Abstract
A bifunctional peptide coating was designed, synthesized, and evaluated as a potential pro-healing stent coating. The bifunctional peptide consisted of a short 28-mer sequence that on the N-terminus, has a motif with affinity for polystyrene binding, and at the C-terminus, a motif that was shown to selectively bind human endothelial cells but not platelets. Results showed that the selective coating, a polystyrene binding peptide terminated in RRETAWA (FFSFFFPASAWGSSGSSGK(biotin)CRRETAWAC), bound endothelial cells quantitatively as well as the common RGD motif, but unlike RGD, it did not show any preference for platelet adherence. Follow-up work examining the difference in cell line selectivity between endothelial cells, whose binding should be encouraged, and smooth muscle cells, whose binding should be deprecated in a stenting application, did identify a temporal preference of the RRETAWA-terminated peptide coating for endothelial cells. However, the in vivo implications of this apparent selectivity need to be examined in more detail before definitive conclusions can be drawn. The positive in vitro results encourage the continued development of other novel coatings that mimic biological structures and/or signaling capabilities to direct cellular processes on the surface of synthetic materials.
Introduction
Bioactive coatings hold the promise of improving clinical outcomes by enhancing device integration with the surrounding biology.1–6 A coating of this type on a stent would ideally display relevant biomimetic ligands to entice adhesion of endothelial cells, reduce inflammation, and prevent thrombosis, thereby increasing the healing rates beyond those obtainable with an uncoated device. However, to date there are only a few stent coatings that have sought to employ this strategy.4 Most stents in clinical trials, or that are currently available, rely on either: 1) a non-fouling coating to prevent cellular adhesion and subsequent inflammation,7–8 or 2) use the elution of a cytotoxic drug to reduce the overproliferation of smooth muscle cells.9–10 Both of these strategies increase the healing time by slowing the re-endothelialization process.
A stent coating created from a bioactive ligand, such as an integrin binding motif or appropriate antibody, would produce a “pro-healing” stent that encourages interactions with the surrounding cells and biology instead of impeding and avoiding these interactions. One recent example of this approach was a stent coated with a CD34 antibody (OrbusNeich's Genous Bio-engineered R stent) which was designed to recruit circulating endothelial progenitor cells (EPCs) from the bloodstream to accelerate the natural healing process. Clinical trials with the coated stent have shown that it is both safe and effective for implantation,11–13 however, further randomized trials still need to be accomplished to determine its efficacy over the more common bare metal, or drug-eluting stents. A further complication of this approach is the reliance on the capture of circulating endothelial progenitor cells to promote re-endothelialization. Morbidities ranging from diabetes to hypertension to old age can reduce the amount of these cells and reduce the efficacy of the capture technology.14 Additionally, the CD34 antibodies have also been shown to capture other progenitor cells, for example smooth muscle progenitor cells, which can exaggerate restenosis.15
Another approach to create a pro-healing stent would be to reduce the binding of platelets to an implanted stent, thereby reducing the inflammatory response and allowing surrounding endothelial cells to properly re-endothelialize the material. This approach could be accomplished by creating a stent coating from a peptide ligand motif that is known to promote endothelial cell adherence and migration. The classic and most ubiquitous of these integrin binding motifs is a trimer sequence of arginine-glycine-aspartic acid (RGD).16–17 RGD has been used successfully as a surface coating in many in vivo applications to promote device integration (e.g., orthopedic, dental),18 but cardiovascular use has not been favorable.19–22 The RGD sequence binds a little less than half of all known human integrins and so lacks the ability to differentiate between endothelial cells and platelets, 23 carrying the deleterious risk of thrombus formation. A more specific peptide sequence is needed to ensure that cell-line specific and appropriate interactions can be controlled by a surface coating. Ideally, for cardiovascular applications, a ligand can be identified that only interacts with integrins uniquely present on endothelial cells but is absent, or in reduced quantity, on platelets, inflammatory cells, and smooth muscle cells.
