, including mescaline, psilocybin and lysergic acid diethylamide (LSD), act at serotonin 5-HT2A receptors (5-HT2ARs). Metabotropic glutamate receptor 2/3 (mGluR2/3) ligands show efficacy in modulating the responses induced by activation of 5-HT2ARs. The formation of a 5-HT2AR-mGluR2 complex suggests a functional interaction that affects the hallucinogen-regulated cellular signaling pathways. Here, we tested the cellular and behavioral effects of hallucinogenic 5-HT2AR agonists in mGluR2 knockout (mGluR2-KO) mice. Mice were intraperitoneally injected with the hallucinogens DOI (2 mg/kg) and LSD (0.24 mg/kg), or vehicle. Head-twitch behavioral response, expression of c-fos, which is induced by all 5-HT2AR agonists, and expression of egr-2, which is hallucinogen-specific, were determined in wild type and mGluR2-KO mice. [3H]Ketanserin binding displacement curves by DOI were performed in mouse frontal cortex membrane preparations. Head twitch behavior was abolished in mGluR2-KO mice. The high-affinity binding site of DOI was undetected in mGluR2-KO mice. The hallucinogen DOI induced c-fos in both wild type and mGluR2-KO mice. However, the induction of egr-2 by DOI was eliminated in mGlu2-KO mice. These findings suggest that the 5-HT2AR-mGluR2 complex is necessary for the neuropsychological responses induced by hallucinogens.
Keywords: Hallucinogenic drugs, LSD, Serotonin 5-HT2A receptor, Metabotropic glutamate mGlu2 receptor, G protein-coupled receptor (GPCR), Schizophrenia and psychosis
Hallucinogenic drugs, such as mescaline, psilocybin and lysergic acid diethylamide (LSD) induce profound alterations of human consciousness, emotion and cognition [12,16,27]. Inactivation of serotonin 5-HT2AR signaling by either genetic or pharmacological approaches results in markedly reduced behavioral responses to hallucinogenic drugs in both rodent models [10,18,34] and humans [33]. Thus, although hallucinogens bind other receptor subtypes [16], the 5-HT2A receptor is considered as necessary for the unique behavioral activity of these chemicals.
Metabotropic glutamate receptors mGlu2/3 have been the target of considerable attention regarding the molecular mechanism underlying psychosis [1,6,23,25]. We have recently reported that 5-HT2AR and mGluR2 are co-expressed in the same population of cortical neurons [14]. We found that 5-HT2AR and mGluR2 form a receptor complex in mouse and human brain, and activation of mGluR2 inhibits hallucinogen-specific neuronal signaling pathways [14]. Based on this and other findings [1,17,31], it has been proposed that mGluR2 agonists modulate, through a mechanism that requires the 5-HT2AR-mGluR2 complex, the signaling pathways induced by hallucinogenic 5-HT2AR agonists. Here we demonstrate that mice with disrupted mGluR2 signaling capacity (mGluR2-KO mice) are insensitive to the cellular and behavioral effects of hallucinogens. This observation suggests that the 5-HT2AR-mGluR2 complex, and not the 5-HT2AR alone, is the molecular target responsible for the actions of hallucinogenic drugs.
Experiments were performed on adult (10–14 weeks old) male 129S6/SvEv mice. 5-HT2A mice have been previously described [18,19]. mGluR2-KO mice were obtained from the RIKEN BioRe-source Center, Japan (see [26,36] for details). mGluR2-KO mice were backcrossed for at least ten generations onto a 129S6/SvEv background. All subjects were offspring of heterozygote breeding. Animals were housed at 12 h light/dark cycle at 23 °C with food and water ad libitum. The Institutional Animal Use and Care Committee approved all experimental procedures. 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI; Sigma–Aldrich) was dissolved in saline and injected intraperitoneally (i.p.). Lysergic acid diethylamide (LSD; Sigma-Aldrich) was injected i.p. after suspension in a minimal amount of DMSO and made up to volume with normal saline.
