Abstract
Gut barrier dysfunction may occur in short bowel syndrome (SBS). We hypothesized that systemic exposure to flagellin and lipopolysaccharide (LPS) in SBS might regulate specific immune responses. We analyzed serial serum samples obtained from parenteral nutrition (PN)-dependent patients with SBS versus non-SBS control serum. Serum from 23 adult SBS patients was obtained at baseline and 4, 8, 12, 16, 20, and 24 wk in a trial of modified diet with or without growth hormone. Control serum was obtained from 48 healthy adults and 37 adults requiring PN during critical illness. Serum flagellin was detected by an ELISA recognizing an array of gram-negative flagellins, and LPS was detected by limulus assay. Serum flagellin- and LPS-specific immunoglobulin levels (IgM, IgA, and IgG) were determined by ELISA. Serum flagellin and LPS were undetectable in control subjects. In contrast, serum flagellin, LPS, or both were detected in 14 SBS patients (61%) during one or more time points [flagellin alone, 5/23 (22%); LPS alone, 6/23 (26%); or flagellin + LPS, 3/23 (13%)]. Flagellin-specific serum IgM, IgA, and IgG levels were markedly increased in SBS patients compared with both control populations and remained elevated during the 6-mo study period. LPS-specific IgA was significantly higher in SBS patients compared with healthy controls; LPS-specific IgM, IgA, and IgG levels each decreased over time in association with PN weaning. We conclude that adults with PN-dependent SBS are systemically exposed to flagellin and LPS, presumably from the gut lumen. This likely regulates innate and adaptive immune responses to these specific bacterial products.
Keywords: parenteral nutrition, intestinal barrier function
short bowel syndrome (SBS) occurs after massive small bowel resection and is an important clinical entity, with significant morbidity and mortality (13, 36). SBS is characterized by chronic diarrhea and malabsorption, dehydration and malnutrition, and, in patients requiring parenteral nutrition (PN), frequent infections (13, 36). Unfortunately, SBS and PN administration are each associated with infection by enteric gram-negative bacteria, suggesting that failure of the gut barrier occurs (2, 4, 5, 18, 20, 22, 24, 25, 33, 34).
A critical function of the intestinal epithelium is to prevent luminal bacteria and their constituents from movement to underlying tissue via transcellular or paracellular pathways (bacterial translocation) (3, 36). Based on evidence derived largely from animal models (36) and limited human studies (2, 4, 14, 18, 20, 33, 35, 38), common events that occur in human SBS may contribute to gut barrier dysfunction. These include small bowel bacterial overgrowth (SBBO), systemic or local infection, splanchnic hypoperfusion, gut mucosal inflammation, use of PN, lack of enteral feeding, and malnutrition, all of which may cause erosion of mucosal anatomic and immune barriers (36). In addition, we hypothesize that there may be translocation of bacterial products [e.g., flagellin and endotoxin/lipopolysacccharide (LPS)] that may worsen intestinal barrier function (6, 8, 9, 15, 21).
The bacterial constituent flagellin is the monomeric subunit of flagella found on motile gut bacteria (11). Our previous studies show that, upon colonization by pathogens such as Salmonella typhimurium, flagellin interacts with basolateral toll-like receptor-5 (TLR5), resulting in gut epithelial cells secreting cytokines and chemokines [e.g., interleukin-8 (IL-8)] that recruit polymorphonuclear neutrophils. These events may mediate local gut mucosal inflammation (8, 9, 11). Flagellin from commensal bacteria might be able to activate gut epithelial cells as well as cells in the lamina propria in states of gut epithelial barrier dysfunction.
Recent reports by ourselves and others demonstrate that some patients with Crohn’s disease (CD) exhibit substantially elevated serum immunoglobulin A (IgA) and IgG titers against flagellin derived from Escherichia coli and other commensal bacteria (10, 17, 29, 32). Bacterial flagellin may be a major antigen in CD, because flagellin-specific CD4+ T cells were shown to induce severe colitis after transfer into naive severe combined immunodeficiency (SCID) mice (11). In addition, otherwise healthy mice injected with gram-negative bacteria have detectable serum flagellin and LPS in blood, whereas systemic injection of flagellin and LPS each potently induce inflammatory cytokines, oxidative stress, organ failure, and a sepsislike syndrome in these animals (6, 15).
Given the potential for gut barrier dysfunction in SBS, we hypothesized that clinically stable patients with severe chronic SBS could exhibit systemic localization of the gram-negative bacterial products flagellin and LPS in serum with secondary activation of specific B cell-mediated adaptive immune responses. Thus we sought to 1) determine whether flagellin and LPS are detectable in serum of PN-dependent adult SBS patients compared with healthy control subjects and 2) evaluate whether SBS patients demonstrate an increased adaptive immune exposure to both flagellin and LPS via measurement of specific immunoglobulins (IgM, IgA, and IgG) directed at these bacterial antigens.
METHODS
Study Subjects
Healthy control subjects
Adults without history of bowel resection or other significant past medical or surgical history were recruited as healthy control subjects. These individuals underwent a screening medical history and physical examination by a physician (T. R. Ziegler) in the General Clinical Research Center (GCRC) of Emory University Hospital. Blood and urine samples were obtained for a comprehensive plasma chemistry profile, complete blood count, and urinalysis. A total of 48 subjects without evidence of acute or chronic disease were identified, and serum obtained from these individuals was analyzed in the current study, as outlined below.
PN-requiring control subjects without SBS
As a second control group, serum was analyzed for antibodies to flagellin from patients receiving PN but without SBS, enrolled in a study reported elsewhere (37). The patients were adults requiring PN, admitted to the surgical intensive care unit following cardiac, pancreatic, vascular, or colonic operations.