To address this limitation, the RRETAWA sequence was identified and shown to bind the α5β1 integrin that is present on the surface of endothelial cells, but lacking on platelets.24–27 This RRETAWA sequence was discovered through the directed use of a phage display library that evaluated billions of random peptide sequences to determine peptide sequences that had a specific affinity for the α5β1 integrin.24 The resulting sequence, RRETAWA, has no known natural related sequences and it appears to be specific for human α5β1 and not the murine version of the integrin. While more than 15 years have passed since its discovery, the sequence has received little applied interest with the notable exception being the work of the Marchant group.25 As mentioned, the RRETAWA sequence binds α5β1 strongly, and this integrin is predominantly present on endothelial cells (ECs) but not platelets. The motif does possess some affinity for the αvβ3 integrin that is present on both endothelial cells (ECs) and platelets, but this interaction is 5 orders-of-magnitude weaker than the binding to α5β1 and so platelet adherence is tempered overall.
We are designing, synthesizing, and evaluating peptide coatings that function at the material/biological interface to control cellular responses on surfaces such as metals and plastics.28–33 These bifunctional, two-domain peptide coatings must recognize and bind both the surface, through non-covalent adsorption, while simultaneously providing a mechanism to adhere to and interact with the surrounding biology. In this manner, these peptides serve to create an interface that allows for appropriate biological interactions with materials. Previously, we demonstrated the creation of bifunctional peptide coatings by first identifying an adsorptive material binding domain through a phage display technique.34–35 The material domain was then combined with a known biological functionality to produce an interfacial surface coating. These resulting peptides were found to regulate the interactions that occurred at the biological-material interface including: suppression of apoptosis,30 prevention of fouling,28,32 release of therapeutics from a surface,31 or promotion of endothelialization.29,33 While we have previously shown endothelialization of both metal and plastic implantable materials through the use of an RGD interfacial peptide, these coatings would be suboptimal for cardiovascular use as they would encourage non-specific cellular adherence that would capture circulating platelets. Herein, we describe a new peptide coating comprised of a surface adhesion domain for polystyrene, FFSFFFPASAWGS, conjugated to a RRETAWA sequence to create an endothelial cell-selective coating.
Experimental Methods
Peptide synthesis
Peptides were commercially synthesized by solid-phase peptide synthesis techniques. The resultant peptides were purified to at least 95 % purity and included high-performance liquid chromatography and mass spectroscopy analysis. The biotinylated peptides for affinity constant calculation were synthesized using a C-terminal biotin attached through the epsilon amide of a lysine residue.
Peptide apparent dissociation constants
The wells of a polystyrene plate were treated for 1 hour with a 1 % non-fat milk mixture. Milk was applied first to ensure the peptides only bound to their preferred sites and did not produce random non-specific adsorptions. Varying the order at which the blocking is applied does not vary the final results more than an order of magnitude. The wells were washed 3x with a PBS-Tween 20 (Sigma; St. Louis, MO) (0.5 %) solution, followed by serial logarithmic dilutions of peptides 1 and 2 in PBS (Mediatech; Herndon, VA), which were placed in triplicate into the wells of a polystyrene plate for 2 hours. The wells were again washed 3x with PBS-Tween 20 (0.5 %) followed by a 30 minute treatment with a 1:500 dilution of streptavidin-alkaline phosphatase (SA-AP) (USB Corporation; Cleveland, OH) in PBS. After a through wash with PBS-Tween 20 (0.5 %), p-nitrophenyl phosphate (pNPP) (Sigma; St. Louis, MO) tablets were dissolved in buffered saline and added to all the wells. The absorbance of each dilution was measured using a platereader (AD340C; Beckman Coulter; Fullerton, CA) at 405 nm, then the absorbance versus log concentration was plotted to yield a sigmoidal binding curve. A four parameter sigmoidal equation was fit to each curve using a custom written MATLAB® (MathWorks; Natick, MA) routine and the inverse of the concentration at the half point of the sigmoidal model curve was extracted as the apparent dissociation constant (“relative Kd”).
