Introduction
In order to carry out the most effective and safe neurosurgical interventions for patients with brain lesions, precise information about the unique structural and functional anatomy of that patient is essential. Eloquent cortical areas cannot be recognized solely by identifying known anatomical landmarks even in non-lesional brains [1-3]. In patients with mass lesions or longstanding epilepsy, physical distortion or compensatory reorganization can further alter normal relationships [4-6]. In order to select patients for surgery, plan the operative approach, and successfully execute the surgical goal, detailed information regarding the individual structural and functional anatomy and the relationship to the lesion is potentially very useful. Thus a number of brain mapping methods which have been developed for neuroscientific brain mapping efforts have been adapted to serve neurosurgical considerations. There are fundamental differences in how these methods are applied to neurosurgical patients and problems. Unlike group studies for neuroscience in which inferences are made about a population based on a sample, neurosurgical planning requires single-subject specific information, spatial precision, adequate accommodation for impaired task performance, prioritization of sensitivity over specificity, and robustness in the face of mass lesions. In the last decade much progress has been made towards fulfilling these needs using several techniques.
In this chapter, we will elaborate on the particular opportunities and challenges associated with brain mapping as it applies to patient selection, presurgical planning and intraoperative guidance. We will discuss the principal methods briefly noting in particular relative strengths and weaknesses. We will then review approaches for mapping cortical motor, sensory and language areas, as well as the associated white matter connections. We will also discuss some practical and technical considerations including patient-specific concerns, issues related to data acquisition, analysis and interpretation, and visualization considerations for planning and intraoperative use. We will then discuss the specific role of brain mapping in patients with specific pathologic processes including brain tumors, vascular malformations, and medically refractory epilepsy.
Brain Mapping Techniques
Direct cortical stimulation (DCS), and the intracarotid amytal (IAT) or Wada test are often considered the gold standard procedures for functional mapping and language lateralization respectively [7]. Their status as gold standard is based on the long experience with these procedures as well as the way in which these procedures localize eloquent brain. Both procedures involve deactivation, in which a brain region, either localized in the case of DCS, or nearly hemispheric in the case of IAT, is taken off line, and the patient's neurologic function tested. In this way, the procedures mimic what deficits might be expected after resection. Although these procedures have proven to be effective, they are highly invasive mapping techniques which carry significant risk of morbidity, and which necessitate active patient cooperation during testing. Functionally impaired patients or those with an altered level of consciousness may find it difficult to perform the tasks thus decreasing the value and broad applicability of these procedures. DCS of the grey matter within the depth of the sulci is also usually not readily accessible. Furthermore, DCS is conducted either within the surgical procedure or a short time before it, leaving little time for effective surgical planning or for the contemplation of alternative therapeutic strategies [8].
Thus, the recent development of less invasive mapping techniques has provided an appealing alternative or adjunct for many neurosurgeons. Most of these techniques map the brain by recruiting the functionality of a particular brain region through a behavioral task (paradigm) and measuring consequent brain changes. For instance, during functional MRI (fMRI) or magneto-encephalography (MEG), patients are asked to perform a task such as a language, visual, or movement paradigm, while changes in blood flow, metabolism, or electric activity in activated brain regions is measured. Although this general approach can demonstrate all functional brain regions that are involved in the execution of a particular task, it cannot differentiate between brain regions that are essential for execution of the task and those that are merely playing a supportive role. On the other hand, inhibition (blocking) methods, like DCS or IAT, temporarily disrupt a specific brain region from functioning, thereby testing for an inducible neurological deficit. Transcranial Magnetic Stimulation (TMS) may act as an inhibition method, e.g. for language mapping, but more commonly is used for neurosurgical mapping as a direct activator, e.g for motor mapping. This method stimulates a given brain region directly, rather than by engaging the subject in a behavioral paradigm, and can thus depict the causal relationship of the brain tissue in question to the task execution. Yet, this approach also has limitations. Even direct mapping of the primary motor cortex with TMS may not show all of the areas involved in motor performance. Inhibition methods may fail to demonstrate sufficiency. For instance, the disruption of face motor pathways, or even of the temporalis muscle by TMS could cause speech arrest without interfering with language function per se. Finally, diffusion tensor imaging (DTI) while not a functional mapping study, is able to provide information on the location and trajectory of white matter tracts which may inform interpretation of functional brain areas. Thus, it is critical that the neurosurgeon understand the strengths and weaknesses of each approach in order to most benefit from pre-surgical mapping.
Whether the mapping technique is an observational one such as positron emission tomography (PET), MEG, or fMRI, an inhibition technique like TMS, or an advanced structural map like DTI, these brain mapping modalities are rapidly acquiring an expanded clinical role in the surgical planning phase. Each technique is based on different physiological properties thus providing different types of functional maps. Furthermore, each imaging modality is characterized by a specific set of advantages and disadvantages related to spatial and temporal resolution, to the degree of invasiveness, and to the costs of implementation. Combining different functional mapping methods, while resource and time intensive, may provide optimal information by offsetting the strengths of one technique against the weaknesses of another or by integrating functional and structural information. With all of these methods, when used for surgical planning, consideration must be given to sensitivity and specificity: in order to not miss an eloquent brain region, it is particularly important to avoid false negatives, thus maximizing sensitivity, sometimes at the expense of specificity.
Understanding the physiologic and technical underpinnings of each mapping method is particularly important in order to use them most effectively for clinical decision-making. For example, PET detects the relative position of radioactively labeled compounds within the patient's body, and can thus provide a wide range of functional and physiological data. PET is emerging as a tool to guide the location of biopsies in order to avoid sampling error leading to undergrading of tumors that may have heterogeneous histologic characteristics. PET is also frequently used to demonstrate areas of interictal hypometabolism associated with epileptogenic foci. Areas under development include radiotracers targeted to specific receptors which may one day guide functional neurosurgery. However, the signal to noise ratio (SNR) and temporal resolution of PET are both relatively poor, and the spatial resolution in best case scenarios is considered to be only moderate. Diffusion tensor imaging on the other hand can quantify the magnitude and direction of water diffusion in brain tissue, and is thus able to demonstrate the location and trajectory of white matter tracts. However, DTI is vulnerable to signal loss artifacts from air spaces and provides little information about the functional status of the depicted white matter tract. As routinely deployed, DTI cannot reliably resolve crossing white bundles and can also have difficulty demonstrating tracts with high curvature. However, by combining DTI with fMRI it may be possible to make inferences about which tracts are important to preserve based on their physical continuity with cortical areas of interest. MEG noninvasively maps brain activity by measuring changes in the local magnetic fields that accompany neuronal activity. MEG is most commonly used in the presurgical evaluation of epileptic patients. In contrast to PET scanning and DTI, the temporal resolution of MEG is excellent on the order of 1ms. However, the spatial resolution is variable and dependent on the model used to approximate the source of the signal and is extremely susceptible to environmental magnetic noise. TMS is an emerging mapping technique which induces neuronal changes by delivering magnetic fields at the scalp which pass unimpeded through the scalp and skull and are able to stimulate the cortex in a manner analogous to DCS. In TMS, electrical activity in a functional brain region may be either stimulated or inhibited depending on the mode of stimulus delivery via a magnetic field change delivered to the scalp. This technique could provide a substantial advantage to the neurosurgeon because it is the only non-invasive modality that can block neuronal function [8]. However, TMS requires dedicated equipment and personnel, has not yet been reliably able to map language function, and may be contraindicated in patients with seizures [9].
Functional MRI as a non-Invasive pre-surgical mapping technique
Presently, the most commonly used functional mapping procedure for surgical planning is fMRI, a functional mapping technique that measures the relative changes in oxygenated and deoxygenated hemoglobin, and thus blood flow, as a surrogate for neuronal activity. Being completely non-invasive and deployable on many clinical MRI scanners, its clinical role has rapidly expanded. The physiological information demonstrated by fMRI can be acquired with and coregistered to corresponding structural images in a relatively straightforward way. The safety and relative ease with which fMRI can be acquired allows its use not only for planning optimal surgical strategy, but may also be used to guide the decision whether to perform surgery and for patient counseling regarding risk. Furthermore, the registration of preoperatively acquired fMRI data into neuronavigational guidance system can help guide the deployment of intraoperative electrocortical stimulation [10-14].
What do we expect from functional mapping in presurgical planning?
The indications for the use of functional brain mapping continue to evolve as technology develops and becomes more broadly applied. In 1999, Lee at al. retrospectively evaluated the impact of fMRI on the treatment plans of 46 patients scheduled for neurosurgical resection of either a tumor or epileptogenic foci [15]. The group determined that fMRI contributed substantially in three key areas: 1) allowing the neurosurgeons to assess the risk of inducing a neurological deficit and thus allowed them to evaluate the actual feasibility of the resection; 2) providing substantial neurosurgical guidance by directing the placement of the bone flap or of subdural grids for mapping or EEG recording; and 3) helping to identify patients who required further evaluation through invasive mapping techniques. Overall, the study concluded that functional MR imaging studies were used in at least one of the three clinical steps in 89% of tumor patients and 91% of epilepsy patients. Petrella et al. conducted a similar prospective study, analyzing the role of sensory-motor fMRI localization on 39 neuro-oncological patients being evaluated for potential neurosurgical resection [16]. The authors reported that fMRI altered patient treatment approaches in 49% of patients and allowed the neurosurgeons to further maximize the extent of tumor resection in 45%.
The impact of PET scanning on the neurosurgical treatment of pediatric patients with brain gliomas has also been investigated [17]. The study group concluded that the addition of PET scans into the neuronavigational guidance system substantially improved the diagnostic yield of glioma biopsies, and optimized designated surgical trajectories. In addition, the number of trials needed to reach gliomas situated in remote locations, such as those near the pineal gland, was significantly less when PET scanning was used. The ability of MEG scanning to predict the epileptogenic foci in patients with refractory seizures has been examined and found to be nearly as helpful as intracranial EEG in correctly predicting the epileptogenic foci (MEG, 57%, intracranial Video-EEG, 62%) [18].
How valid is functional mapping for surgical planning?