Subjects with SBS
Clinically stable adult patients with SBS who chronically required at least 2 days of PN weekly and were able to consume oral diets were eligible for participation in this study. These individuals were enrolled in a double-blind, randomized, controlled trial primarily designed to assess the effects of modified oral diets, with or without recombinant human growth hormone (GH), on gut nutrient absorption and weaning from chronic PN, as we recently reported (7, 31). Entry inclusion criteria were no previous history of stomach or esophageal resection, no current renal impairment (creatinine > 1.8 mg/dl) or hepatic dysfunction (total bilirubin > 1.5 mg/dl), and no history of diabetes mellitus, colonic polyps, colonic adenomas, or cancer (within the previous 5 years). Subjects were ineligible if they had a history of severe chronic infections such as human immunodeficiency virus (HIV) or any infection requiring antibiotics within the prior 2 mo. Subjects with a history of CD had no evidence of worsening disease activity within the prior 2 mo (no significant change in the CD activity index, anti-inflammatory medication dosage, or usual stool volume). The Institutional Review Board of Emory University School of Medicine (Atlanta, GA) approved the study protocol.
SBS Study Protocol
After a screening visit in the GCRC, subjects with SBS were admitted to the inpatient GCRC unit for a 28-day period of metabolic studies, as outlined previously (7, 31). Baseline studies (including colonoscopy with residual distal small bowel and residual midcolonic mucosal biopsies for histological examination) were completed during the initial 7 days, during which the subjects received their usual diet and PN regimen. During the subsequent 23 wk of study, subjects continued their usual PN and were placed on modified oral diets individually designed to improve nutrient absorption, as outlined previously (31, 36). The SBS subjects were also randomized to receive either subcutaneous saline placebo (control; n = 9) or GH therapy (n = 14; Serostim; Serono, Rockland, MA) in a double-blind manner (31). At the end of study week 4, the SBS patients were discharged home; weaning from PN was commenced and oral diet adjusted and advanced as tolerated using standardized methods (7, 31). The subjects returned to the GCRC at weeks 8, 12, 16, 20, and 24 for clinical evaluation and repeat studies. All subjects had a repeat colonoscopy with biopsies as per the baseline period at weeks 4 and 12. Serum samples were obtained from all SBS patients at baseline and at study weeks 4, 8, 12, 16, 20, and 24 visits for analysis of flagellin- and LPS-specific end points.
Serum Analysis
Serum flagellin and LPS
Flagellin was detected by an ELISA that recognizes a broad array of gram-negative flagellins to minimize serotype specificity. We developed this assay using flagellin monomers purified from a human commensal E. coli strain, as previously described (29). Briefly, native flagellin from E. coli subtype F18 was chromatographically purified, and a polyclonal E. coli flagellin antibody was affinity purified from rabbit sera (8). Human sera demonstrate a similar recognition pattern of such flagellin monomers whether isolated from several flagellated E. coli or S. typhimurium strains (11, 29). In previous studies, we used two secondary methods to determine whether flagellin is a target of the adaptive immune response in sera from control subjects and patients with CD (10, 29). Purified flagellin (10 μl of 500 ng/ml sample) was run on SDS-PAGE gels and immunoblotted with control and patient serum as the primary antibody, followed by enhanced chemiluminescence (ECL) detection (29). In addition, whole bacterial lysates (107 colony-forming units/ml) of flagellate or aflagellate E. coli (Invitrogen, Carlsbad, CA) were run on SDS-PAGE gels (10 μl), immunoblotted using control and patient serum as the primary antibody followed by anti-human IgG as the secondary linking antibody, and detected using ECL. The presence of the 45- to 50-kDa flagellin band was verified to be flagellin by its absence in lanes loaded with lysates of aflagellate E. coli. (29).
Serum LPS was detected by limulus assay, using a well-established kit method (Cape Cod Associates, Falmouth, MA) (8). LPS concentration values >10 pg/ml were considered positive.
Serum flagellin- and LPS-specific immunoglobulins
Flagellin- and LPS-specific IgM, IgA, and IgG levels were quantitated by ELISA, as we have previously reported for IgA and IgG (10, 29). Levels of these immunoglobulins likely reflect the amount of systemic exposure to flagellin and LPS and may reflect altered adaptive immune responses potentially linked to gut barrier dysfunction. Microtiter plates were coated overnight with purified protein L (Sigma, St. Louis, MO), a bacterial protein with high affinity for all human immunoglobulins, purified E. coli flagellin (100 ng/well), or purified E. coli LPS (1 μg/well) (8). Serum samples from control and SBS subjects diluted 1:500 were applied to wells coated with flagellin, LPS, or protein L. After incubation and washing, the wells were incubated either with anti-human IgM or IgG coupled to horseradish peroxidase (Amersham) or, in the case of IgA-specific antibodies, using a two-step process of anti-human IgA-biotin followed by avidin-peroxidase. Quantitation of total immunoglobulins was performed using the colorimetric peroxidase substrate tetramethylbenzidine, and optical density (OD) was read at 650 nm with an ELISA plate reader (10, 29). Data are reported as OD corrected by subtracting background (determined by readings in samples lacking serum) and are normalized to each plate’s control sample, which was prepared in bulk, aliquoted, frozen, and thawed daily as used. Standardization was performed using preparations of known concentrations of IgM, IgA, and IgG, (10, 29). There is no serospecificity to measuring anti-flagellin or anti-LPS antibodies this way using ELISA (28).
Statistical Methods
Unpaired Student’s t-tests were used to compare flagellin- and LPS-specific immunoglobulin values in control serum samples compared with baseline serum samples in SBS subjects. Student’s t-tests were also used to compare immunoglobulin values between SBS subjects with or without randomization to GH during the study. Serial data in the total group of SBS patients and in the SBS subgroups (with or without a history of CD or administration of GH) were also analyzed and compared using repeated-measures analysis of variance (ANOVA). Baseline flagellin- and LPS-specific immunoglobulin values in subjects with CD, multiple surgeries for SBO/fistula, and abdominal injury/ischemia were also compared using ANOVA. The prevalence of serum positive for the presence of flagellin or LPS at any time point during the 6-mo study was compared between the SBS subgroups using Fischer’s exact test. Unpaired Student’s t-test were used to assess whether there were any effects of SBS patient age, residual small bowel length, percent residual colon, time since last bowel resection, and time on PN, and positive serum for flagellin or LPS at any time point. Univariate linear regression analysis was used to analyze SBS patient data for the influence of age, residual small bowel length, percent residual colon, time since last bowel resection, and time on PN on baseline flagellin- and LPS-specific IgM, IgA, and IgG levels. Univariate linear regression was also used to relate the weekly PN requirement at baseline and weeks 12, 20, and 24 to flagellin- and LPS-specific IgM, IgA, and IgG levels at these time points. P values ≤0.05 were considered statistically significant. Data are means ± SE.