Longevity Studies
100 µL aliquots of PS-biotin (0.0741mg/mL) were added to 96-well PS microtiter plates in quadruplicate and left to coat surfaces for 2 hours at room temperature. Following three PBS washes to remove unbound peptide, 100 µL aliquots of 100% fetal bovine serum (FBS) and DPBS were added to the wells. Plates were stored at RT on an orbital shaker and liquid levels were topped up with DPBS or 100% FBS once a week to avoid drying out of the wells. Plates were removed after different storage times (1 day, 3 days, 1 week, 2 weeks and 6 weeks) and assayed for the amount of PS-biotin remaining on PS surfaces using modified ELISA. In this assay, wells were washed three times with a buffered saline-Tween 20 solution, and then exposed to a blocking solution for 30 minutes. (1% BSA in TBS) Following three washes in buffered saline-Tween 20, 100 uL streptavidin-conjugated alkaline phosphatase (SA-ALP) was added to wells and incubated for 30 minutes at RT. After a final buffer wash, the chromogenic agent p-nitrophenol phosphate (pNPP) was added to the wells. Plates were incubated at 37°C for 5 minutes and the developed absorbances at 405 nm measured using a microplate reader. Absorbances were normalized using positive control wells freshly coated with PS-biotin on the same day as the assay.
Peptide cyclization
Peptides were cyclized following manufacturer’s instructions for the CLEAR-OX™ resin (Peptide International; Louisville, KY). To begin, 0.4 mg of peptide 1 was dissolved in 4 mL nanopure water, and the pH was adjusted to 8.0 using ammonium hydroxide. A small amount of resin (3x molar excess) was weighed out and dissolved in dichloromethane (DCM) for 1 hour, RT. The resin was then washed with DCM, dimethylformamide (DMF), methanol, deionized water, and finally 1:1 acetonitrile:water. All solvents were purchased in high purity from Pharmco-Aaper (Brookfield, CT). The mixture was then centrifuged, the supernatant was removed and replaced by 2 mL of the peptide solution and shaken for 2 hours, RT. The resin changed from yellow to dark orange upon completion at which point the solution was again centrifuged and the resultant supernatant containing the peptide was noted as the “cyclized” version.
To verify the formation of the disulfide bond, a 100 µL aliquot of the peptide solution was then added to a 100 µL mixture of 4 mg Ellman’s Reagent (Pierce; Rockford, IL) dissolved in a 0.1 M sodium phosphate buffer (pH 8.0) containing 1 mM EDTA (Sigma; St. Louis, MO). After mixing for 15 minutes at RT the absorbance was measured at 405 nm and showed a reduction in the signal for the “cyclized” version exemplifying the loss of the free thiols. The uncyclized peptide (linear version) did not show the signal reduction.
As a final verification, 2 µL aliquots of both the linear and cyclic versions were combined with 20 µL of an α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution. The MALDI matrix was made by dissolving CHCA (Fluka; St. Louis, MO) in 500 µL of acetonitrile (ACN) and 500 µL of water containing 0.1 % trifluoroacetic acid (TFA). The samples were then spotted on a plate and analyzed using a Shimadzu AXIMA CFR MALDI-TOF-MS. Spectra were then examined for their peak values and showed a reduction of 1.7 Da signifying the loss of the protons.
EC, SMC cell culture
Human umbilical vein endothelial cells (HUVEC) and human umbilical artery smooth muscle cells (UASMC) were purchased from Cambrex (North Brunswick, NJ). Endothelial cells arrived at passage 0 and were used for all experiments between passages 3 and 5. Smooth muscle cells arrived at passage 3 and were used for experiments between passages 6 and 8. HUVEC were cultured in an EGM-2 (Cambrex; North Brunswick, NJ) medium kit and UASMC were cultured in an smGM-2 (Cambrex; North Brunswick, NJ) medium kit both according to the manufacturer instruction and using all supplied growth factor, serum, and antibiotic aliquots. The EGM-2 kit contained 2% serum and the smGM-2 kit contained 5% serum and these concentrations were used in all experiments and during the maintenance of the cells.
To determine cell binding, wells of a 96-wells plate were treated with molar equivalents of PS-CRRETAWAC, 1, (0.1 mg/mL), PS-RGD, 3, (0.0666 mg/mL), PS-RGE, 4, (0.0670 mg/mL), PS-Biotin, 2, (0.0674 mg/mL), or deionized water for 2 hours, RT. The wells were then washed 3x with deionized water and blocked for 1 hour at RT with a 1 % bovine serum albumin (BSA) (Sigma; St. Louis, MO) followed by another 3x wash with deionized water. HUVEC were trypsinized, counted, and 5000 total cells (1.6×104 cells/cm2) were added to the plate in sextuplicate and left for 2 hours, at 37 °C and 5 % CO2. After 2 hours, the wells were washed 3x with DPBS to remove unbound cells and replenished with 100 µL fresh medium containing 20 µL CellTiter 96® AQueous One Solution Cell Proliferation MTS assay (Promega; Madison, WI). The plate was then developed at 37 °C 5 % CO2 for 2 hours and the absorbances at 492 nm were measured on a platereader (Beckman Coulter AD 340C). A control plate run in parallel containing 0 through 6000 cells (1.9×104 cells/cm2) in 1000 cell (0.3×104 cells/cm2) increments was used to develop a constant of proportionality relating absorbance to cell count.