Given the relative newness of the functional mapping methods, there remain numerous issues related to the validity and reliability of the methods under different conditions. One particular area of note is that several of these methods evolved out of neuroscience efforts and the vast majority of publications are related to group results. Functional mapping for neurosurgical planning, though, must inform about individual subjects, and the cost for erroneous results may lead to significant morbidity, or on the other extreme to under-treatment of a lesion which might be surgically addressed. Thus ongoing validation of the brain mapping approaches against each other and against the gold standard methods of Wada testing and intraoperative DCS mapping is particularly important. Quite a lot of work has been done examining agreement between mapping approaches and increasingly examining impact on patient care [19-25].
Although fMRI offers substantial advantages to the neurosurgeon, its use in clinical decision-making should bear in mind several technical difficulties. Functional MRI does not assess neural activation directly, but measures the associated variations in the cerebral blood flow using BOLD (blood oxygen level dependent) contrast on T2* weighted images. Thus, an intact autoregulatory response is required for proper signal interpretation. Pathologic processes in the brain that disrupt the neural hemodynamic coupling may alter the concordance between neural activity and the cerebral blood flow. For example, the signal can be disrupted by the mass effect of a lesion, or, large cerebral veins can induce susceptibilities that can also distort the BOLD signal interpretation. These issues can diminish the sensitivity and specificity of functional brain mapping. Furthermore fMRI acquisition and analysis as yet has no accepted standard procedure. There is no standardized battery of behavioral paradigms, and determination of the significant threshold of activation is a subjective process. Finally, the statistical analysis of the data is diverse; different statistical approaches can be followed in the data interpretation. Furthermore, fMRI only depicts the topography of the functional cortical areas, but does not provide information about the white matter connections, that are also as important to maintain the proper neuronal functionality of the patient. Due to these limitations, data interpretation and clinical decisions need to be made with caution.
Several studies demonstrated higher incidence of neurological deficits in patients who underwent resections that were within 0.5 to 2 cm of functional cortex, when fMRI alone was used to map functional regions as opposed to using DCS [5, 26-28]. For instance, Mueller et al. used fMRI to map the functional cortex and showed that post-operative neurological deficits occurred in 0%, 33%, and 50% of cases when the resection margins were beyond 2cm of the eloquent cortex, within 1-2cm of the eloquent cortex, and less than 1cm from the eloquent cortex, respectively[29]. Although these studies advocated intraoperative DCS to map functional cortex, when the spatial distance between the lesion and the functional cortex was less than, or equal to 2cm; it should also be emphasized that these early studies concluded that fMRI was a safe and reliable mapping tool that carried very promising potential for the future.
Statistical analysis in clinical implementations of fMRI
It should be emphasized that the area of activation in fMRI is dependent on the statistical threshold and a multitude of other factors. When the statistical threshold is lowered, the area of activation increases, and when the statistical threshold is raised, the area of activation decreases (figure 1). Thus, fMRI does not measure absolute values, and therefore cannot be used to infer absolute spatial distances. Although a specific description of the statistical methods implemented to analyze fMRI data is beyond the scope of this text, it is important to note that the color-coded maps presented for fMRI studies depict statistical likelihoods, usually as T or Z scores. Additionally, variation exists in the magnitude, shape, and location of a signal across different individuals performing an identical task under controlled conditions [30]. The reason for this varied fluctuation has not yet been precisely determined. Furthermore, there is also intra-subject variability in the signal generation that could be influenced by numerous physiologic and psychological factors, such as caffeine ingestion[31, 32], sleep deprivation[33], level of attention[34], and fatigue[35]. For neuroscience applications, group analysis merges the data across different subjects and random effects analysis helps to diminish the impact of individual variability. For surgical planning however, capturing individual variability is the point of the exercise, but avoiding, or at least being aware of, potential artifacts is critical for accurate inferences.
Figure 1.
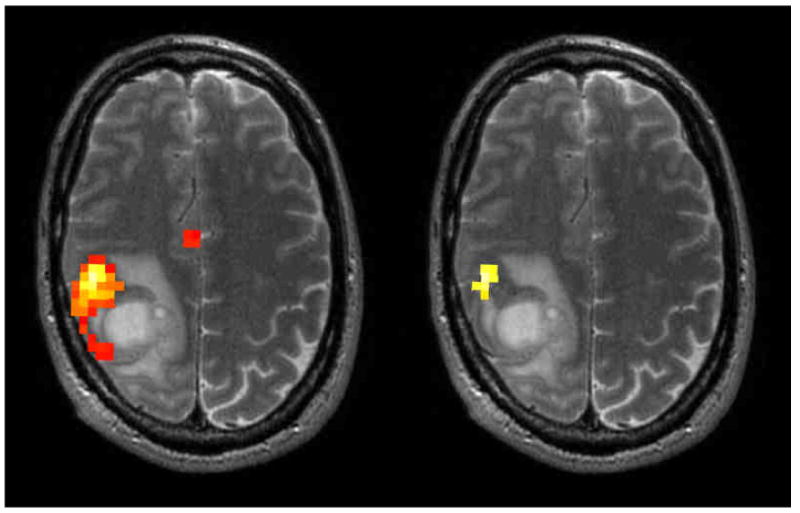
Axial slices of fMRI hand motor mapping in a patient with a right frontal high grade glioma. The same data is shown in both panels at two different statistical thresholds illustrating the difference in the relationship of the activation to the lesion.
Spatial Precision: Smoothing and the inverse solution
Spatial smoothing is a common step implemented during the analysis of fMRI and PET datasets to improve SNR and because it is a prerequisite for standard statistical analysis using the general linear model. For neuro-scientific studies of groups, smoothing is also used to reduce the effect of functional and anatomic variability within and between individual subjects. Smoothing is usually implemented as a convolution of spatial data with a Gaussian kernel. In other words, neighboring voxels within a predefined spatial extension are averaged and “smoothed” into a single voxel value. This processing step optimizes the signal strength and enhances the “blob” appearance of active brain regions on functional maps. This added benefit however, comes at the undesirable cost, particularly for surgical planning, of decreasing the spatial resolution of the functional maps. The merging of activation centers can blur and shift them distorting their anatomical depiction on the functional maps. In addition, smoothing creates partial volume artifacts along the edge of the brain, because high intensity brain tissue voxels are averaged with low intensity voxels outside the brain. Maisog and Chmielowska addressed this particular issue, and successfully described a method to correct the artifacts along the edges of the brain [36]. Other approaches using data driven analysis strategies such as independent component analysis (ICA), have also been proposed which do not require spatial smoothing [37].
Localization of function (or signal sources) based on the observation of recorded signals at the scalp, i.e. MEG and EEG, requires assumptions about a model to which the data is fitted. This is known as the inverse problem of estimating the model parameters based on the data. In the case of MEG and EEG, the inverse solution is ill-posed, thus requiring that some assumptions be included in order to solve the problem. In clinical MEG, one model which is frequently used is the single equivalent current dipole. This model assumes that the generator of the measured signal originates from a single source. In practice, this approximation may result in solutions which do not accurately localize the true source or sources of the signal. Other approaches have been proposed, including distributed solutions, but to date there is no consensus on how best to localize the signals.
In practice, it is important with any mapping method that a measure of the spatial uncertainty be presented with the data. For MEG results, generally only dipoles with goodness of fit of ≥ 70% are displayed. The written report should also be inspected for a description of how many of the dipoles were discarded etc. For fMRI, the unthresholded or dynamically thresholded statistical maps can provide some information about the location of the boundary of an activation, though there remains no established way of demonstrating the degree of uncertainty. In general information about acquisition voxel size and the size of the smoothing kernel if applied, is not given in processed clinical fMRI. Discussion between the individuals acquiring, processing and interpreting the studies is very important in understanding the limits of the data.
Shortcomings and considerations of particular techniques
The validity of any functional mapping data interpretation is dependent on numerous preconditions: physiologic assumptions are accurate, adequate signal to noise, patient cooperation during the data acquisition, and the methods of analysis. Different techniques will be best for different situations and below we describe some technique-specific considerations.
fMRI
Functional MRI indirectly assesses the level of neuronal activation by measuring the relative changes in the concentrations of oxy- and deo-xyhemoglobin present in the microvasculature. However, several studies have suggested that signal distortion can also be generated from larger draining veins not directly involved in the activation process (in the so-called vein effect), a phenomenon that can substantially decrease the precision of the spatial localization[25]. Abduljalil et al. suggested that the use of spin echo sequences can reduce this distortion from the macrovasculature downstream of the activated area [38]. However, spin echo sequences are less sensitive to magnetic susceptibility effects (which are the basis of the BOLD signal), and thus require longer scanning acquisition times, and cover smaller areas.
Head movement during fMRI acquisition severely degrades data quality. Not only can excessive head motion result in supplemental “false” activation, it can also decrease or even obscure “real” activation by altering the signal time course in such a way that the synchronous MR signal from the task paradigm is concealed. This effect can be caused by both abrupt and gradual head movements [39, 40] (figure 2). Krings et al. further showed that motion-related signal artifacts in paretic patients occurred in a significantly higher ratio than in patients without a paresis (72% vs29%) [39]. These are felt to be related to compensatory movement efforts recruiting uninvolved, usually proximal, musculature, usually. We have found that patients have more head motion than healthy controls. In general if the patient's maximal displacement is more than 2mm we repeat the acquisition once to see if better motion parameters can be achieved. If not, then the data is not deemed interpretable.
Figure 2.
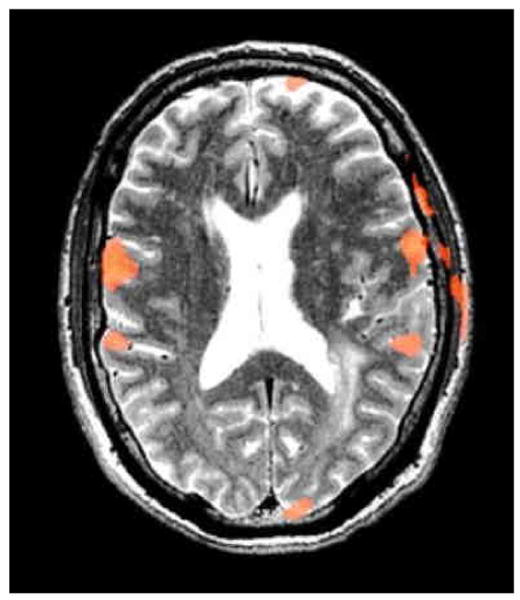
Axial T2 image with superimposed fMRI activations from a scan with excessive head motion (2.28 mm) resulting in artifactual activation in the skull for a vocalized antonym task.
