Abstract
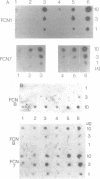
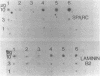
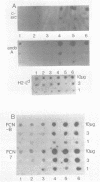
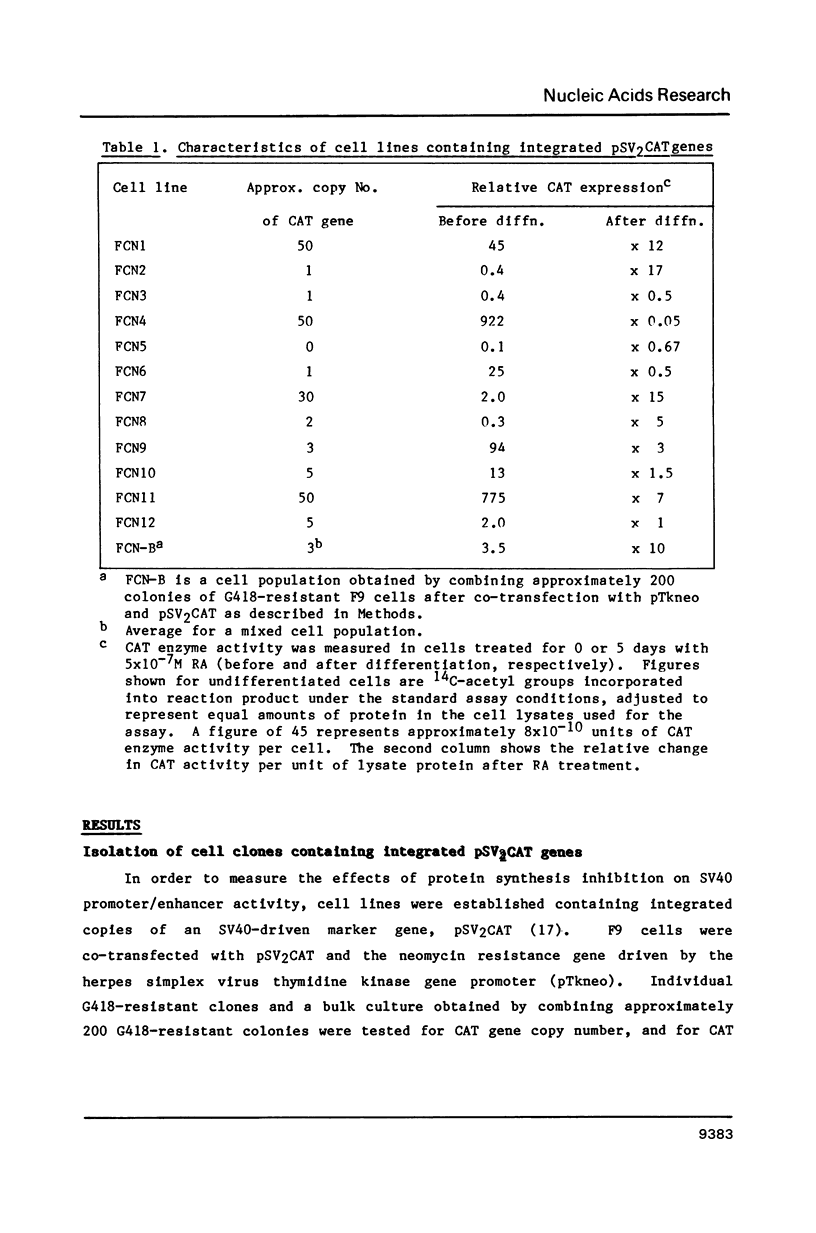
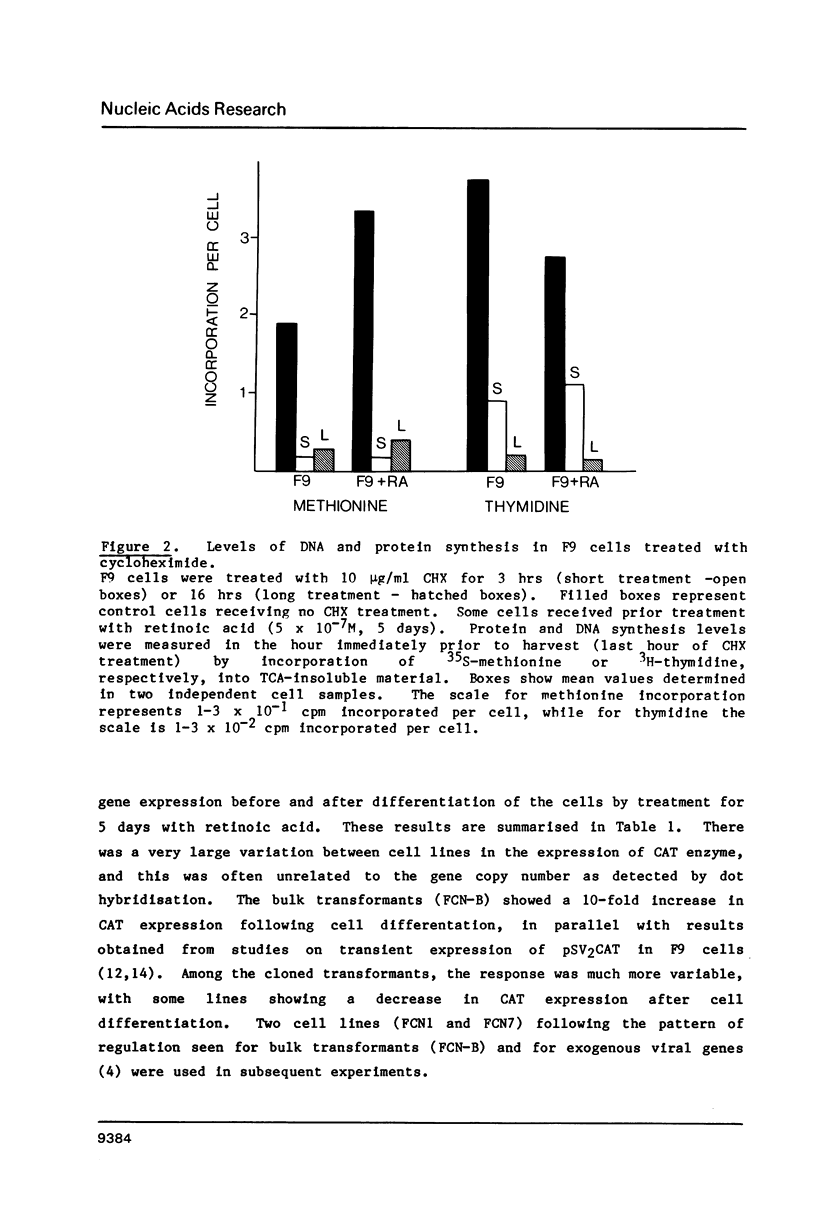
Expression of genes driven by the SV40 promoter/enhancer appears to be under net negative regulatory control in undifferentiated F9 cells, but not in their differentiated derivatives. In cells containing integrated copies of an SV40 promoter-driven marker gene, induction of differentiation by retinoic acid treatment produced a modest increase in transcription from the viral promoter. A much greater increase was observed when differentiated or undifferentiated cells were treated with the protein synthesis inhibitor, cycloheximide. If cycloheximide acts through removal of negative-regulatory molecule(s), then it is apparent that these molecules are present in both differentiated and undifferentiated cells, and that retinoic acid treatment removes only a portion of the total transcriptional repression. RNA levels from a variety of cellular genes activated during F9 cell differentiation were either unaffected or only slightly increased by cycloheximide treatment. This suggests important qualitative or quantitative differences in the regulation mechanism for viral and cellular genes in differentiating F9 cells.