RESULTS
Subject Demographics
The 48 healthy control subjects comprised 23 males and 25 females, with an average age of 40 ± 2 yr. The PN-requiring non-SBS surgical intensive care unit control subjects for anti-flagellin antibodies comprised 24 males and 13 females, with an average age of 55 ± 2 yr. A total of 20 patients were studied after pancreatic operations, 8 patients were studied after cardiac operations (coronary artery bypass grafting or valve replacement), 5 patients were studied after thoracic or abdominal arterial operations (typically aneurysm repair), and 4 patients were studied after partial colonic resections. None of these individuals had evidence of acute infection at the time of the blood sampling; they required PN because of a variety of common factors (high aspiration risk, postoperative ileus, partial bowel obstruction, recent gastrointestinal bleeding, hemo-dynamic instability requiring pressor agents, and/or intolerance to previous enteral feeds).
The demographic data of the SBS patients are shown in Table 1. There were 7 males and 16 females, with an average age of 50 ± 3 yr. All SBS patients had been chronically dependent on PN for an average of 37 ± 8 mo. All had a history of massive small bowel resection, with or without partial or complete colonic resection, for a variety of primary disorders (Table 1). All subjects exhibited significant malabsorption at entry (mean stool wet weight = 2,406 ± 280 g/day) and thus had clinically significant SBS (7, 31). The time since the last bowel resection before baseline studies averaged 36 ± 9 mo (range: 3–155 mo). Residual postduodenal small bowel length was estimated by review of operative reports and barium studies and averaged 108 ± 21 cm. A total of eight SBS patients had no residual colon, nine had estimated residual colonic length of 50–75% of normal, and six had an intact colon with intact ileal-cecal valve. Seven SBS patients had a history of CD, which had led to massive bowel resection, and all were clinically and histologically quiescent (Table 1).
Table 1.
SBS patient demographic data
| Subject | Age, yr | Sex, M/F | Diagnosis | Residual Postduodenal Small Bowel Length, cm |
Approximate Colon, % | Time Since Last Bowel Resection, mo |
Time on PN, mo |
|---|---|---|---|---|---|---|---|
| 1 | 32 | M | Abdominal injury | 70 (jejunum-ileum) | 50 | 3 | 3 |
| 2 | 37 | M | Abdominal injury | 50 (jejunum) | 50 | 10 | 11 |
| 3 | 55 | F | Multiple surgeries for SBO/fistula | 105 (jejunum) | 0 | 11 | 11 |
| 4 | 52 | F | Crohn’s disease | 350 (jejunum-ileum) | 0 | 108 | 108 |
| 5 | 35 | F | Crohn’s disease | 180 (jejunum-ileum) | 100 | 40 | 36 |
| 6 | 57 | F | Multiple surgeries for SBO/fistula | 75 (jejunum-ileum) | 100 | 15 | 18 |
| 7 | 37 | F | Multiple surgeries for SBO/fistula | 35 (jejunum) | 100 | 7 | 18 |
| 8 | 63 | F | Multiple surgeries for SBO/fistula | 35 (jejunum-ileum) | 100 | 10 | 21 |
| 9 | 72 | F | Mesenteric ischemia | 60 (jejunum) | 100 | 22 | 20 |
| 10 | 67 | M | Mesenteric ischemia | 75 (jejunum) | 50 | 23 | 26 |
| 11 | 32 | M | Crohn’s disease | 185 (jejunum-ileum) | 50 | 7 | 7 |
| 12 | 29 | M | Crohn’s disease | 140 (jejunum-ileum) | 0 | 6 | 6 |
| 13 | 57 | F | Mesenteric ischemia | 15 (ileum) | 100 | 16 | 19 |
| 14 | 43 | F | Multiple surgeries for SBO/fistula | 350 (jejunum-ileum) | 0 | 7 | 7 |
| 15 | 32 | F | Mesenteric ischemia | 15 (jejunum) | 50 | 34 | 34 |
| 16 | 52 | F | Mesenteric ischemia | Duodenum only | 0 | 11 | 24 |
| 17 | 35 | M | Abdominal injury | 75 (ileum) | 50 | 136 | 136 |
| 18 | 58 | F | Crohn’s disease | 300 (jejunum-ileum) | 0 | 96 | 48 |
| 19 | 61 | M | Mesenteric ischemia | 135 (jejunum) | 50 | 31 | 34 |
| 20 | 56 | F | Multiple surgeries for SBO | 20 (jejunum-ileum) | 50 | 77 | 77 |
| 21 | 54 | F | Crohn’s disease | 75 (jejunum) | 0 | 21 | 21 |
| 22 | 47 | F | Crohn’s disease | 150 (jejunum) | 0 | 155 | 155 |
| 23 | 55 | F | Multiple surgeries for SBO/fistula | 60 (jejunum) | 75 | 8 | 37 |
| Mean ± SE | 49.5±2.7 | 7 M, 16 F | 7 Crohn’s disease | 108±21 | 48±8 (n = 8 with no colon) | 36±9 | 37±8 |
M, male; F, female; PN, parenteral nutrition; SBO, small bowel obstruction. Patients with <100% of colon did not have an ileal cecal valve in situ.
Detection of Flagellin and LPS in Serum of SBS Patients
Flagellin and LPS were not detectable in the serum of the 48 control subjects. However, a total of 61% (14/23) of SBS subjects had either flagellin or LPS detected in serum at least once during the 24-wk protocol, and several patients exhibited serial positive tests for these molecules (Table 2). Flagellin alone was detected in the sera of 5 of 23 SBS patients (22%), LPS alone was detected in 6/23 (26%), and flagellin and LPS were detected in 3/23 (13%). Two of these subjects were positive for both flagellin and LPS in the same serum sample. Flagellin was detected in serum at least once in eight SBS patients during the study (35% incidence), and LPS was detected at least once in nine patients (39% incidence) (Table 2). Randomization to GH did not influence the incidence of detectable flagellin and LPS (not shown). At least one serum sample was positive for flagellin and/or LPS in 3 of 7 subjects with a history of CD (43% incidence), but 11 of 16 subjects without a history of CD were also positive (69% incidence). Serum with detectable flagellin and/or LPS occurred in 4 of 8 SBS subjects without a residual colon (50% incidence) and in 10 of 15 of SBS subjects with residual colon in situ (67%). Patient age, residual small bowel or colonic length, time since last bowel resection surgery, or time on PN in subjects did not influence the incidence of serum positive for flagellin or LPS during the 6-mo study (not shown).