SMC, EC imaging
Wells of a 24-well plate were treated with 500 µL of molar equivalents of PS-CRRETAWAC, 1, PS-RGD, 3, PS-RGE, 4, and PS-Biotin, 2, as well as water and 20 µg/mL bovine fibronectin (Invitrogen; Carlsbad, CA). After 2 hours the wells were washed 3x with DPBS and blocked for an additional hour with 1 % BSA solution, and washed again with 3x DPBS. HUVEC, UASMC, and a 50:50 mix of both cell types (30,000 total cells each format; 1.5×104 cells/cm2) were added to the wells in EGM-2, smGM-2, and a 50:50 mix of EGM-2:smGM-2, respectively for 1 hour at 37 °C, 5 % CO2. After 1 hour the cells were washed 3x with DPBS and the remaining cells were fixed for 15 minutes in 3.7 % paraformaldehyde (Fluka; St. Louis, MO) in DPBS. The wells were again washed with DPBS and blocked for 1 hour with a 1 % non-fat milk solution. An additional wash was performed and the plate was incubated for 1 hour, 37 °C and 5 % CO2, in a 1:100 dilution of mouse anti-PECAM-1 antibody (Millipore; Billerica, MA) in the 1 % milk solution. PECAM-1 was chosen as it is present on endothelial cells but not on muscle cells.36 The wells were then washed and a second incubation step occurred with a 1:200 dilution of goat anti-mouse Alexa Fluor 594 conjugate (Invitrogen; Carlsbad, CA) also in the milk solution. All immunostaining was accomplished per the manufacturer’s standard protocols. Finally, the wells were washed and imaged on an Olympus IX70 (Olympus; Melville, NY) phase contrast microscope with attached CCD camera (Spot Diagnostics 11.2 Color Mosaic), epifluorescence, and appropriate filters. The fluorescent and brightfield images were merged with ImageJ software (NIH; Bethesda, MD).
Platelet imaging
Wells of a 24-well plate were treated with molar equivalents of PS-CRRETAWAC, 1, and PS-RGD, 3, as well as water and 20 µg/mL bovine fibronectin (Invitrogen; Carlsbad, CA) in triplicate for 2 hours, RT. The wells were washed, blocked with 2 % BSA for 30 minutes, and washed again. Platelet rich plasma (PRP; 274×103 platelets/µL) was obtained from Research Blood Components (Brighton, MA) and used the day of collection. The PRP was supplied in sodium citrate, but before use was centrifuged at 1800g for 10 minutes, the supernatant was aspirated, and then the platelet pellet was resuspended in 1% BSA. Right before adding to the treated wells to achieve a final concentration of 1×108 platelets/cm2, a bolus of CaCl2 and MgCl2 was added to produce final concentrations of 2 mM and 1 mM, respectively. The platelet containing solutions were then incubated for 30 minutes at 37 °C. The wells were washed with 1% BSA to remove any unbound platelets and a 3.7 % paraformaldehyde (Fluka; St. Louis, MO) solution in DPBS was added for 20 minutes. After an additional wash, the wells were blocked with a 1 % BSA solution and washed again before being incubated for 1 hour with a mouse anti-CD41a antibody (Millipore; Billerica, MA) in the 1 % BSA solution. The wells were then washed and a second incubation step occurred with a 1:200 dilution of goat anti-mouse Alexa Fluor 594 conjugate (Invitrogen; Carlsbad, CA) also in the BSA solution. Images were taken on an Olympus IX70 (Olympus; Melville, NY), with attached CCD camera (Spot Diagnostics 11.2 Color Mosaic) and epifluorescence using appropriate filters and brightfield imaging at 40× magnification (443 µm by 332 µm). Platelets were then counted by hand and verified using a custom MATLAB® (MathWorks; Natick, MA) routine. A test with adenosine diphosphate (ADP) showed increased platelet coagulation and confirmed the activity of the platelets.