Bold fMRI reliance on susceptibility imaging is a double-edged sword. Susceptibility artifacts for example are created at the junction of air and tissue interfaces, such as in the vicinity of the tympanomastoid air cells of the middle fossa, and from the nasal and sinus airspaces in the orbitofontal cortex causing geometric distortions and drop-outs in signal intensity [41]. Furthermore susceptibility artifacts can also be evident in post-surgical patients due to the presence of surgical clips, titanium plates, metal dust from a skull drill, or prior blood products. The presence of these paramagnetic objects can create significant susceptibility that can mask the signal generated from the surrounding neural cortex. Thus constant caution is required in the fMRI data interpretation of postsurgical patients. The customary habit of overlaying the functional brain maps on T1 weighted conventional images can be furthermore troublesome, because the artifacts may no longer be discernible.
MEG
MEG scanners and the shielded room required to house them are very expensive, thus limiting broad clinical applicability outside of a few centers. Currently, the cost of installing a MEG scanner can be more than $2 million; MEG scanners also require dedicated personnel. A further drawback of MEG scanners is their extreme susceptibility to the surrounding magnetic fields, such as the magnetic noise of the hospital, and even the earth's own magnetic field.
The clinical applicability of MEG is further hindered by the relatively large spatial distance that separates the source of the signal and the MEG detectors; decreasing both the spatial resolution, and the accuracy of signal source localization [42]. Furthermore, interpreting MEG signals is also problematic whenever multiple sources in the brain become active simultaneously. Then, an infinite number of ways could be used to interpret the signal. For instance Liu et al. showed that several signals that in reality were generated from several sources that were 1-3 cm apart were erroneously portrayed as a single superimposed signal on MEG scanners [43].
PET
PET scanners are characterized by a low spatial and temporal resolution. Furthermore the SNR of PET scanners is also relatively weaker than the SNR of other noninvasive mapping techniques such as fMRI. The temporal resolution is especially limited because a substantial temporal delay exists between the imaging phase of the procedure and the visualization phase of the metabolically active foci that discern the neuronal changes. Moreover, PET is a relatively invasive procedure that invokes the administration of a radioactively labeled tracer. Thus, certain patient populations such as children are excluded from PET imaging. Similarly to MEG scanning, PET imaging is also very expensive, and also requires dedicated personnel. The equipment of PET scanners is also very highly priced. Furthermore, a complete PET scanning platform would require a cyclotron to generate radioactive tracers. Similar to fMRI, PET scanning is also an imaging modality that is hemodynamically based. Thus, it is also a technique that is susceptible to signal distortions from uncoupling issues. Finally, PET scanning is also an observational technique and can thus not differentiate functional areas that are essential for the task execution from those areas that are only supportive to the task execution.
DTI
DTI is presently the only method which can allow the visualization of white matter tracts in vivo. However, several technical limitations still hinder its complete clinical applicability for surgical planning. For instance, 3D tractrography is generally “seeded” over the whole brain creating a large mass of fibers which obscure visualization. Several approaches have been developed to aid in the selective visualization of tracts including seeding from specific anatomical or functional landmarks. Nevertheless, for tracts in close vicinity to a brain lesion it may be difficult to tell whether the tract is running within the lesion or just beyond it. In a recent study, Golby et al. tried to address these issues by developing an interactive software tool that can show all white bundle tracts within a specific distance from the tumor boundary, and allows the clinician to instantaneously redefine the spatial distance required, enabling the clinician to view all the tracts layers around a specific brain lesion [44]. In addition the software also included the ability to place “seedpoints” in regions of interests, which also allow the clinician to visualize all the tracts passing through that particular location. Since DTI depends on the fractional anisotropy of white matter to define areas of tightly bundled fibers, anything which lowers the anisotropy, such as tumor infiltration or edema may falsely obscure the presence of preserved tracts. Also, like some of the functional mapping methods, the visualization of DTI results depends on setting several thresholds such as the FA and curvature stopping thresholds. Thus there is a subjective element to which tracts may be visualized. Furthermore DTI does not provide any information that relates to the interactions between white bundle tracts and functional cortical regions. Without a priori knowledge acquired from some sort of functional mapping such as fMRI, it can be hard to determine the reciprocal interactions between white bundle tracts and the functional cortex. Furthermore DTI cannot identify eloquent structures within the depicted white bundle tracts, nor can it recognize tracts that are necessary for task execution.
Practical and Technical Considerations
Hardware
All of the brain mapping techniques require dedicated hardware. For many centers, fMRI will require the least capital investment since fMRI can be performed on most modern clinical scanners and technical personnel can be trained in additional skills. DTI can also be acquired in addition to any routine clinical studies. In the past ten years, the most commonly available magnetic field strength was 1.5T. Today, however, 3T field strength is widely being recognized as the optimal strength for most clinical applications [45]. As a notable advantage, the signal to noise ratio (SNR) is substantially higher. The signal at 3T, is expected to be four times stronger than at 1.5T. Unfortunately, the noise also increases two fold, and thus only results in a net two times factor increase in the SNR. However, this higher resolution in the imaging scans can have important clinical implications, and provides neurosurgeons with a substantial advantage during the surgical planning phase [46]. Finally, at 3T spatial resolution can be improved, thus allowing more precise mapping. Advances in coil design have now made multi-channel (8- or 16 channel, or even 32-channel) head coils fairly available. The increase in SNR from the multi-channel coils can be used to shorten acquisition times or to allow for smaller voxels. Shortening the data acquisition phase can decrease the occurrence of motion artifacts since it is easier for patients to tolerate and makes it more feasible to acquire both structural and functional images in one sitting [47]. Overall, designing fMRI scanning paradigms involves making tradeoffs about acquisition time, voxel size and scan coverage. While higher field strength and multi-channel coils can provide more SNR, the issues of how to effectively maximize acquisitions remain important.
Compared to fMRI, PET and MEG require significant investment. Both methods require very expensive scanners, dedicated and specially shielded space, and specially trained personnel. Institutions which already have these suites set up, either for existing clinical applications or research efforts are likely the sites where these types of studies will be performed in the near future. In addition functional PET scanning will require the support of nuclear chemists and a cyclotron to maximize the potential. TMS set ups are relatively much less expensive to acquire, but have very limited penetration in clinical sites to date.
Software
Software plays a critical role in the acquisition and analysis of data from all the brain mapping methods. In addition to the basic processing software, there are also frequently issues of separate software systems which need to work together. The most common format for processed images remains DICOM ((Digital Imaging and Communications in Imaging), Medical Imaging and Technology Alliance, Arlington, VA), although other formats such as NIfTI (Neuroimaging informatics Technology Initiative, jointly sponsored by NINDS and NIMH) are also being developed. It is particularly important to have personnel with good working knowledge of the software. In going from the acquisition phase on the imaging device to a processing computer and then to a display or navigation system, there are numerous steps in which potential inconsistencies can be introduced. An audit trail of data integrity is particularly important when multiple platforms are used. Some groups have developed fully automated pipelines for data analysis and display which can help to insure quality control and streamline workflows. The most commonly used software packages for fMRI and PET have been Brain Voyager [48] and SPM (Statistical Parametric Mapping, Wellcome Department of Neurology, London, UK). Although these software packages are able to import DICOM images, perform 2D and 3D statistical data analysis, and analyze single and multiple subject data sets; these applications were mainly designed for research use, and they were not tailored for the clinical interpretation of single subject data or for use by clinicians. With the expanding role of fMRI in the clinical domain, multiple manufactures have begun to develop software tailored for clinical applications. Applications have been designed for use on the major MRI scanner platforms as well as by third party vendors. Most packages include both stimulus delivery for controlling behavioral paradigms and analysis and visualization software. These software packages, unlike the earlier research packages designed for neuroscience research, do not generally focus on strategies for analyzing multiple subject data such as normalization and random effects statistics, nor is there the degree of customization generally available in research platforms. Rather, since they have been designed for clinical application the focus is on single subject acquisition, standardization of procedures, ease of use, and regulatory compliance. Several applications have the ability to monitor and display the activation maps with only minimal delay, thus providing direct visualization of statistical t maps as the data is being acquired. A substantial advantage of this feature is its ability to monitor the quality of the data at the time of the study. Thus, if poor fMRI maps are being generated, the study can be stopped and any complicating factors such as stimulus display problems, misunderstanding of task instructions, excessive head motion, etc. can be addressed before the patient leaves the suite. The post-acquisition packages can perform a semi-automatic in depth analysis of the datasets that are based in part on the older research software. Thresholding can be performed at the time of acquisition or ideally can be dynamically changed by the interpreting clinician. After analysis, data may be represented as contoured or segmented structures on the 2D images or as 3D colored functional maps. In turn, the data can be viewed in any plane, or may be projected onto a reconstruction of the individual's cortical surface thereby providing a useful surgical planning tool to the neurosurgeon (figure 3).
Figure 3.
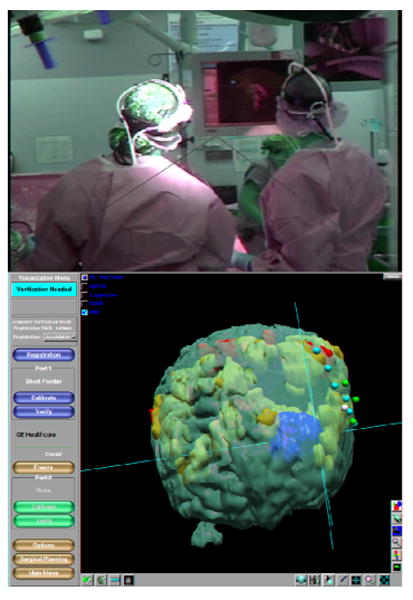
OR view showing screen where navigational data including fMRI and ECS locations is visible to the surgeon (top panel) and navigation system slice view (middle). Outlines of segmented structures including the fMRI and tumor may also be visualized through the operating microscope if tracked (bottom).