Table 2.
Detection of flagellin and LPS in serum of adult SBS patients
| SBS Patient | CD Hx | Residual Colon | Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Yes | Flagellin | ||||||
| 2 | No | Yes | Flagellin | ||||||
| 3 | No | No | LPS | Flagellin | LPS | ||||
| 4 | Yes | No | Flagellin | Flagellin | Flagellin | Flagellin | Flagellin | Flagellin | |
| 5 | Yes | Yes | |||||||
| 6 | No | Yes | Flagellin | ||||||
| 7 | No | Yes | |||||||
| 8 | No | Yes | LPS | ||||||
| 9 | No | Yes | LPS | ||||||
| 10 | No | Yes | LPS | LPS | LPS | ||||
| 11 | Yes | Yes | Flagellin | Flagellin | Flagellin | ||||
| 12 | Yes | No | Flagellin + LPS | ||||||
| 13 | No | Yes | Flagellin + LPS | Flagellin + LPS | |||||
| 14 | No | No | LPS | ||||||
| 15 | No | Yes | LPS | ||||||
| 16 | No | No | |||||||
| 17 | No | Yes | LPS | LPS | |||||
| 18 | Yes | No | |||||||
| 19 | No | Yes | |||||||
| 20 | No | Yes | |||||||
| 21 | Yes | No | |||||||
| 22 | Yes | No | |||||||
| 23 | No | Yes |
CD Hx, history of Crohn’s disease (no subject had active disease during study); SBS, short bowel syndrome.
Increased Serum Flagellin- and LPS-Specific Immunoglobulin Levels in SBS Patients
Anti-flagellin immunoglobulins
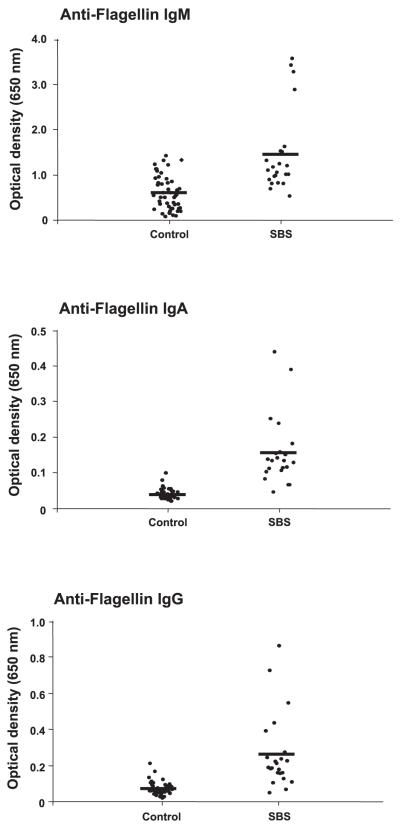
SBS patients demonstrated markedly increased serum anti-flagellin IgM, IgA, and IgG levels compared with values in healthy control subjects and patients in the critical care unit receiving PN (Table 3 and Fig. 1). Levels for these immunoglobulins in the healthy subjects and the critical care unit patients were similar (Table 3). The level of elevated flagellin-specific immunoglobulins in the SBS patients was substantially greater than what we previously observed in CD patients (29). The increase in anti-flagellin IgM was approximately twofold in SBS patients compared with healthy control subjects (Table 3). However, serum levels of anti-flagellin IgG, and especially IgA, were more profoundly increased (~3-fold and 5-fold, respectively; Fig. 1). SBS patient age, residual small bowel length, percent residual colon, presence or absence of CD, time since last bowel resection, and time on PN were unrelated to the baseline flagellin-specific IgM, IgA, and IgG levels as shown by linear regression analysis. The flagellin-specific immunoglobulins remained elevated and stable over time from the baseline week through study week 24 (Table 3). Use of GH did not influence the anti-flagellin immunoglobulin titers at any serial time point (not shown). Baseline flagellin-specific immunoglobulin values in subjects with SBS due to CD, multiple surgeries for SBO/fistula, and abdominal injury/ischemia were similar [IgM: 1.49 ± 0.30, 1.45 ± 0.41, and 0.98 ± 0.01, respectively (not significant, NS); IgA: 0.18 ± 0.05, 0.16 ± 0.06, and 0.21 ± 0.08, respectively (NS); and IgG: 0.25 ± 0.06, 0.21 ± 0.05, and 0.18 ± 0.03, respectively (NS)]. As reported in our preliminary communication (37), the study protocol of individualized modified diet resulted in improved nutrient absorption and a corresponding decrease in PN needs in SBS patients over time, without a significant effect of GH. PN requirements in the entire group of SBS patients averaged 10.5 ± 1.2 l/wk at baseline and were weaned to 3.0 ± 0.6 l/wk by week 12 and 2.5 ± 0.5 l/wk by week 24 (P < 0.01, baseline vs. weeks 12 and 24, respectively). The volume of PN infused per week was positively correlated with anti-flagellin IgG levels at week 12 of the study (r2 = 0.20; P = 0.052).
Table 3.
Flagellin-specific immunoglobulins in SBS patients and control subjects
| Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | |
|---|---|---|---|---|---|---|---|
| SBS IgM | 1.45±0.19* | 1.22±0.14 | 1.31±0.14 | 1.27±0.16 | 1.29±0.15 | 1.28±0.13 | 1.29±0.15 |
| Healthy control IgM | 0.60±0.05 | ||||||
| ICU control IgM | 0.36±0.41 | ||||||
| SBS IgA | 0.16±0.03* | 0.15±0.02 | 0.16±0.02 | 0.14±0.02 | 0.16±0.02 | 0.15±0.02 | 0.17±0.03 |
| Healthy control IgA | 0.04±0.01 | ||||||
| ICU control IgA | 0.05±0.03 | ||||||
| SBS IgG | 0.26±0.04* | 0.20±0.02 | 0.23±0.03 | 0.21±0.03 | 0.21±0.02 | 0.22±0.03 | 0.24±0.03 |
| Healthy control IgG | 0.07±0.01 | ||||||
| ICU control IgG | 0.05±0.04 |
Data are means ± SE, expressed as optical density readings.