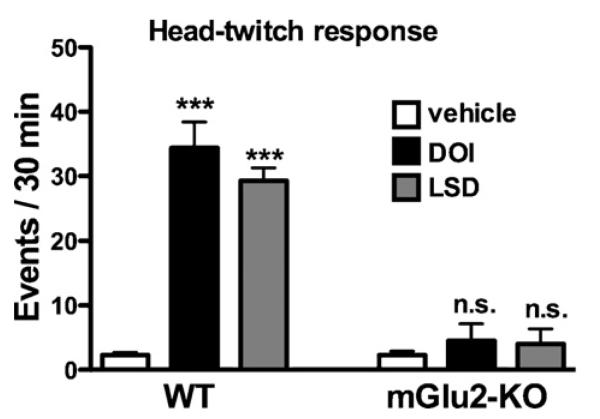
Head-twitch behavior is known to be reliably and robustly elicited by hallucinogenic 5-HT2AR agonists in rodents [18,19]. We first assayed the head-twitch response induced by DOI and LSD in wild type and mGluR2-KO mice (Fig. 1). Two-way ANOVA indicated a statistical significance for the effects of the treatment [F(2,19) = 31.05; p < 0.001] and genotype [F(1,19) = 74.10; p < 0.001]. Significance was also found for the interaction between treatment and genotype [F(2,19) = 20.05; p < 0.001]. The post hoc analysis revealed that DOI and LSD activated a significant head-twitch response in wild type mice (p < 0.001). Notably, no significant head-twitch response was detected in mGluR2-KO mice for any of these two agonists (p > 0.05).
Fig. 1.
Behavioral response to hallucinogens DOI and LSD. Wild type and mGluR2-KO mice (n = 4–5 per treatment group) were injected with vehicle, DOI (2 mg/kg) or LSD (0.24 mg/kg), and the head-twitch response was scored 15 min after injection for 30 min. ***p < 0.001; Bonferroni's post hoc test of two-way ANOVA. Data are means ± S.E.M.
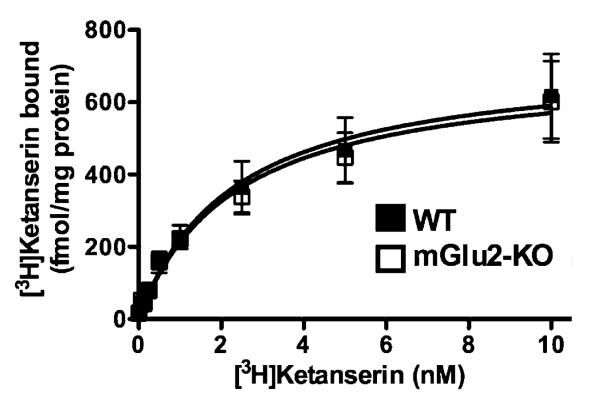
The decreased head-twitch response following administration of hallucinogens led us to examine the level of expression of 5-HT2AR in mGluR2-KO mice. Equilibrium binding saturation experiments were performed to determine the binding affinity (KD) and receptor density (Bmax) of 5-HT2ARs in wild type and mGluR2-KO mouse frontal cortex membrane preparations (Fig. 2; for experimental details, see [14]). Neither Bmax nor KD values of the binding of [3H]ketanserin, a 5-HT2AR antagonist, were significantly changed in mGluR2-KO mice, which demonstrates that level of expression of 5-HT2AR is not affected in the absence of mGluR2 (Bmax:wild type, 724.5±93 fmol/mg protein;mGluR2-KO, 701.5±80 fmol/mg protein. KD: wild type, 2.27±0.8 nM;mGluR2-87 KO, 2.30±0.71 nM).
Fig. 2.
Expression of 5-HT2AR in mGluR2-KO mice. [3H]Ketanserin binding saturation curves in wild type (black) and mGluR2-KO (white) mouse frontal cortex membrane preparations (n = 6 per group).