DTI visualization has been developed as part of many major research software packages including DTIStudio, FSL, Camino, BrainVisa, MedINRIA, and Slicer. These packages have many features and are highly customizable, but require expert users and are not designed for clinical use. More recently, commercial software designed for clinical use including BrainLab and Siemens syngo DTI Tractography, has become available. Most clinicians are interested in viewing the tractograms which show the output of tractography processing. However, reference to the less processed images such as the fractional anisotropy (FA) maps and the 2D images representing the degree and direction of anisotropy as glyphs my also be useful.
The software for processing, analysis, and display of MEG data is usually included with the MEG hardware. As such these FDA-approved software suites are the most commonly used for clinical work. There are also numerous research software platforms developed by both commercial and academic efforts.
Software for TMS, also, has been developed by both commercial and academic initiatives. The software is used to control the delivery of the magnetic fields as well as to create a digital recording of the events of the session. TMS has also been integrated with frameless navigation for greater spatial accuracy (NexStim, Helsinki, Finland).
Integrating Functional Data into Surgical Guidance Systems
To provide multi-modality image guidance to the neurosurgeon during the surgical procedure, navigational systems have developed the capacity to display multiple datasets including functional data together with any structural data into a single multimodality platform [49]. The use of such systems for surgical planning before the case is probably underappreciated presently. Using the navigation planning system workstation, or research software such as Slicer, the datasets can be merged and viewed. When multiple types of studies acquired at separate sittings on separate platforms are used accurate co-registration of datasets is critical. Regions, activations, or tracts of interest and the lesion can be highlighted and a surgical plan can be developed. Most commercial neuro-navigational guidance systems portray the area of interest as a graphic illustration and both 2D and 3D models (figures 3 and 4). In addition, a graphical overlay may be visualized through the surgical microscope provided that the microscope can be tracked by the navigation system. This capacity is available for most modern operating microscopes, though there may be additional components which will need to be purchased. Studies in the literature are beginning to demonstrate the added clinical value of integrating multimodal data into neuronavigational guidance systems [50].
Figure 4.
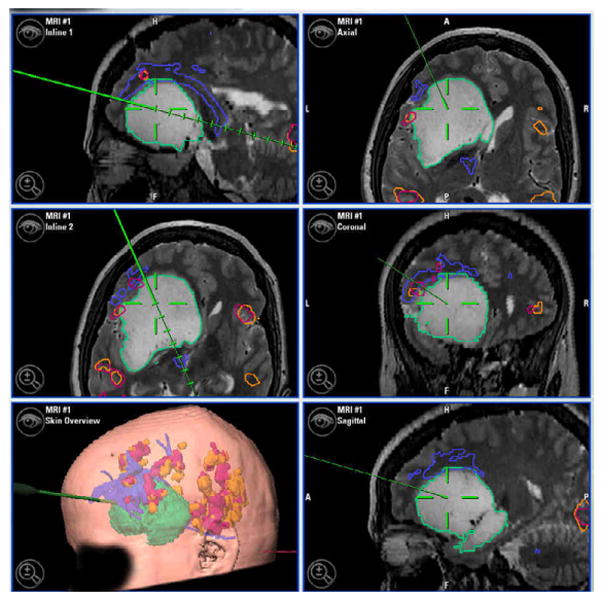
Intra-operative display of pre-and intra-operatively acquired data co-registered to patient and displayed by navigation system 3D viewer. Numbered foreground dots indicate intraoperative cortical stimulation locations. Large green model is segmented tumor. Blue model superior to green is tractography. Orange and red models are functional MRI activations from language tasks in the patient's first and second language. The surgeon can choose a combination of viewpoints which is particularly important to appreciate the three dimensional relations between functional locations and the tumor. Clockwise from top-right: standard orthogonal slices; 3D viewer; axial and sagittal in-line views in the plane of the navigation probe.
Although a large number of groups have reported success in integrating multimodality data into a single neuronavigational system, several technical challenges still hinder its practical clinical application. For instance, the large amount of functional and structural data that is depicted by each imaging modality can result in incomprehensible data compilations that can make it difficult to identify the precise anatomical and functional features from each imaging technique, particularly within the constraints of the operative setting. This issue may be exacerbated when the surgical planning phase is not performed by the neurosurgeon conducting the operation or when several different people take part in the creation of the functional maps. Such a large amount of data also can frequently slow down the speed with which the navigation system updates the tracking display meaning that the surgeon must wait a few seconds after pointing the probe in the field. While this is not a serious downside, it should be noted when many datasets have been loaded into the system.
The integration of functional data such as fMRI, DTI, or MEG into a neuronavigational guidance system is no longer unusual, due to the availability of faster performing computers, better software/hardware equipment, faster networking, and broader availability of high field MRI scanners. For instance, Jannin et al. successfully integrated MEG, fMRI, and anatomical MR images into a single neuronavigational system to track functional regions in 11 patients with lesions near the central sulcus [51]. In a similar study Kamada et al. also showed that fMRI MEG, and DTI functional data could also be incorporated into a single integrative platform to provide image guidance during the surgical procedure [52]. In a recent study, Rasmessen et al. successfully integrated fMRI and DTI data into a single navigational system, and reported that the incorporation of the functional data into surgical planning provided valuable additional information in a user-friendly way [53]. Even the direct injection of coregistered fMRI images into a surgical microscope with instantaneous portrayal of the functional maps onto the surface of the exposed surgical field is currently available [54].
Yet, several technical limitations are still present; for instance it is challenging for the neurosurgeon to visualize the relationship of the 3D activation maps to the 3D tumor model while the procedure is ongoing (both 2D and 3D views may be helpful). In addition, since viewing the monitor with the structural and functional data cannot be performed simultaneously with surgical action, the neurosurgeon visualizes the neuronavigational data intermittently, thereby limiting his/her ability to comprehend and absorb the data efficiently. In the future, advanced visualization methods, perhaps adopted from military or gaming applications, may help to overcome some visualization challenges.
Guidance of the Surgical Procedure and the Problem of Brain Shift
Perhaps, the most important factor that limits the utility of pre-operative mapping studies is the problem of brain shift. Following the opening of the dural flap, surgical manipulation, CSF drainage, edema, and issues related to gravity and positioning causes the brain to shift, causing an anatomical discrepancy between the preoperatively-acquired images and the surgical field. This displacement is further exacerbated by the progression of the surgical procedure, during which anatomical displacement greater than 10mm can occur within an hour of dural opening [55-59]. Reinges et al. also showed that the anatomical displacement of superficial cortical structures did not correlate with the displacement of deeper brain structures further diminishing the utility of surgical guidance [60]. Several research groups have tried to address these issues by designing algorithms that attempt to correct these anatomical discrepancies [1]. New information may be used to update the images to account for brain shift (e.g. US.) (See figure 5).
Figure 5.
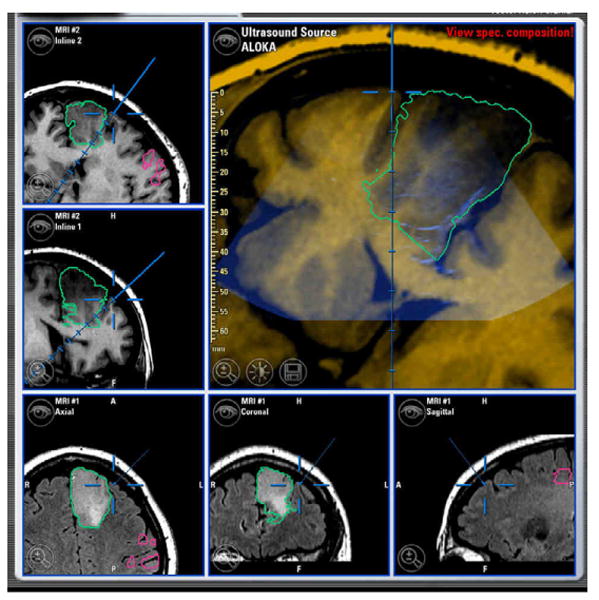
Neuro-navigation screen with co-registered intra-operative ultrasound overlaid on structural MRI images. Ultrasound may be used to perform updated registration to account for some of the brain shift occurring during surgery.
One solution is the acquisition of intraoperative imaging to allow the neurosurgeon to register the intraoperatively acquired anatomical scans to the pre-operatively obtained functional images to further increase the precision and accuracy of the information made intraoperatively available to the neurosurgeon [1, 61]. Where available, intraoperative MRI will thus provide a substantial advantage, but given the amount of time and patient cooperation needed for fMRI, this is unlikely to be possible. Rather, the pre-operative functional mapping and tract data may be updated by non-rigidly warping it based on the updated structural image which uses a segmentation-based model rather than updated imaging [62, 63]. Intraoperative DTI has been acquired and has shown displacements on the order of 1- 1.5 cm can occur [64].
Clinical Considerations
Selecting the Appropriate Paradigm for Presurgical Motor Mapping
It is critical that the neurosurgeon ordering a brain mapping study for the presurgical evaluation of a patient convey the clinical aspects of the case to the radiological staff performing the study. Important considerations include the clinical status of the patient, particularly any deficits which may limit the performance of the task, or which may influence interpretation of the results. The location of the lesion and the planned trajectory are also important to select the tasks to be tested. The functional architecture of the cerebral cortex involved in voluntary motor movements includes primary motor cortex (M1), the supplementary motor area (SMA), primary somatosensory cortex (S1), and the premotor area [46, 65]. Within both the M1 and S1 cortex, each body part is disproportionally represented within a distinctive topographic region. The hand region for instance is within the upper portion of the convexity of the motor cortex (the omega region), while the leg and tongue regions are respectively on the medial and lateral borders. Thus, the performance of finger tapping paradigms will activate the upper portions of the motor cortex, and will usually not activate the medial and lateral parts. These regions can be mapped using tasks such as toe wiggling or tongue movement respectively [29, 66, 67]. We use toe wiggling rather than movement at the ankle joint to minimize any transmission of movement to the head which results in excessive head motion in the z axis. Similarly, lip pursing avoids excessive movement of the jaw or oropharynx which can cause artifacts.