P ≤ 0.001, SBS (n = 23) vs. control subjects (n = 48) and critically ill patients without SBS requiring parenteral nutrition (n = 37). SBS patients have short bowel syndrome; controls are healthy subjects with intact intestine; ICU controls are adults without SBS requiring PN following cardiac, vascular, or colonic surgery in the surgical intensive care unit.
Fig. 1.
Patients with short bowel syndrome (SBS) demonstrate markedly elevated flagellin-specific immunoglobulin levels in serum. Flagellin (Flag)-specific immunoglobulins M, A, and G (IgM, IgA, and IgG) were quantitated in serum obtained from clinically stable adults with parenteral nutrition (PN)-dependent severe SBS (n = 23) and compared with samples obtained from 48 healthy adults (control) and 37 critically ill patients requiring PN and without SBS, as outlined in methods. Serum levels of flagellin-specific IgM, IgA, and IgG were markedly increased in SBS patients compared with values in control subjects (each P < 0.001).
Anti-LPS immunoglobulins
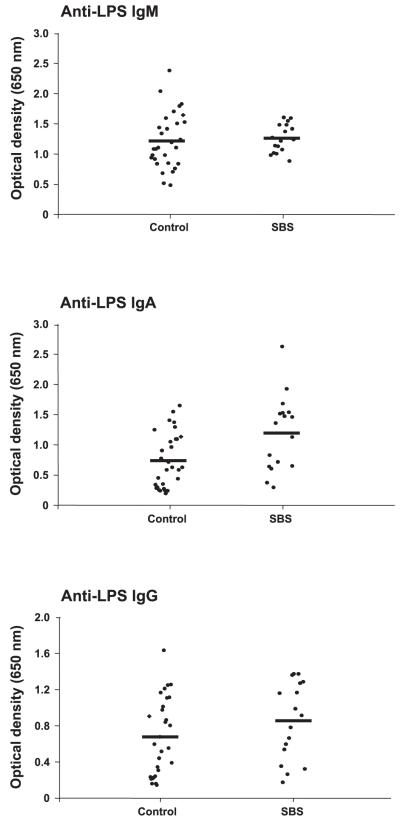
Serum anti-LPS IgA levels were significantly increased (~1.6-fold) in SBS patients compared with healthy control subjects (Table 4 and Fig. 2). In contrast to the increase in anti-flagellin IgM and IgG levels demonstrated by SBS patients, serum anti-LPS IgM and IgG titers were similar between these groups (Table 4 and Fig. 2). Baseline LPS-specific immunoglobulin values in subjects with SBS due to CD, multiple surgeries for SBO/fistula, and abdominal injury/ischemia were also similar [IgM: 1.34 ± 0.13, 1.29 ± 0.08 and 1.20 ± 0.09, respectively (NS); IgA: 1.45 ± 0.43, 0.94 ± 0.23, and 1.22 ± 0.20, respectively (NS); and IgG: 1.06 ± 0.18, 0.63 ± 0.20, and 0.89 ± 0.15, respectively (NS)]. SBS patient age was positively correlated with baseline anti-LPS IgM levels (r2 = 0.21; P = 0.035); otherwise, there were no correlations between baseline anti-LPS immunoglobulin levels and residual small bowel length, percent residual colon, presence or absence of CD, time since last bowel resection, or time on PN. Use of GH did not influence the anti-LPS immunoglobulin titers at any serial time point (not shown). However, in contrast to the chronic elevation in anti-flagellin antibody levels, anti-LPS IgM, IgA, and IgG levels all modestly decreased during the 6-mo study (Tables 3 and 4). This pattern was particularly evident when samples obtained at week 8 or earlier were compared with samples obtained during the latter phase of the study (weeks 20 and 24; Table 4). The volume of PN infused per week was positively, but not significantly, correlated with anti-LPS IgA levels at week 12 (r2 = 0.21; P = 0.062), with anti-LPS IgA levels at week 20 (r2 = 0.21; P = 0.085), and with anti-LPS IgG levels at week 20 (r2 = 0.23; P = 0.071). However, at week 24, there was a robust and statistically significant positive correlation with PN volume infused per week and the serum levels of both anti-LPS IgM (r2 = 0.55; P = 0.009) and anti-LPS IgG (r2 = 0.41; P = 0.034).
Table 4.
LPS-specific immunoglobulins in SBS patients and control subjects
| Baseline | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | |
|---|---|---|---|---|---|---|---|
| SBS IgM | 1.26±0.06 | 1.35±0.07† | 1.37±0.06†‡ | 1.23±0.08 | 1.31±0.08 | 1.24±0.08 | 1.32±0.06 |
| Control IgM | 1.21±0.08 | ||||||
| SBS IgA | 1.19±0.15*§ | 1.23±0.15§ | 1.29±0.13§ | 1.16±0.13 | 1.29±0.12 | 1.11±0.11 | 1.06±0.09 |
| Control IgA | 0.74±0.08 | ||||||
| SBS IgG | 0.85±0.10 | 0.91±0.12‡§ | 0.92±0.11‡§ | 0.81±0.11§ | 0.90±0.11 | 0.77±0.10 | 0.73±0.1.0 |
| Control IgG | 0.67±0.08 |
Data are means ± SE, expressed as optical density readings.
P ≤ 0.001, SBS (n = 23) vs. control subjects (n = 48).
P < 0.05 vs. week 12.
P < 0.05 vs. week 20.
P < 0.05 vs. week 24.
Fig. 2.
Patients with SBS demonstrate elevated lipopolysaccharide (LPS)-specific IgA levels in serum. LPS-specific IgM, IgA, and IgG were quantitated in serum obtained from clinically stable adults with PN-dependent severe SBS (n = 23) and compared with samples obtained from 48 healthy adults, as outlined in methods. Serum titers of LPS-specific IgA were modestly, but significantly, increased in SBS patients compared with control subjects (P < 0.006). In contrast, anti-LPS IgG and IgM levels in serum were similar in SBS patients and control subjects.