Full text
PDF




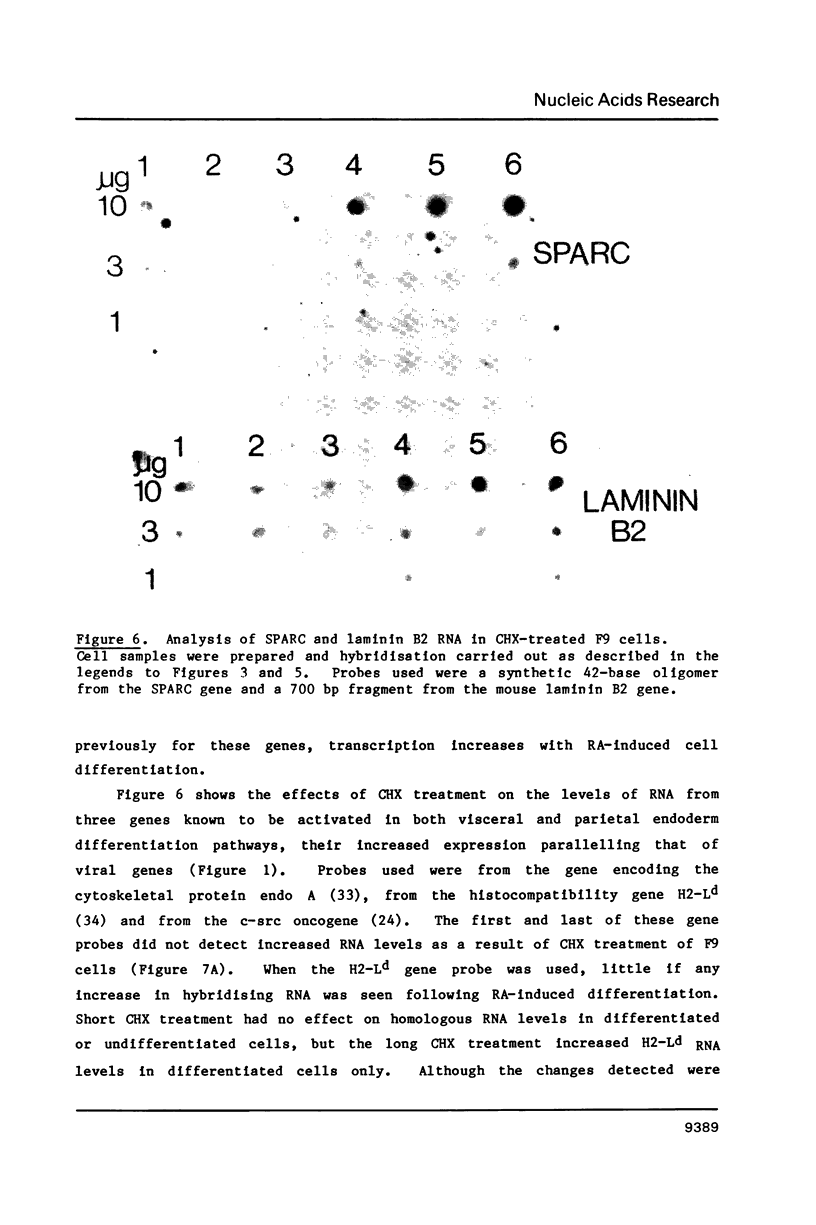
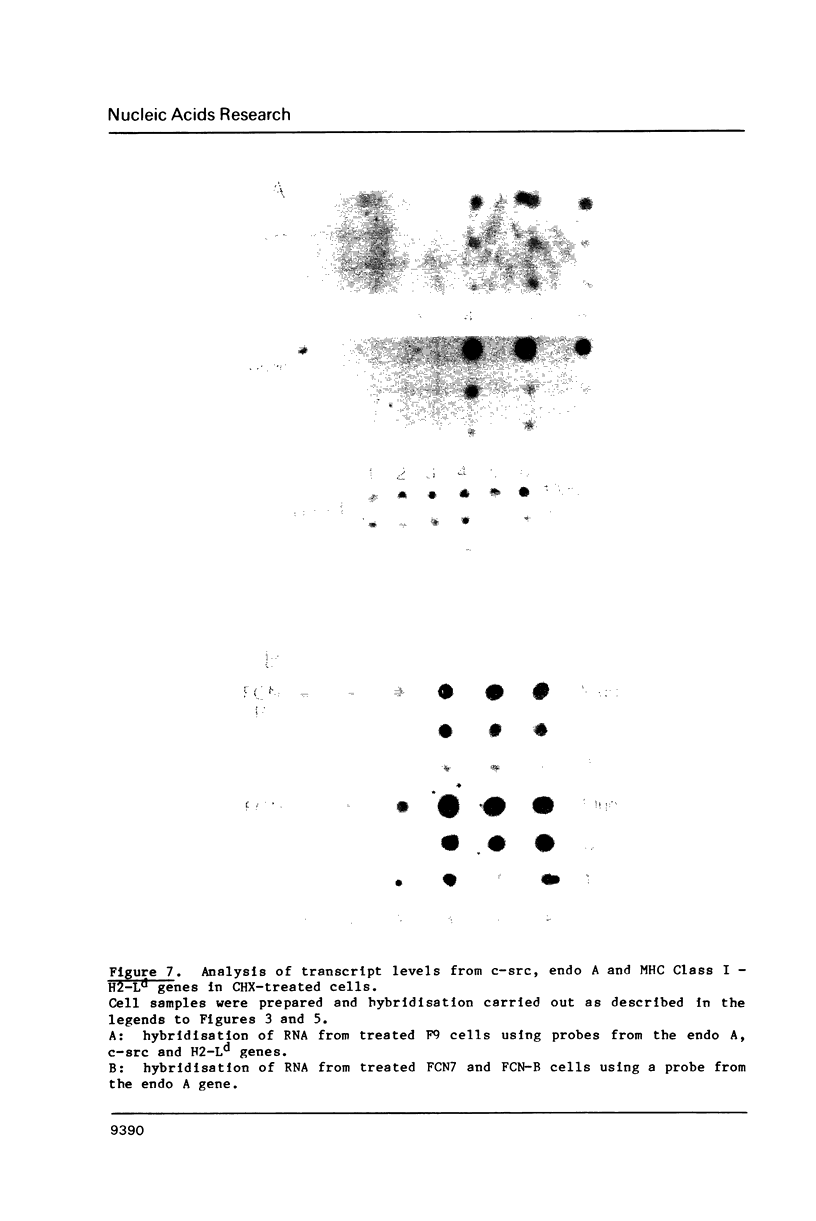





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P. Polyoma regulatory region: a potential probe for mouse cell differentiation. Cell. 1985 Dec;43(3 Pt 2):561–562. doi: 10.1016/0092-8674(85)90225-9. [DOI] [PubMed] [Google Scholar]
- Barlow D. P., Green N. M., Kurkinen M., Hogan B. L. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Jacob F. Molecular cloning of a cDNA sequence encoding a trophectoderm-specific marker during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2328–2332. doi: 10.1073/pnas.79.7.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Babinet C. Negative regulation of early polyomavirus expression in mouse embryonal carcinoma cells. J Virol. 1986 Sep;59(3):761–763. doi: 10.1128/jvi.59.3.761-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Duprey P. Spontaneous differentiation of murine teratocarcinoma stem cells at low temperature. J Cell Physiol. 1986 Nov;129(2):230–236. doi: 10.1002/jcp.1041290215. [DOI] [PubMed] [Google Scholar]
- Dandolo L., Vasseur M., Kress C., Aghion J., Blangy D. Temperature dependent expression of polyoma virus in murine embryonal carcinoma cells. J Cell Physiol. 1980 Oct;105(1):17–24. doi: 10.1002/jcp.1041050104. [DOI] [PubMed] [Google Scholar]
- Dony C., Kessel M., Gruss P. Post-transcriptional control of myc and p53 expression during differentiation of the embryonal carcinoma cell line F9. Nature. 1985 Oct 17;317(6038):636–639. doi: 10.1038/317636a0. [DOI] [PubMed] [Google Scholar]