It is also important to consider the reciprocal neuronal connectivity between the precentral and postcentral gyri for patients who have limited motor function due to the mass effect or anatomic location of a brain lesion. It may be reasonable to perform passive sensory stimulations tasks on such patients. Brushing or stroking a body part such as vibrotactile stimulation can be performed to initiate activity in the S1 cortex [68]. Electrical stimulation of the median and tibial nerves has also been shown to elicit a response from the sensory cortex [69]. Although sensory paradigms are more likely to favor the post central sensory gyri; the reciprocal connectivity between the pre and post central gyri can also activate the motor cortex. Such interconnections likely underlie the finding that motor paradigms such as finger tapping can also activate the post-central sensory cortex. Furthermore, in paralytic patients that have impaired sensation, Stippich et al. showed that mere imagination of task execution can elicit a response from the SM1 cortex [70].
The execution of task paradigms may be problematic for patients who, due to language, attention or other deficits are unable to follow the task instructions. For example, it has been shown that the timing of motor performance impacts the resultant maps significantly [71]. Although the length of the task block can be extended (from 16s to 30s for instance) to partially compensate for the fluctuating performance, task execution may still prove to be totally unreliable [25]. Liu et al. recently addressed these issues, and offered an alternative approach for pre-surgical motor mapping which relies on measuring spontaneous synchronized changes in brain activation [72]. This approach builds on a finding first described by Biswal et al. in which fMRI was used to measure slow spontaneous brain activity fluctuations to localize distinctive functional brain regions [73]. The participant is asked to close their eyes while fMRI volumes are continuously acquired. Post-processing then examines the functional connectivity based on temporal correlations between specific functional regions of the brain (such as with the motor network described above). This “task-free” approach which has come to be known as fcMR carries great potential for presurgical planning. However, further patient studies will be required to test whether the robustness seen in healthy subjects extends reliably to neurosurgical patients[74]. Another potential advantage of this approach is that multiple brain functions could possibly be probed within a single 20 minute scanning session. Furthermore, the procedure reduces the need for patient compliance. Patients are simply asked to rest or fixate on a crosshair. In certain cases, FcmR has even been conducted on patients who were under the effects of general anesthesia [72]. FcMR imaging has even been performed on human infants which may allow this approach to be used in children too young to comply with traditional fMRI or other mapping techniques [75].
Other groups have used TMS for pre-surgical motor mapping. Although TMS suffers from some disadvantages, by being a technique characterized by poor spatial resolution, and one that can increases seizure risks in patients, it is the only non-invasive mapping technique that is based on direct brain stimulation or inhibition. In 2004, Neggers et al. successfully incorporated TMS into a frameless stereotactic navigational system, and showed that the TMS results correlated to within 5mm of functional mapping by DCS [20]. In 1997, Krings et al. also investigated the correlation between DCS and TMS in two patients that had tumors infiltrating the functional M1 area, and showed that the correlation was within 1cm between the two techniques [76].
In addition, DTI can also been used to understanding of the patient's motor system. The depicted tractography can demonstrate the corticospinal tracts enabling the neurosurgeon to avoid the resection of displaced white bundle tracts that are affected by either the edema or the mass-effect of the lesion. DTI can also be helpful during the resection of low grade gliomas. Some groups have shown that functional white bundle tracts may still be present within the tumor lesion and tractography can allow the surgeon to plan the resection of the low grade gliomas while preserving the integrity of the tracts [77]. DTI data has also been combined with fMRI to illustrate motor cortical areas with the associated descending tracts [52, 78-80]. Tummala et al. successfully used DTI to resect brain tumor lesions in two pediatric patients near the optic radiation. No postoperative neurological deficit was reported in either patient [81].
Approaches for Language Mapping
Language mapping includes both the determination of the “dominant” hemisphere (or determination of bilateral support for language) and the localization of language areas. The IAT remains the gold standard for establishing language (and memory) dominance in patients [7]. However there are significant complications associated with this invasive test which is also uncomfortable and frightening for patients. A recent study published in 2008 by Loddenkemper et al. conducted a retrospective evaluation of 677 patients that underwent IAT, and showed that 74 patients (10.9%) developed complications (encephalopathy (7.2%), seizures (1.2%), strokes (0.6%), transient ischemic attacks (0.6%), localized hemorrhage at the catheter insertion site (0.6%), carotid artery dissections (0.4%), or allergic reaction to contrast (0.3%) [82]. It can sometimes be difficult to definitively determine language dominance due to altered cerebrovascular flow: Smith et al. evaluated 166 patients undergoing IAT finding that cross flow of amobarbital to the contralateral hemisphere occurred to a certain degree in 61 (36.7%) patients [83]. Functional MRI has a reported overall concordance with IAT testing between 90%-100%[21, 84-89]. Woermann et al. for instance, compared fMRI data results to IAT in 100 epilepsy patients and found a 91% concordance rate between the two tests [90]. In a different study, Fernandez et al. showed that fMRI was as reliable as IAT in its ability to lateralize the regional and global language functions of all investigated patients [91]. Furthermore, Deblaere et al. utilized a relatively low field (1.0T) MRI scanner to lateralize language functions in twenty epilepsy patients, demonstrating that fMRI acquired at relatively low magnetic field strength could lateralize language function with a 100% concordance to IAT [92]. Nevertheless, interpretation of the studies is complicated by issues of which regions of interest should be measured and with what metric. Furthermore, several study groups have combined fMRI with DTI data results to further shed light on the anatomical and physiological causes of asymmetrical language lateralization. Vernooij et al. and Powell et al. have both proposed that language lateralization is related to structural asymmetry of the white matter tracts in the arcuate fasciculus which can be measured by DTI [93, 94], though these findings have not yet led to clinical protocols. Changes in fMRI activation patterns and white matter measures have also been found to reflect handedness[95]. These findings suggest that DTI and measures of white matter structure may one day be useful for predicting structure-function asymmetries in the brain reflecting language lateralization in patients.
Other groups have also shown that MEG can be used as another non-invasive mapping technique to lateralize language function. MEG is usually used to map the functional cortex that is involved in language reception; in addition to providing the important advantage of enabling the neurosurgeon to tract the temporal course of language activation (figure 6). In 2004, Papinacolaou et al. investigated the concordance between MEG and IAT in 100 epilepsy patients and found the rate to be 93% [96]. The group concluded that MEG was reliable enough as a noninvasive mapping technique to replace IAT. In another similar study conducted by Maestu et al. which used a Spanish-based version of the test, the concordance rate between MEG and IAT was found to be 88% [19]. In a recent study conducted by Doss et al., the concordance between MEG and IAT was found to be 86%, while the sensitivity and specificity values were reported to be 80% and 100%, respectively [22].
Figure 6.
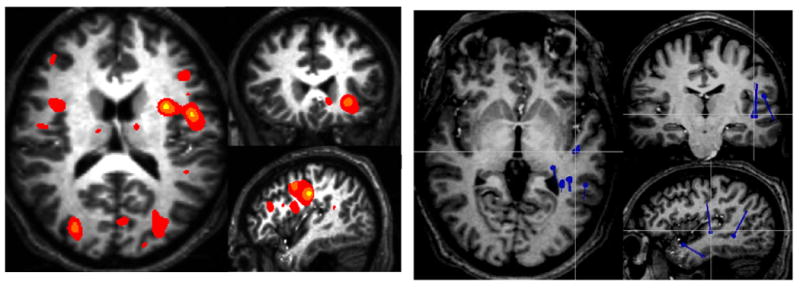
Functional MRI (left) and MEG (right) language maps obtained from the same subject performing the same task (vocalized antonym generation with visual stimuli). While both studies are consistent with left language lateralization, the fMRI demonstrates frontal activations more clearly wheras the MEG shows more temporal dipole sources
Language Localization
To date, no single standardized robust clinical fMRI language battery has been developed, and no universal paradigm has been defined to plan for the surgical resection of brain lesions near Broca's or Wernicke's areas. For instance different groups have implemented different paradigms such as picture naming[97], silent word generation[97, 98], rhyme detection[99], semantic-decision[97, 99, 100], word stem completion[101], and silent reading tasks[102]. Moreover, localization of language function is challenging primarily because of the functional heterogeneity found in peri-Sylvian language areas. The proximity of the brain lesion to the language cortex furthermore complicates interpretation of the functional language localization because of the possibility of lesion-induced plasticity, false negatives related to edema or mass effect, or poor performance due to language impairments. Additionally, as with all fMRI the language-associated region of activation is dependent on the statistical threshold selected, something which remains largely arbitrary. As with all fMRI, lowering the statistical threshold increases the area of activation, and vice-versa when the threshold is increased. Furthermore the extent of activation is also influenced by the baseline task. If a resting baseline is used then generally more extensive activations including sensory and attention areas will be seen whereas if a higher level baseline task (e.g. non-word letter strings) then a more restricted activation pattern is likely to be seen. Thus when implementing fMRI for language mapping great care must be taken in selecting active and control tasks and these choices must be incorporated into the review of the final functional maps by the clinician. For these reasons, the validation of fMRI against direct electrocortical stimulation techniques has produced sensitivity and specificity results that have generally been lower than those produced with the motor-sensory brain mapping. Several groups have shown that both the specificity [99]and sensitivity[100] can be increased by having the patients to perform multiple linguistic tasks.