DISCUSSION
Our data are the first to show detectable flagellin in the blood of SBS patients and an enhanced adaptive immune response to the bacterial products flagellin and LPS. We hypothesize that the intermittent presence in serum of flagellin and/or LPS upregulates immunoglobulin levels to these mediators due to gut barrier dysfunction in SBS. An increased adaptive immune response to flagellin has also been observed in CD (10, 17, 29, 32), a condition in which both immune dysregulation and gut barrier dysfunction occur.
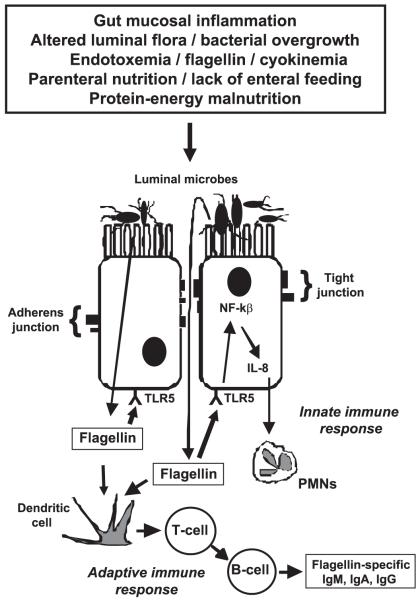
Concomitant with elevated levels of flagellin- and LPS-specific immunoglobulin, patients with SBS demonstrated detectable, albeit intermittent, flagellin and LPS in serum over the 6-mo period of blood sampling. The markedly elevated flagellin-specific IgM, IgA, and IgG and LPS-specific IgA levels during a time frame when serum was intermittently positive for flagellin and/or LPS suggest that the increased antibody titers were theoretically regulated by exposure to flagellin and LPS in the systemic circulation and/or to more proximal local exposure at the basolateral membrane of gut epithelia (Fig. 3). Based on the risk factors for gut barrier failure in SBS outlined in Fig. 3, we speculate that the flagellin and LPS seen by the gut-associated immune system in our patients may have occurred as a result of low-level, intermittent bacterial translocation across the gut mucosa. There were no statistically significant differences in anti-flagellin IgM, IgA, or IgG titers in SBS patients who had serum positive for flagellin at any of the seven serial serum sample time points (baseline and weeks 4, 8, 12, 16, 20, and 24) compared with anti-flagellin titers in SBS patients without any of the serum samples positive for flagellin (not shown). Similarly, there were no statistically significant differences in anti-LPS IgM, IgA, or IgG titers in SBS patients with any serum sample positive for LPS compared with patients without any positive serum samples for LPS (not shown). Our interpretation of these data is that increased anti-flagellin and anti-LPS titers in SBS patients negative for flagellin and LPS in our protocol likely reflect a long-term adaptive immune response to intermittent translocation of these products that would not necessarily correlate with positivity for flagellin or LPS at specific time points when serum was collected.
Fig. 3.
Theoretical schema of gut barrier dysfunction and translocation of flagellin in human SBS. Various events common in SBS, such as gut mucosal inflammation, small bowel bacterial overgrowth, endotoxemia, cytokinemia, malnutrition, parenteral feeding, malnutrition, and/or relative lack of enteral food stimulation may theoretically cause movement of flagellated luminal bacteria via transcellular pathways, or paracellular movement following disruption of tight junction and adherens junction proteins may result in translocation of bacteria to the basolateral membrane of gut epithelial cells. Alternatively, flagellin itself may traverse these barriers. Flagellin may interact with basolateral toll-like receptor-5 (TLR5) to induce an innate immune response in which gut epithelial cells secrete cytokines and chemokines [e.g., interleukin-8 (IL-8)] after activation of nuclear factor-κB (NF-κB). This, in turn, may recruit polymorphonuclear neutrophils (PMNs) to mediate local inflammation at the epithelial level and may contribute to further gut barrier dysfunction. Flagellin also stimulates an adaptive immune response via antigen-presenting cells and T cells, resulting in B cell production of flagellin-specific IgM, IgA, and IgG.
The overall increase in flagellin-specific immunoglobulins in SBS patients compared with healthy control subjects was substantially greater than the differences between groups observed for LPS-specific immunoglobulins (Figs. 1 and 2; Tables 3 and 4). These differential results suggest that SBS-associated adaptive immune responses target flagellin more than LPS. We did not find that PN use or time on PN influenced the incidence of serum positive for flagellin or LPS in the SBS subjects. However, we did observe an intriguing positive correlation between infused PN dose and anti-flagellin IgG levels at week 12 and with all LPS-specific immunoglobulins at later time points of the study. This suggests the possibility that PN use itself may influence either basolateral/systemic exposure to both of these mediators or the immune response to them. On the other hand, the amount of PN delivered may also be reflective of the extent of underlying intestinal mucosal function, surface area, and/or injury, and it is possible that this is causing the antibody positivity seen in our subjects. More detailed studies in SBS patients are needed to assess the impact of PN use and enteral feeding regimens on gut barrier function, gram-negative bacterial infection, and the presence of bacterial products and specific immunoglobulins in blood (4, 23, 24).
We did not do blood cultures in our clinically stable SBS subjects and thus do not have data to test whether positive serum for flagellin and LPS is due to concomitant presence of gram-negative bacteria in the blood. Nonetheless, our prior in vitro data (8) showed that flagellin itself is capable of translocating from the apical to the basolateral membrane in human colonic epithelial cells, where it induces a proinflammatory innate immune response (Fig. 3). In addition, Alexander et al. (1) demonstrated that Candida albicans, E. coli, or endotoxin instilled into Thiry-Vella loops of thermally injured guinea pigs and rats was capable of transcellular movement to the basolateral membrane. Therefore, it is possible that flagellin and LPS alone traversed the gut epithelial barrier in at least some of our SBS patients. This in turn would stimulate specific immune responses that were not observed in our one-time sampling of 48 healthy subjects or 37 intensive care unit patients. The increase in anti-flagellin immunoglobulins of IgM, IgA, and IgG classes in the SBS patients compared with the PN-requiring intensive care unit patients points to SBS (and not PN use) as the most important associated factor in the flagellin-specific adaptive immune responses.