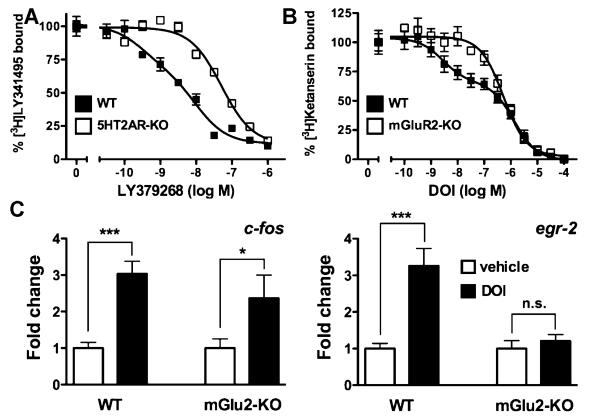
We next determined the affinity of the mGlu2/3 agonist LY379269 displacing [3H]LY341495 in wild type and 5-HT2AR-KO mice (Fig. 3A), and that of the 5-HT2AR agonist DOI displacing [3H]ketanserin binding in wild type and mGluR2-KO mice (Fig. 3B; for experimental details, see [14]). Competition binding experiments of [3H]LY341495 were best described by a two-site model in wild type mouse frontal cortex membrane preparations [F(2,28) = 4.71; p < 0.05]. However, displacement of [3H]LY341495 binding by LY379268 was best described by a one-site model in 5-HT2AR-KO mice [F(2,16) = 0.62; p = 0.55]. The low affinity binding site for LY379268 did not differ between wild type and 5-HT2AR-KO mice (Fig. 3A). Similarly, competition binding experiments of [3H]ketanserin were best described by a two-site model in wild type mouse frontal cortex membrane preparations [F(2,79) = 19.05; p < 0.001]. Interestingly, displacement of [3H]ketanserin binding by DOI was best described by a one-site model in mGluR2-KO mice [F(2,79) = 0.89; p = 0.41]. The low affinity site for DOI did not differ between the wild type and mGluR2-KO mice (Fig. 3B). The changes in high-affinity binding suggested that the cellular responses induced hallucinogenic 5-HT2AR agonists may be altered in mGluR2-KO mice. This hypothesis was tested by measuring the gene response to hallucinogens in mouse frontal cortex.
Fig. 3.
Cellular response to hallucinogenic 5-HT2AR agonist DOI. (A) LY379268 displacement of [3H]LY341495 binding was performed in wild type (black) and 5-HT2AR-KO (white) mouse frontal cortex membrane preparations. Competition curves were analyzed by nonlinear regression to derive dissociation constants for the high and low affinity states of the receptor. One-site model or two-site model as a better description of the data was determined by F test. Two-site model, p < 0.001. A two-site model provided a better description of the data in wild type mice. Wild type mice: Ki-high (logM), −9.51±0.45; Ki-low (logM), −7.95±0.26; % high-affinity binding sites, 36.6±1; and 5HT2AR-KO mice: Ki-low (logM), −7.60±0.07 (n = 3–5). (B) DOI displacement of [3H]ketanserin binding was performed in wild type (black) and mGluR2-KO (white) mouse frontal cortex membrane preparations. A two-site model provided a better description of the data in wild type mice. Wild type mice: Ki-high (logM), −8.56±0.28; Ki-low (logM), −6.31±0.16; % high-affinity binding sites, 35.9±4; and mGluR2-KO mice: Ki-low (logM), −6.72±0.08 (n = 5–6). (C) Cellular response in mouse frontal cortex assayed by qRT-PCR. Wild type or mGluR2-KO mice were injected with vehicle (white) or DOI (black; 2mg/kg). Changes in expression levels are reported as fold change over vehicle. *p < 0.05; ***p < 0.001; Bonferroni's post-hoc test of two-factor ANOVA (n = 5–6 per group). Data are means±S.E.M.
We have previously demonstrated that hallucinogenic 5-HT2AR agonists induce a unique pattern of gene expression in mouse cerebral cortex that predicts the behavioral effect of the tested ligand [18,19]. Here we examined the role of mGluR2 in the cellular responses induced by hallucinogens in mouse frontal cortex, a region involved in acute psychotic episodes [17]. As previously shown [18,19], the hallucinogen DOI induced expression of c-fos and egr-2 in wild type mice (Fig. 3C; for primer pair sequences, see [14]). In mGluR2-KO mice, we found that DOI also induces the expression of c-fos, whereas regulation of egr-2 was abolished (Fig. 3C).