Disease-Specific Considerations
Gliomas
The diffuse infiltrative nature of gliomas can distort the normal anatomy of the brain and induce several pathological modifications to the cerebral vasculature. High grade gliomas distort the biochemical environment in the brain and alter the normal composition of ATP, pH, glucose and lactate levels. HGG also induce neovascularization by secreting substances such as Vascular Endothelial Growth Factor (VEGF)[103] and rennin[104], promoting the formation of new blood vessels. However the architecture of the newly formed blood vessels is weak, disorganized, and immature due to a decreased amount of perivascular cells. As a result, focal hemorrhage and fluid transudation leak into the extracellular space, increase the oxygen availability for activated neural cells [105-108]. The normal oxygen extraction levels of activated neural areas are thus diminished, and the BOLD signal generation is in its turn also decreased due to a relatively lower concentration of deoxyhemoglobin causing an uncoupling effect between neural activation and autoregulatory vascular responses. In addition, the mass effect of the rapidly growing tumor can compress the surrounding vasculature, and thus furthermore alter the overall BOLD signal effect. In 2003, Signorelli et al. showed that in post-operative patients, the BOLD signal of activated neural areas that were close to the resection cavity witnessed an increase in the signal intensity when compared to the preoperative functional maps [23]. Several reports in the literature have shown that uncoupling can substantially decrease the reliability of fMRI in HGG, and could be wrongfully interpreted as brain plasticity.
Generally functional imaging is very useful for low grade gliomas which may contain functional tissue and for which an understanding of the surgical risks often is the key determinant of the surgical plan. The slow growing nature of low grade gliomas can allow for a functional adaptation and a cortical topographic reorganization to occur which can make interpretation of unusual activation configurations difficult. Russell et al. examined the incidence of transient weakness or SMA syndrome in 27 consecutive patients that underwent resection of tumors neighboring or infiltrating the SMA [109]. The incidence of an SMA syndrome was significantly higher among patients with low grade gliomas, when compared to patients with high grade gliomas, presumably, because infiltrated tissue within the SMA retained some functionality. MEG studies in LGG may be particularly helpful in patients who also have refractory seizures by allowing both functional mapping and a seizure onset localization in a single study.
Diffusion tensor imaging also may be particularly helpful in LGG since preserved tracts seen within lesion (Figure 7). DTI can differentiate infiltration of tracts by the tumor from displacement or destruction of tracts by the tumor. However, edema or tumor infiltration, by lowering FA, may lead to lack of tract visualization when there is actually a tract present.
Figure 7.
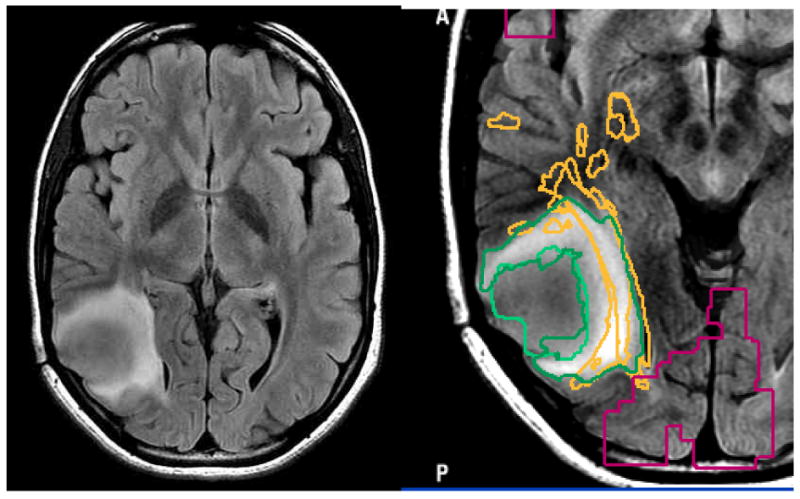
Axial FLAIR image of a patient with an intermediate grade glioma show two distinct regions of abnormality, making it unclear what should be the surgical goal.
Vascular malformations
Arteriovenous malformations (AVM) are the most common cerebrovascular deformity, and consist of an interconnecting complex of arteries and veins that are devoid of a capillary network. fMRI has proven to be a reliable brain mapping tool that has produced results that closely resembles those of intraoperative brain mapping techniques [110, 111]. Additionally, functional mapping has also been reported in the preoperative planning of patients undergoing resections of cavernous hemangiomas [78, 112]. However, assessing the functional integrity of the brain territories that surround an AVM with fMRI may not always be simple. Because AVM are a direct arteriovenous shunting system, they are characterized by a blood flow that has a high velocity, and a low resistance. The blood pressure within the arterial portion of the AVM is characteristically hypotensive, while being hypertensive in the draining venous portion. Because of this irregular pressure difference, the feeding pressure of the artery is relatively lower than normal. This can cause a net reduction in the cerebral perfusion pressure in the regions that surround the AVM. Such vascular perturbations do not necessarily decrease the neurologic functionality of the surrounding brain tissues, but can disrupt the BOLD signal, and obscure activation in brain regions that are functionally active. Thus, functional mapping with fMRI may erroneously label functionally active brain regions as inactive. This problem is especially important in patients that have AVM with severe flow anomalies (Figure 8). For instance, Juenger et al. recently published a case-report that showed that MEG and TMS recordings were more reliable than fMRI data in mapping functional regions within the vicinity of an AVM [113]. In this case report, the validity of presurgical mapping techniques was assessed by comparing each of fMRI, MEG and TMS data results to the results of DCS. However many patients have AVMs with only moderate or even inconsiderate flow irregularities. In such patients, Pouratian et al. showed that the correlation between direct electrocortical stimulation and fMRI can be very high, with a 100% and 66.7% sensitivity and specificity respectively [114]. In a further attempt to minimize the risk of fMRI based surgical planning for the resection of AVM lesions, Cannestra et al. proposed subcatergorizing AVM into three distinctive groups based on the spatial distance between the AVM and the eloquent brain cortex as defined by fMRI [115]. The first group (low risk) included patients that had at least one complete gyrus that separated the AVM from the functionally active brain regions. In the second group (high risk), the association between the AVM and the functional brain regions is profound. In the third group (indeterminate risk), the AVM and eloquent cortex were bordering each other. For the low risk patients, the study group concluded that fMRI alone may be used to plan the surgical resection of the AVM. Patients of the second (high risk) group are generally considered to be inoperable and are usually referred for radiotherapy, while the AVM lesions in the patients of the third group are performed through direct electrocortical stimulation, because the lesion is too close to the eloquent cortex to rely on fMRI (less than 1cm).
Figure 8.
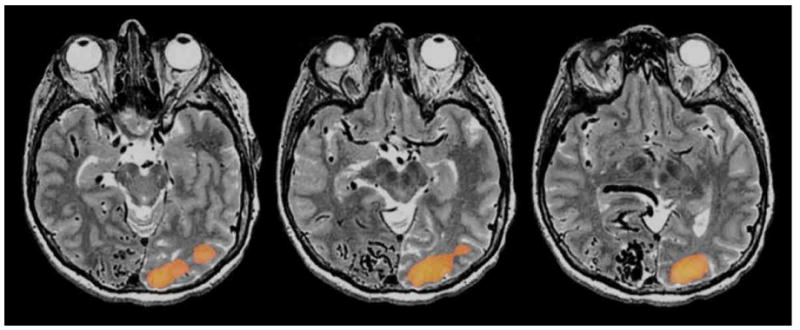
Visual fMRI scans of a patient with a large occipital AVM. A whole filed flashing checkerboard paradigm was delivered. Although the patient had preserved visual perception in the left visual field the patient did not have any visual activation in the right occipital cortex depicted on fMRI
Epilepsy
In the presurgical evaluation of epileptic patients, functional brain mapping is particularly important since the target of resection is usually brain tissue rather than tumor or other tissue. Brain mapping can be used to assess numerous functions for pre-operative patient selection and procedure planning. The IAT and more recently fMRI including fcMRI have been used to assess memory dominance and risk for post-operative memory loss in patients undergoing temporal lobectomy and amygdalohippocampectomy [116, 117].
The IAT, fMRI and MEG have been used for language lateralization and localization. Compared to a control population, patients with temporal lobe epilepsies have been shown to have more bilateral language activation, during the execution of language paradigms, with less lateralization to either cerebral hemisphere. The degree of activation in the homologous language region of the contralateral hemisphere of these patients was significantly higher than t in healthy subjects [118]. Localization of the seizure onset is particularly important in the evaluation of epilepsy patients. MEG, being a modality that assesses neuronal activity directly, can be used to localize interictal discharges which may help to localize the seizure focus. Although the correlation is still not perfect, MEG interictal spike recordings are increasingly being used to identify seizure foci. For instance, Mamelak et al. used interictal MEG recordings to localize seizure foci to the correct lobe and thereby guide subdural grid placement [119]. In addition several study groups have utilized FDG-PET and SPECT, to identify the seizure foci by analyzing the interactal cellular metabolism, and the ictal-interictal metabolism differences respectively [120, 121]. FMRI has developed some role in the localization of the epileptogenic zone. Krings et al. used the fMRI to examine the spatial and temporal course of a patient undergoing a seizure, and were thus able to successfully identify the location of epileptogenic activation. In a recent promising development, fMRI has been combined with EEG to identify the epileptogenic foci in patients with frequent interictal discharges. So called spike triggered fMRI can lateralize medial temporal lobe epilepsies, as well as to identify seizure foci by supplementing the spatial resolution of the MRI to the temporal resolution of EEG in the localization of the seizure foci [122, 123].
Conclusion
The development of numerous functional mapping techniques now gives neurosurgeons many options for pre-operative planning. Integrating functional and anatomical data can inform patient selection and surgical planning and makes functional mapping much more accessible than when only invasive studies such as IAT and DCS were available. However, the applications of functional mapping to neurosurgical patients is still evolving. Acquisition, processing, interpretation and visualization of the functional images remain complex require understanding of the underlying physiologic and imaging characteristics. In particular, the neurosurgeon will also have to accustom him/herself to interpreting highly processed data. Successful implementation of functional image guided procedures requires efficient interactions between neurosurgeon, neurologist, radiologist, neuropsychologist, and others but promises to enhance the care of our patients.