Intestinal permeability to sugar markers in our SBS patients may have provided some insight into gut barrier function over time, but these assays are very difficult to interpret in SBS subjects, who exhibit rapid bowel transit and potential bacterial overgrowth, each of which affects assay results (38). In previous studies, we demonstrated that intravenous injection of purified E. coli endotoxin resulted in an abnormal increase in intestinal permeability to lactulose and mannitol in healthy adults (21). This observation supports the potential effect of systemic LPS to itself worsen gut barrier function. However, data correlating gut permeability changes to sugar markers with infection or bacterial translocation in humans have been inconsistent (5, 18, 19, 38). Previous in vivo studies indicate that administration of both purified flagellin and LPS can induce organ failure and acute-phase cytokine responses (6, 15, 21). CD is associated with elevated serum flagellin-specific IgA and IgG immunoglobulins (17, 29, 32); however, a dominant-negative TLR5 polymorphism decreases adaptive IgA and IgG responses to flagellin and is negatively associated with CD risk (10). It is also possible that circulating flagellin or LPS may be mechanisms contributing to the increased circulating cytokines observed in PN-dependent SBS patients (12, 16).
Limited available data show that SBS patients commonly exhibit SBBO (14). Such abnormal microbial flora may be a risk factor for gut-derived systemic infection, as suggested in animal models and some clinical studies (2, 14, 35, 36). We did not test for the presence of SBBO in our study subjects, but our data raise the possibility that stimulation of local innate cytokine-mediated inflammatory responses by flagellin or LPS (Fig. 3) may contribute to low-grade inflammation observed in small bowel mucosa of SBS patients with documented SBBO (14). Of interest, systemic administration of LPS inhibits the adaptive small bowel intestinal growth response following massive small bowel resection in rats (30). Thus studies to determine a potential link between the presence of SBBO and systemic flagellin or LPS, the immune response to these antigens, and intestinal adaptation in patients with SBS would be of interest.
The intestinal resection procedures our SBS patients experienced before the study likely exposed them to systemic flagellin and LPS and thus may have contributed to some degree to the elevation in serum IgA and IgG immunoglobulins. In mice, nonintestinal surgical stress alone (30% partial hepatectomy) shifted the cecal E. coli population to a more adherent phenotype that disrupted gut barrier function and was reversed by antibiotics (27). Also, surgical manipulation of the colon was recently associated with a very high incidence (80%) of positive bacterial cultures in mesenteric lymph nodes of adult patients undergoing colorectal surgery compared with surgical controls (11%) (26). In our study, the average time since the last intestinal operation averaged 36 ± 9 mo (Table 1), and anti-flagellin IgM (a more acute-phase immunoglobulin) was increased compared with control subjects. Also, the baseline flagellin-specific and LPS-specific antibody titers were independent of residual small bowel or colonic length or the time since last bowel resection. In light of the systemic detection of flagellin and LPS in our patients over a 6-mo period of observation, as well as elevated specific IgM levels, we believe that the adaptive immune responses observed are largely driven by the intermittent exposure to these bacterial products in the bloodstream.
We conclude that patients with SBS are serially and intermittently exposed to increased systemic levels of the gram-negative bacterial products flagellin and LPS, in all probability due to gut barrier dysfunction. Systemic exposure to flagellin and LPS likely regulates innate and adaptive immune responses to each of these agents. Additional studies with more frequent blood sampling in adult and pediatric SBS patients are needed to determine the incidence, prevalence, and natural history of detectable flagellin and LPS in serum. Studies on the underlying mechanisms and route of entry of these into the systemic circulation and on the immune, cytokine, and organ-specific responses to these mediators would also be of interest. Finally, agents that may improve gut barrier function, such as specific nutrients and growth factors (36), could be tested as methods to decrease the systemic presence of these bacterial antigens in SBS.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of the Emory GCRC nursing and bionutrition staff for care of the subjects.
GRANTS
This work was supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK55850 (to T. R. Ziegler), R01 DK061417 (to A. T. Gewirtz), R01 DK002802 (to S. V. Sitaraman), Emory Epithelial Pathobiology Research Development Center Grant R24 DK064399, Emory GCRC Grant M01 RR00039, and a grant from Serono, Inc. (to T. R. Ziegler).
REFERENCES
- 1.Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, Pyles T, Childress CP, Ash SK. The process of microbial translocation. Ann Surg. 1990;212:496–510. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briet F, Flourie B, Achour L, Maurel M, Rambaud JC, Messing B. Bacterial adaptation in patients with short bowel, and colon in continuity. Gastroenterology. 1995;109:1446–1453. doi: 10.1016/0016-5085(95)90629-0. [DOI] [PubMed] [Google Scholar]
- 3.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AL, Moukarzel A, Goodson B, Herzog F, Pollack P, Reyen L, Alvarez M, Ament ME, Gornbein J. Catheter-related infections associated with home parenteral nutrition and predictive factors for the need for catheter removal in their treatment. JPEN J Parenter Enteral Nutr. 1994;18:297–230. doi: 10.1177/014860719401800403. [DOI] [PubMed] [Google Scholar]
- 5.D’Antiga L, Dhawan A, Davenport M, Mieli-Vergani G, Bjarnason I. Intestinal absorption and permeability in paediatric short-bowel syndrome: a pilot study. J Pediatr Gastroenterol Nutr. 1999;29:588–593. doi: 10.1097/00005176-199911000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Eaves-Pyles T, Murthy K, Liaudet L, Virag L, Ross G, Soriano FG, Szabo C, Salzman AL. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of pro-inflammatory mediators and cardiovascular dysfunction. J Immunol. 2001;166:1248–1260. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Estívariz C, Luo M, Bazargan N, Gu LH, Sitaraman SV, Klapproth JM, Jones DP, Leader LM, Galloway JR, Ziegler TR. Effects of modified oral diet and human growth hormone on nutrient absorption and parenteral nutrition needs in adults with severe short bowel syndrome: results of a randomized, double-blind, placebo-controlled clinical trial (Abstract) Gastroenterology. 2006;130(Suppl 2):A68. [Google Scholar]