- Durkin M. E., Phillips S. L., Chung A. E. Control of laminin synthesis during differentiation of F9 embryonal carcinoma cells. A study using cDNA clones complementary to the mRNA species for the A, B1 and B2 subunits. Differentiation. 1986;32(3):260–266. doi: 10.1111/j.1432-0436.1986.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E., Vasseur M., Blangy D. Polyoma virus mutants as probes of variety among mouse embryonal carcinoma cell lines. Differentiation. 1982;22(1):62–65. doi: 10.1111/j.1432-0436.1982.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A., Adamson E. D. Evidence for the existence of an early common biochemical pathway in the differentiation of F9 cells into visceral or parietal endoderm: modulation by cyclic AMP. Dev Biol. 1986 Apr;114(2):492–503. doi: 10.1016/0012-1606(86)90213-7. [DOI] [PubMed] [Google Scholar]
- Grover A., Adamson E. D. Roles of extracellular matrix components in differentiating teratocarcinoma cells. J Biol Chem. 1985 Oct 5;260(22):12252–12258. [PubMed] [Google Scholar]
- Grover A., Oshima R. G., Adamson E. D. Epithelial layer formation in differentiating aggregates of F9 embryonal carcinoma cells. J Cell Biol. 1983 Jun;96(6):1690–1696. doi: 10.1083/jcb.96.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Jenkins J. R., Hogan B. L. In vitro synthesis of laminin and entactin polypeptides. J Biol Chem. 1983 May 25;258(10):6543–6548. [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Belin D., Strickland S. The production of distinct forms of plasminogen activator by mouse embryonic cells. Dev Biol. 1982 Mar;90(1):154–159. doi: 10.1016/0012-1606(82)90220-2. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin F., Pinon H., Reiss C., Kress C., Montreau N., Blangy D. Common features of polyomavirus mutants selected on PCC4 embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1799–1803. doi: 10.1002/j.1460-2075.1985.tb03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J., Levine A. J. Alterations in the developmental potential of embryonal carcinoma cells in mixed aggregates of nullipotent and pluripotent cells. Cell. 1979 Jun;17(2):337–346. doi: 10.1016/0092-8674(79)90160-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Cedar H., Flavell R., Grosveld F. Regulated expression of an introduced MHC H-2K bm1 gene in murine embryonal carcinoma cells. Nature. 1984 Aug 2;310(5976):415–418. doi: 10.1038/310415a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Singer P. A., Trevor K., Oshima R. G. Molecular cloning and characterization of the Endo B cytokeratin expressed in preimplantation mouse embryos. J Biol Chem. 1986 Jan 15;261(2):538–547. [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J., Lockett T. J., Kelly J., Lewy D. Competition studies with repressors and activators of viral enhancer function in F9 mouse embryonal carcinoma cells. Nucleic Acids Res. 1987 May 26;15(10):4307–4324. doi: 10.1093/nar/15.10.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Lockett T. J. SV40 enhancer activation during retinoic acid-induced differentiation of F9 embryonal carcinoma cells. EMBO J. 1985 Dec 30;4(13B):3831–3837. doi: 10.1002/j.1460-2075.1985.tb04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Tabor J. M., Oshima R. G. Identification of mRNA species that code for extra-embryonic endodermal cytoskeletal proteins in differentiated derivatives of murine embryonal carcinoma cells. J Biol Chem. 1982 Aug 10;257(15):8771–8774. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]