Acknowledgments
Funding Disclosure: NIH 1U41RR019703-01A2, NIH P01-CA67165, Brain Science Foundation, Klarman Family Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Archip N, et al. Non-rigid alignment of pre-operative MRI, fMRI, and DT-MRI with intraoperative MRI for enhanced visualization and navigation in image-guided neurosurgery. Neuroimage. 2007;35(2):609–24. doi: 10.1016/j.neuroimage.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo SS, et al. Evaluating requirements for spatial resolution of fMRI for neurosurgical planning. Hum Brain Mapp. 2004;21(1):34–43. doi: 10.1002/hbm.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolls ET, Grabenhorst F, Franco L. Prediction of subjective affective state from brain activations. J Neurophysiol. 2009;101(3):1294–308. doi: 10.1152/jn.91049.2008. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki S, et al. Identification of pre- and postcentral gyri on CT and MR images on the basis of the medullary pattern of cerebral white matter. Radiology. 1991;179(1):207–13. doi: 10.1148/radiology.179.1.2006278. [DOI] [PubMed] [Google Scholar]
- 5.Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50(2):164–9. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- 6.Steinmetz H, Furst G, Freund HJ. Variation of perisylvian and calcarine anatomic landmarks within stereotaxic proportional coordinates. AJNR Am J Neuroradiol. 1990;11(6):1123–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Wada J. A new method for determination of the side of cerebral speech dominance: a preliminary report on the intracarotid injection of sodium amytal in man Iqkaa te Seibutzuquki. 1949;14:221–222. [Google Scholar]
- 8.Tharin S, Golby A. Functional brain mapping and its applications to neurosurgery. Neurosurgery. 2007;60(4 Suppl 2):185–201. doi: 10.1227/01.NEU.0000255386.95464.52. discussion 201-2. [DOI] [PubMed] [Google Scholar]
- 9.Sack AT, Linden DE. Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Res Brain Res Rev. 2003;43(1):41–56. doi: 10.1016/s0165-0173(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 10.Nimsky C, et al. Integration of functional magnetic resonance imaging supported by magnetoencephalography in functional neuronavigation. Neurosurgery. 1999;44(6):1249–55. doi: 10.1097/00006123-199906000-00044. discussion 1255-6. [DOI] [PubMed] [Google Scholar]
- 11.Maldjian JA, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr. 1997;21(6):910–2. doi: 10.1097/00004728-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 12.McDonald JD, et al. Integration of preoperative and intraoperative functional brain mapping in a frameless stereotactic environment for lesions near eloquent cortex. Technical note. J Neurosurg. 1999;90(3):591–8. doi: 10.3171/jns.1999.90.3.0591. [DOI] [PubMed] [Google Scholar]
- 13.Gumprecht H, et al. Neuronavigation and functional MRI for surgery in patients with lesion in eloquent brain areas. Minim Invasive Neurosurg. 2002;45(3):151–3. doi: 10.1055/s-2002-34341. [DOI] [PubMed] [Google Scholar]
- 14.Schulder M, et al. Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg. 1998;89(3):412–8. doi: 10.3171/jns.1998.89.3.0412. [DOI] [PubMed] [Google Scholar]
- 15.Lee CC, et al. Assessment of functional MR imaging in neurosurgical planning. AJNR Am J Neuroradiol. 1999;20(8):1511–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Petrella JR, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006;240(3):793–802. doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- 17.Pirotte B, et al. PET imaging in the surgical management of pediatric brain tumors. Childs Nerv Syst. 2007;23(7):739–51. doi: 10.1007/s00381-007-0307-8. [DOI] [PubMed] [Google Scholar]
- 18.Wheless JW, et al. A comparison of magnetoencephalography, MRI, and V-EEG in patients evaluated for epilepsy surgery. Epilepsia. 1999;40(7):931–41. doi: 10.1111/j.1528-1157.1999.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 19.Maestu F, et al. Spanish language mapping using MEG: a validation study. Neuroimage. 2002;17(3):1579–86. doi: 10.1006/nimg.2002.1235. [DOI] [PubMed] [Google Scholar]
- 20.Neggers SF, et al. A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage. 2004;21(4):1805–17. doi: 10.1016/j.neuroimage.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Arora J, et al. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–41. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 22.Doss RC, et al. Lateralizing language with magnetic source imaging: validation based on the Wada test. Epilepsia. 2009;50(10):2242–8. doi: 10.1111/j.1528-1167.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- 23.Signorelli F, et al. Technical refinements for validating functional MRI-based neuronavigation data by electrical stimulation during cortical language mapping. Minim Invasive Neurosurg. 2003;46(5):265–8. doi: 10.1055/s-2003-44454. [DOI] [PubMed] [Google Scholar]
- 24.Giussani C, et al. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 66(1):113–20. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- 25.Tieleman A, et al. Preoperative fMRI in tumour surgery. Eur Radiol. 2009;19(10):2523–34. doi: 10.1007/s00330-009-1429-z. [DOI] [PubMed] [Google Scholar]
- 26.FitzGerald DB, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol. 1997;18(8):1529–39. [PMC free article] [PubMed] [Google Scholar]
- 27.Haglund MM, et al. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34(4):567–76. doi: 10.1227/00006123-199404000-00001. discussion 576. [DOI] [PubMed] [Google Scholar]
- 28.Roux FE, et al. Usefulness of motor functional MRI correlated to cortical mapping in Rolandic low-grade astrocytomas. Acta Neurochir (Wien) 1999;141(1):71–9. doi: 10.1007/s007010050268. [DOI] [PubMed] [Google Scholar]
- 29.Mueller WM, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery. 1996;39(3):515–20. doi: 10.1097/00006123-199609000-00015. discussion 520-1. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz H, Seitz RJ. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29(12):1149–61. doi: 10.1016/0028-3932(91)90030-c. [DOI] [PubMed] [Google Scholar]
- 31.Liu TT, et al. Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage. 2004;23(4):1402–13. doi: 10.1016/j.neuroimage.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 32.Behzadi Y, Liu TT. Caffeine reduces the initial dip in the visual BOLD response at 3 T. Neuroimage. 2006;32(1):9–15. doi: 10.1016/j.neuroimage.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24(19):4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbetta M, et al. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11(8):2383–402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tartaglia MC, Narayanan S, Arnold DL. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol. 2008;15(4):413–9. doi: 10.1111/j.1468-1331.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 36.Maisog JM, Chmielowska J. An efficient method for correcting the edge artifact due to smoothing. Hum Brain Mapp. 1998;6(3):128–36. doi: 10.1002/(SICI)1097-0193(1998)6:3<128::AID-HBM2>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tie Y, et al. Group independent component analysis of language fMRI from word generation tasks. Neuroimage. 2008;42(3):1214–25. doi: 10.1016/j.neuroimage.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abduljalil AM, et al. Macroscopic susceptibility in ultra high field MRI. II: acquisition of spin echo images from the human head. J Comput Assist Tomogr. 1999;23(6):842–4. doi: 10.1097/00004728-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Krings T, et al. Functional MRI for presurgical planning: problems, artefacts, and solution strategies. J Neurol Neurosurg Psychiatry. 2001;70(6):749–60. doi: 10.1136/jnnp.70.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajnal JV, et al. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994;31(3):283–91. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- 41.Devlin JT, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11(6 Pt 1):589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- 42.Sharon D, et al. The advantage of combining MEG and EEG: comparison to fMRI in focally stimulated visual cortex. Neuroimage. 2007;36(4):1225–35. doi: 10.1016/j.neuroimage.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp. 2002;16(1):47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golby AJ, et al. Interactive Diffusion Tensor Tractography Visualization for Neurosurgical Planning. Neurosurgery. doi: 10.1227/NEU.0b013e3182061ebb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin W, A H, Chen Y, et al. Practcal consideration for 3T imaging. Magnetic Resonance Imaging Clin of N Am. 2003;(11):615–639. doi: 10.1016/s1064-9689(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 46.Alkadhi H, et al. Plasticity of the human motor cortex in patients with arteriovenous malformations: a functional MR imaging study. AJNR Am J Neuroradiol. 2000;21(8):1423–33. [PMC free article] [PubMed] [Google Scholar]
- 47.Bellgowan PS, et al. Improved BOLD detection in the medial temporal region using parallel imaging and voxel volume reduction. Neuroimage. 2006;29(4):1244–51. doi: 10.1016/j.neuroimage.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 48.Goebel R. Brainvoyager: A program for analyzing and visualizing functional and structural magnetic resonance data sets. Neuroimage. 1996;3(3) [Google Scholar]
- 49.O'Donnell LJ, et al. The fiber laterality histogram: a new way to measure white matter asymmetry. Med Image Comput Comput Assist Interv. 13(Pt 2):225–32. doi: 10.1007/978-3-642-15745-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JS, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007;61(5):935–48. doi: 10.1227/01.neu.0000303189.80049.ab. discussion 948-9. [DOI] [PubMed] [Google Scholar]
- 51.Jannin P, et al. Integration of sulcal and functional information for multimodal neuronavigation. J Neurosurg. 2002;96(4):713–23. doi: 10.3171/jns.2002.96.4.0713. [DOI] [PubMed] [Google Scholar]
- 52.Kamada K, et al. Visualization of the eloquent motor system by integration of MEG, functional, and anisotropic diffusion-weighted MRI in functional neuronavigation. Surg Neurol. 2003;59(5):352–61. doi: 10.1016/s0090-3019(03)00018-1. discussion 361-2. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen IA, Jr, et al. Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien) 2007;149(4):365–78. doi: 10.1007/s00701-006-1110-0. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan R, et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery. 2004;55(4):904–14. doi: 10.1227/01.neu.0000137331.35014.5c. discusssion 914-5. [DOI] [PubMed] [Google Scholar]
- 55.Nabavi A, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48(4):787–97. doi: 10.1097/00006123-200104000-00019. discussion 797-8. [DOI] [PubMed] [Google Scholar]
- 56.Nimsky C, et al. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47(5):1070–9. doi: 10.1097/00006123-200011000-00008. discussion 1079-800. [DOI] [PubMed] [Google Scholar]
- 57.Nimsky C, et al. Intraoperative compensation for brain shift. Surg Neurol. 2001;56(6):357–64. doi: 10.1016/s0090-3019(01)00628-0. discussion 364-5. [DOI] [PubMed] [Google Scholar]
- 58.Hata N, et al. Three-dimensional optical flow method for measurement of volumetric brain deformation from intraoperative MR images. J Comput Assist Tomogr. 2000;24(4):531–8. doi: 10.1097/00004728-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Colen RR, Kekhia H, Jolesz FA. Multimodality intraoperative MRI for brain tumor surgery. Expert Rev Neurother. 10(10):1545–58. doi: 10.1586/ern.10.145. [DOI] [PubMed] [Google Scholar]