- 8.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 9.Gewirtz AT, Simon PO, Schmitt CK, Taylor LJ, Hagedorn CH, O’Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–G1163. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz AT. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol. 2006;22:8–12. doi: 10.1097/01.mog.0000194791.59337.28. [DOI] [PubMed] [Google Scholar]
- 12.Hise ME, Compher C, Harlan L, Kohlmeier JE, Benedict SH, Gajewski B, Brown JC. Inflammatory mediators and immune function are altered in home parenteral nutrition patients. Nutrition. 2006;22:97–103. doi: 10.1016/j.nut.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen PB, Mortensen PB. Enhancing bowel adaptation in short bowel syndrome. Curr Gastroenterol Rep. 2002;4:338–347. doi: 10.1007/s11894-002-0085-0. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman SS, Loseke CA, Lupo JV, Young RJ, Murray ND, Pinch LW, Vanderhoof JA. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr. 1997;131:356–361. doi: 10.1016/s0022-3476(97)80058-3. [DOI] [PubMed] [Google Scholar]
- 15.Liaudet L, Murthy KG, Mabley JG, Pacher P, Soriano FG, Salzman AL, Szabo C. Comparison of inflammation, organ damage, and oxidant stress induced by Salmonella enterica serovar Muenchen flagellin and serovar Enteritidis lipopolysaccharide. Infect Immun. 2002;70:192–198. doi: 10.1128/IAI.70.1.192-198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling PR, Khaodhiar L, Bistrian BR, Keane-Ellison M, Thibault A, Tawa N. Inflammatory mediators in patients receiving long-term home parenteral nutrition. Dig Dis Sci. 2001;46:2484–2489. doi: 10.1023/a:1012388206553. [DOI] [PubMed] [Google Scholar]
- 17.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Boyle CJ, MacFie J, Dave K, Sagar PS, Poon P, Mitchell CJ. Alterations in intestinal barrier function do not predispose to translocation of enteric bacteria in gastroenterologic patients. Nutrition. 1998;14:358–362. doi: 10.1016/s0899-9007(97)00488-7. [DOI] [PubMed] [Google Scholar]
- 20.O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Dwyer ST, Michie HM, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin alters intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe SJ, Burnes JU, Thompson RL. Recurrent sepsis in home parenteral nutrition patients: an analysis of risk factors. JPEN J Parenter Enteral Nutr. 1994;18:256–263. doi: 10.1177/0148607194018003256. [DOI] [PubMed] [Google Scholar]
- 23.Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. New developments in the diagnosis of bloodstream infections. Lancet Infect Dis. 2004;4:751–760. doi: 10.1016/S1473-3099(04)01205-8. [DOI] [PubMed] [Google Scholar]
- 24.Piedra PA, Dryja DM, LaScolea LJ., Jr. Incidence of catheter-associated gram-negative bacteremia in children with short bowel syndrome. J Clin Microbiol. 1989;27:1317–1319. doi: 10.1128/jcm.27.6.1317-1319.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierro A, van Saene HK, Donnell SC, Hughes J, Ewan C, Nunn AJ, Lloyd DA. Microbial translocation in neonates and infants receiving long-term parenteral nutrition. Arch Surg. 1996;131:176–179. doi: 10.1001/archsurg.1996.01430140066018. [DOI] [PubMed] [Google Scholar]
- 26.Reddy BS, Gatt M, Sowdi R, MacFie J. Surgical manipulation of the large intestine increases bacterial translocation in patients undergoing elective colorectal surgery. Colorectal Dis. 2006;8:596–600. doi: 10.1111/j.1463-1318.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- 27.Rocha F, Laughlin R, Musch MW, Hendrickson BA, Chang EB, Alverdy J. Surgical stress shifts the intestinal Escherichia coli population to that of a more adherent phenotype: role in barrier regulation. Surgery. 2001;130:65–73. doi: 10.1067/msy.2001.115360. [DOI] [PubMed] [Google Scholar]
- 28.Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 29.Sitaraman SV, Klapproth JM, Moore DA, 3rd, Landers C, Targan S, Williams IR, Gewirtz AT. Elevated flagellin-specific immunoglobulins in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G403–G406. doi: 10.1152/ajpgi.00357.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sukhotnik I, Yakirevich E, Coran AG, Siplovich L, Krausz M, Sabo E, Kramer A, Shiloni E. Lipopolysaccharide endotoxemia reduces cell proliferation and decreases enterocyte apopotosis during intestinal adaptation in a rat model of short-bowel syndrome. Pediatr Surg Int. 2002;18:615–619. doi: 10.1007/s00383-002-0862-8. [DOI] [PubMed] [Google Scholar]
- 31.Tangpricha V, Luo M, Fernandez-Estivariz C, Gu LH, Bazargan N, Klapproth JM, Sitaraman SV, Galloway JR, Leader LM, Ziegler TR. Growth hormone favorably affects bone turnover and bone mineral density in patients with short bowel syndrome undergoing intestinal rehabilitation. JPEN J Parenter Enteral Nutr. 2006;30:480–486. doi: 10.1177/0148607106030006480. [DOI] [PubMed] [Google Scholar]
- 32.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2006;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Terra RM, Plopper C, Waitzberg DL, Cukier C, Santoro S, Martins JR, Song RJ, Gama-Rodrigues J. Remaining small bowel length: association with catheter sepsis in patients receiving home total parenteral nutrition: evidence of bacterial translocation. World J Surg. 2000;24:1537–1541. doi: 10.1007/s002680010274. [DOI] [PubMed] [Google Scholar]
- 34.Ugur A, Marashdeh BH, Gottschalck I, Brøbech Mortensen P, Staun M, Bekker Jeppesen P. Home parenteral nutrition in Denmark in the period from 1996 to 2001. Scand J Gastroenterol. 2006;41:401–407. doi: 10.1080/00365520500441247. [DOI] [PubMed] [Google Scholar]
- 35.Vanderhoof JA, Young RJ, Murray N, Kaufman SS. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J Pediatr Gastroenterol Nutr. 1998;27:155–160. doi: 10.1097/00005176-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez-Estivariz C, Griffith DP, Galloway JR, Wischmeyer PE. Parenteral glutamine increases serum heat shock protein-70 in critically ill patients. Intensive Care Med. 2005;31:1079–1086. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123:1313–1319. doi: 10.1001/archsurg.1988.01400350027003. [DOI] [PubMed] [Google Scholar]