This study demonstrates that mGluR2 is necessary for at least some of the cellular and behavioral responses induced by hallucinogenic 5-HT2AR agonists. We found that the head-twitch response was not produced by the hallucinogens DOI and LSD in mGluR2-KO mice. We also demonstrated that the hallucinogenic gene response signature required the expression of mGluR2, and that the high-affinity binding sites for LY379268 and DOI were undetectable in the absence of 5-HT2AR or mGluR2, respectively, in mouse frontal cortex. Although mouse models of neuropsychiatric disturbances have limitations [3,12], these findings suggest that the 5-HT2AR requires the expression of mGluR2 to induce hallucinogenic-dependent psychoactive states.
5-HT2AR and mGluR2 are members of the G protein-coupled receptor (GPCR) superfamily, also referred to as seven transmembrane receptors [28,29]. The ternary complex model (receptor [R], agonist, and G protein [G]) has typically been the most commonly used pharmacological model to describe the mechanism of activation of GPCRs [16,21]. In this model, the receptor exists in two conformational states: an inactive state, R, and an active state R*G. Agonists show a higher affinity for R*G than for R, whereas neutral antagonists show roughly equal affinities for the two states [21]. According to the ternary complex model, agonists present a biphasic pattern displacing radiolabeled antagonists (see also Fig. 3A and B), and the fraction of high-affinity binding site is decreased when R*G complexes are uncoupled by the non-hydrolyzable analog of guanosine triphosphate GTPγS [14,15]. However, recent findings provide evidence indicating that GPCR dimers lead to positive and negative ligand-dependent cooperative binding [2,4,20,24,32], for which theoretical pharmacological models have proposed GPCRs as dimeric/oligomeric structures [8,11,13]. We have previously demonstrated that mGluR2 activation increases the affinity of 5-HT2AR agonists, while, by contrasts, 5HT2AR activation decreases the affinity of mGluR2 agonists in mouse brain cortex and tissue culture preparations ([14]; similar results were obtained in the present study–data not shown). In frontal cortex membrane preparations, hereweshow that the biphasic displacement curve of [3H]ketanserin by DOI became monophasic in mGluR2-KO mouse, and that the biphasic displacement curve of [3H]LY341495 by LY379268 became monophasic in 5-HT2AR-KO mouse. Although further investigation is necessary, concurrently these binding data support the expression of 5-HT2AR and mGluR2 as a GPCR heterocomplex in mouse frontal cortex, and provide an explanation for the functional crosstalk observed between the components of the receptor complex.
The G protein subtypes activated by 5-HT2AR and mGluR2 are mainly Gq/11 and Gi/o, respectively [5,7]. However, some reports have implicated pertussis toxin-sensitive Gi/o proteins in the cel1lular responses mediated by 5-HT2AR activation [22,30]. In mouse cortical primary neurons, we previously discovered that the signaling elicited by hallucinogen and non-hallucinogen 5-HT2AR agonists causes induction of c-fos and requires Gq/11-dependent signaling [18]. However, the signaling of hallucinogenic 5-HT2AR agonists also induces egr-2, which is Gi/o-dependent [18]. Our data now demonstrate that the induction of c-fos by DOI was not affected. In contrast, the induction of egr-2 was abolished in mGluR2-KO mice, which provide the first demonstration that the hallucinogen-specific signaling signature is affected in the absence of mGluR2 in whole animal models, and suggest that the 5-HT2AR-mGluR2 complex is necessary for the responses induced by LSD-like drugs. It is important to note that the level of expression of 5-HT2AR as determined by [3H]ketanserin binding saturation curves was unaffected in mGluR2-KO mouse frontal cortex, which further supports the hypothesis that expression of mGluR2 is necessary for the cellular and behavioral effects induced by hallucinogenic 5-HT2AR agonists. Our data do not exclude the possibility that the closely related mGluR3 also plays a role in the unique effects induced by LSD-like hallucinogens. Thus, althoughmGluR2, and not mGluR3, has been shown to be responsible for the antipsychotic like responses induced by the mGlu2/3 agonists LY379268 [35] and LY404039 [9], further investigation is necessary to determine the pharmacological and behavioral effects of hallucinogens in wild-type and mGluR3-KO mice.