- 60.Reinges MH, et al. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien) 2004;146(4):369–77. doi: 10.1007/s00701-003-0204-1. discussion 377. [DOI] [PubMed] [Google Scholar]
- 61.Kekhia H, C R, Oguro S, Black PM, Golby AJ, Jolesz FA. A Comparison Between Intraoperative MRI and Standard Frameless Stereotaxy in Glioma Surgery [Google Scholar]
- 62.Archip N, et al. Compensation of geometric distortion effects on intraoperative magnetic resonance imaging for enhanced visualization in image-guided neurosurgery. Neurosurgery. 2008;62(3 Suppl 1):209–15. doi: 10.1227/01.neu.0000317395.08466.e6. discussion 215-6. [DOI] [PubMed] [Google Scholar]
- 63.Zhuang DX, et al. A Sparse Intraoperative Data-Driven Biomechanical Model to Compensate for Brain Shift during Neuronavigation. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nimsky C, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56(1):130–7. doi: 10.1227/01.neu.0000144842.18771.30. discussion 138. [DOI] [PubMed] [Google Scholar]
- 65.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106(4):283–96. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 66.Nimsky C, et al. Usefulness of Intraoperative Ultra Low-Field Magnetic Resonance Imaging In Glioma Surgery Comments. Neurosurgery. 2008;63(4):266–267. doi: 10.1227/01.NEU.0000313624.77452.3C. [DOI] [PubMed] [Google Scholar]
- 67.Hirsch J, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery. 2000;47(3):711–21. doi: 10.1097/00006123-200009000-00037. discussion 721-2. [DOI] [PubMed] [Google Scholar]
- 68.Golaszewski SM, et al. Human brain structures related to plantar vibrotactile stimulation: a functional magnetic resonance imaging study. Neuroimage. 2006;29(3):923–9. doi: 10.1016/j.neuroimage.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 69.Gasser TG, et al. A novel passive functional MRI paradigm for preoperative identification of the somatosensory cortex. Neurosurg Rev. 2004;27(2):106–12. doi: 10.1007/s10143-003-0318-1. [DOI] [PubMed] [Google Scholar]
- 70.Stippich C, Ochmann H, Sartor K. Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and motor execution by functional magnetic resonance imaging. Neurosci Lett. 2002;331(1):50–4. doi: 10.1016/s0304-3940(02)00826-1. [DOI] [PubMed] [Google Scholar]
- 71.Feigl GC, et al. Real-time 3T fMRI data of brain tumour patients for intra-operative localization of primary motor areas. Eur J Surg Oncol. 2008;34(6):708–15. doi: 10.1016/j.ejso.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, et al. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg. 2009;111(4):746–54. doi: 10.3171/2008.10.JNS08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 74.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fransson P, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104(39):15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krings T, et al. Stereotactic transcranial magnetic stimulation: correlation with direct electrical cortical stimulation. Neurosurgery. 1997;41(6):1319–25. doi: 10.1097/00006123-199712000-00016. discussion 1325-6. [DOI] [PubMed] [Google Scholar]
- 77.Skirboll SS, et al. Functional cortex and subcortical white matter located within gliomas. Neurosurgery. 1996;38(4):678–84. discussion 684-5. [PubMed] [Google Scholar]
- 78.Moller-Hartmann W, et al. Preoperative assessment of motor cortex and pyramidal tracts in central cavernoma employing functional and diffusion-weighted magnetic resonance imaging. Surg Neurol. 2002;58(5):302–7. doi: 10.1016/s0090-3019(02)00869-8. discussion 308. [DOI] [PubMed] [Google Scholar]
- 79.Parmar H, Sitoh YY, Yeo TT. Combined magnetic resonance tractography and functional magnetic resonance imaging in evaluation of brain tumors involving the motor system. J Comput Assist Tomogr. 2004;28(4):551–6. doi: 10.1097/00004728-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 80.Witwer BP, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97(3):568–75. doi: 10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]
- 81.Tummala RP, et al. Application of diffusion tensor imaging to magnetic-resonance-guided brain tumor resection. Pediatr Neurosurg. 2003;39(1):39–43. doi: 10.1159/000070879. [DOI] [PubMed] [Google Scholar]
- 82.Loddenkemper T, Morris HH, Moddel G. Complications during the Wada test. Epilepsy Behav. 2008;13(3):551–3. doi: 10.1016/j.yebeh.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Isabel M, Smith JM, Allan J. Intracarotid Amobarbital Memory Protocol: Mutened Dysphasia, and Variations in Arterial Distribution of the Drug do Not Affect Recognition Results. Journal of Epilepsy. 1993;6:75–84. [Google Scholar]
- 84.Binder JR, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46(4):978–84. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 85.Bahn MM, et al. Localization of language cortices by functional MR imaging compared with intracarotid amobarbital hemispheric sedation. AJR Am J Roentgenol. 1997;169(2):575–9. doi: 10.2214/ajr.169.2.9242780. [DOI] [PubMed] [Google Scholar]
- 86.Hertz-Pannier L, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48(4):1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- 87.Benson RR, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52(4):798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 88.Fernandes MA, et al. Comparing language lateralization determined by dichotic listening and fMRI activation in frontal and temporal lobes in children with epilepsy. Brain Lang. 2006;96(1):106–14. doi: 10.1016/j.bandl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Sabbah P, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage. 2003;18(2):460–7. doi: 10.1016/s1053-8119(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 90.Woermann FG, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez G, et al. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60(6):969–75. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- 92.Deblaere K, et al. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology. 2004;46(6):413–20. doi: 10.1007/s00234-004-1196-0. [DOI] [PubMed] [Google Scholar]
- 93.Vernooij MW, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–76. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 94.Powell HW, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Propper RE, et al. A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain Cogn. 73(2):85–92. doi: 10.1016/j.bandc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papanicolaou AC, et al. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg. 2004;100(5):867–76. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- 97.Deblaere K, et al. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology. 2002;44(8):667–73. doi: 10.1007/s00234-002-0800-4. [DOI] [PubMed] [Google Scholar]
- 98.Vikingstad EM, et al. Cortical language lateralization in right handed normal subjects using functional magnetic resonance imaging. J Neurol Sci. 2000;175(1):17–27. doi: 10.1016/s0022-510x(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 99.Seghier ML, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23(3):140–55. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber B, et al. Presurgical language fMRI in patients with drug-resistant epilepsy: effects of task performance. Epilepsia. 2006;47(5):880–6. doi: 10.1111/j.1528-1167.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 101.Palmer ED, et al. An event-related fMRI study of overt and covert word stem completion. Neuroimage. 2001;14(1 Pt 1):182–93. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- 102.Gaillard WD, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59(2):256–65. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- 103.Plate KH, et al. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59(4):520–9. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 104.Ariza A, et al. Renin in glioblastoma multiforme and its role in neovascularization. Am J Clin Pathol. 1988;90(4):437–41. doi: 10.1093/ajcp/90.4.437. [DOI] [PubMed] [Google Scholar]
- 105.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2(4):266–76. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 106.Shweiki D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 107.Plate KH, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–8. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 108.Helmlinger G, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3(2):177–82. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 109.Russell SM, Kelly PJ. Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery. 2007;61(1 Suppl):358–67. doi: 10.1227/01.neu.0000279229.58449.d1. discussion 367-8. [DOI] [PubMed] [Google Scholar]
- 110.Latchaw RE, et al. Functional magnetic resonance imaging as a management tool for cerebral arteriovenous malformations. Neurosurgery. 1995;37(4):619–25. doi: 10.1227/00006123-199510000-00003. discussion 625-6. [DOI] [PubMed] [Google Scholar]
- 111.Maldjian J, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral arteriovenous malformations before surgical or endovascular therapy. J Neurosurg. 1996;84(3):477–83. doi: 10.3171/jns.1996.84.3.0477. [DOI] [PubMed] [Google Scholar]
- 112.Duffau H, Fontaine D. Successful resection of a left insular cavernous angioma using neuronavigation and intraoperative language mapping. Acta Neurochir (Wien) 2005;147(2):205–8. doi: 10.1007/s00701-004-0357-6. discussion 208. [DOI] [PubMed] [Google Scholar]
- 113.Juenger H, et al. Misleading functional magnetic resonance imaging mapping of the cortical hand representation in a 4-year-old boy with an arteriovenous malformation of the central region. J Neurosurg Pediatr. 2009;4(4):333–8. doi: 10.3171/2009.5.PEDS08466. [DOI] [PubMed] [Google Scholar]
- 114.Pouratian N, et al. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002;97(1):21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- 115.Cannestra AF, et al. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55(4):804–12. doi: 10.1227/01.neu.0000137654.27826.71. discussion 812-4. [DOI] [PubMed] [Google Scholar]
- 116.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 117.Yu HY, et al. The Wada memory test and prediction of outcome after anterior temporal lobectomy. J Clin Neurosci. 17(7):857–61. doi: 10.1016/j.jocn.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 118.Adcock JE, et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 119.Mamelak AN, et al. Magnetoencephalography-directed surgery in patients with neocortical epilepsy. J Neurosurg. 2002;97(4):865–73. doi: 10.3171/jns.2002.97.4.0865. [DOI] [PubMed] [Google Scholar]
- 120.Paesschen WV. Ictal spect. Epilepsia. 2004:35–40. doi: 10.1111/j.0013-9580.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 121.Theodore WH, et al. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography. Ann Neurol. 1992;32(6):789–94. doi: 10.1002/ana.410320613. [DOI] [PubMed] [Google Scholar]
- 122.Salek-Haddadi A, et al. Studying spontaneous EEG activity with fMRI. Brain Res Brain Res Rev. 2003;43(1):110–33. doi: 10.1016/s0165-0173(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 123.Salek-Haddadi A, et al. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53(5):663–7. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]