In conclusion, we have shown that the behavioral responses to hallucinogenic 5-HT2A agonists are absent inmGlu2-KOmice. The cognitive and perceptual changes induced by hallucinogenic drugs exhibit similarities with the endogenous psychosis of schizophrenia [12,17]. The level of expression of 5-HT2A and mGlu2 has been found to be dysregulated in postmortem human brain of untreated schizophrenic subjects [14]. Deciphering the molecular mechanism of action of hallucinogens should provide new inquiries to understand the molecular and cellular mechanisms that underlie the complex clinical phenotype of schizophrenia.
Acknowledgements
NIMH 5R01MH084894 (J.G.M.), NIDA P01 DA12923 (S.C.S.), NARSAD (J.G.M.), and the Maltz Family Foundation (J.G.M.) participated in the funding of this study. We are grateful to Dr. J.A. Gingrich for his gift of 5-HT2AR-KO mice. J.L.M was the recipient of a postdoctoral fellowship from Ministerio de Ciencia e Innovación, Spain.
References
- 1.Aghajanian GK. Modeling “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology (Berl.) 2009;206:575. doi: 10.1007/s00213-009-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, Barberis C, Durroux T. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol. Pharmacol. 2006;70:1783. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- 3.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J. Biol. Chem. 2001;276:22621. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- 5.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Conn PJ, Jones CK. Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology. 2009;34:248. doi: 10.1038/npp.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 8.Durroux T. Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol. Sci. 2005;26:376. doi: 10.1016/j.tips.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid ( LY404039) J. Pharmacol. Exp. Ther. 2008;326:209. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 10.Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II. Reassessment of LSD false positives. Psychopharmacology (Berl.) 1995;121:357. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- 11.Franco R, Casado V, Mallol J, Ferre S, Fuxe K, Cortes A, Ciruela F, Lluis C, Canela EI. Dimer-based model for heptaspanning membrane receptors. Trends Biochem. Sci. 2005;30:360. doi: 10.1016/j.tibs.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 2008;29:445. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Giraldo J. On the fitting of binding data when receptor dimerization is suspected. Br. J. Pharmacol. 2008;155:17. doi: 10.1038/bjp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ. Quantitative stoichiometry of G-proteins activated by mu-opioid receptors in postmortem human brain. Eur. J. Pharmacol. 2002;452:21. doi: 10.1016/s0014-2999(02)02242-2. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Maeso J, Sealfon SC. Agonist-trafficking and hallucinogens. Curr. Med. Chem. 2009;16:1017. doi: 10.2174/092986709787581851. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 2003;23:8836. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenakin T. Efficacy at G-protein-coupled receptors. Nat. Rev. Drug Discov. 2002;1:103. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- 22.Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J. Neurochem. 2003;86:980. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 23.Marek GJ. Metabotropic glutamate 2/3 receptors as drug targets. Curr. Opin. Pharmacol. 2004;4:18. doi: 10.1016/j.coph.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno JL, Sealfon SC, Gonzalez-Maeso J. Group II metabotropic glutamate receptors and schizophrenia. Cell. Mol. Life Sci. 2009;66:3777. doi: 10.1007/s00018-009-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4170. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols DE. Hallucinogens. Pharmacol. Ther. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Perez DM. The evolutionarily triumphant G-protein-coupled receptor. Mol. Pharmacol. 2003;63:1202. doi: 10.1124/mol.63.6.1202. [DOI] [PubMed] [Google Scholar]
- 29.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 30.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001;92:179. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 31.Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prezeau L. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28:2195. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 34.Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl.) 1990;100:417. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- 35.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl.) 2008;196:431. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 36.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]