Results and Discussion
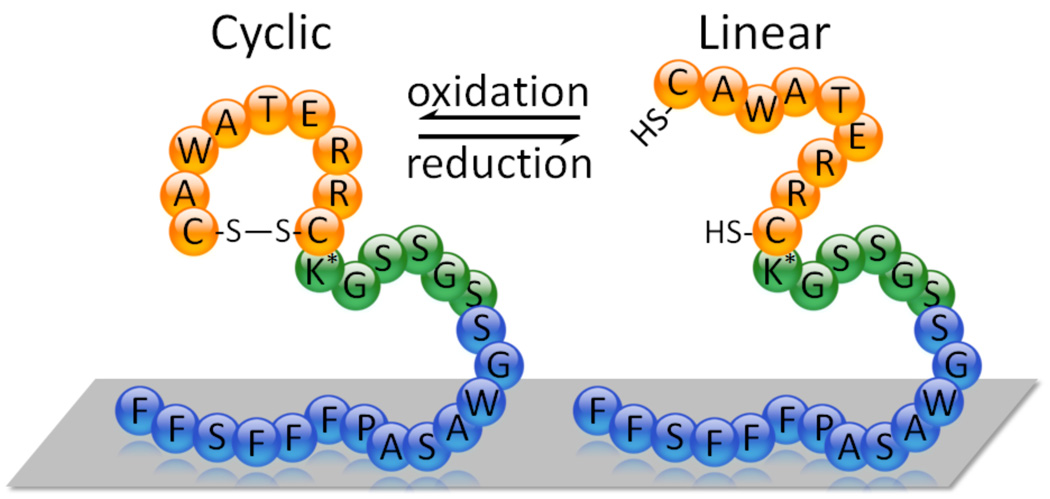
This cell-selective coating contains a peptide domain that binds polystryene. The polystyrene (PS) binding domain was chosen for these initial studies since PS is a surrogate of the polymeric coating present on drug eluting stents (i.e., poly(styrene-b-isobutylene-b-styrene)), and PS allows for easier characterization of the peptide coating’s functioning as the material is both optically clear and readily obtainable. We have identified the PS binding peptide, FFSFFFPASAWGS, previously in our laboratory using phage display.30 Due to the modularity of the interfacial peptide approach, our coating is easily modified to use physisorptive domains for other surfaces including metals and polyglycolic acid.29,33 The resulting synthesized peptide containing both the PS and endothelial cell binding domains, FFSFFFPASAWGSSGSSGK(biotin)CRRETAWAC (PS-CRRETAWAC; 1), was evaluated for its ability to bind ECs as opposed to platelets, of primary interest, and smooth muscle cells (SMCs), of secondary interest (Figure 1). The peptide coating can readily be non-covalently adsorbed onto surfaces under mild aqueous conditions and so this technique has two main advantages: 1) it is amenable to use with a variety of sensitive biologics because it does not necessitate the use of caustic chemicals, solvents, extreme temperatures, or surface pretreatments; and 2) is readily applied and patterned onto any surface geometry through a variety of methods (e.g., dipcoating, spraying, or ink-jet printing). The RRETAWA was produced flanked by two cysteine residues, producing CRRETAWAC, because upon oxidation, a disulfide bond is formed which presents the RRETAWA motif in a cyclic format characteristic of many ligand sequences. Generally, cyclic presentations have been found to function better than their linear counterparts as they replicate the conformational loops found in native proteins. Figure 1 shows the CRRETAWAC structure attached to our surface adsorptive protein in both its linear and cyclic conformations. The PS-CRRETAWAC peptide, 1, was commercially synthesized in the “linear” version containing two free thiols and later cyclized using a CLEAR-OX resin. An Ellman’s reagent test confirmed the removal of the free thiols in this “cyclized” version, as did MALDI-MS.
Figure 1.
Schematic of the PS-CRRETAWAC peptide in the cyclic and linear configurations with complete sequence shown (K* = biotinylated lysine). Not to scale.
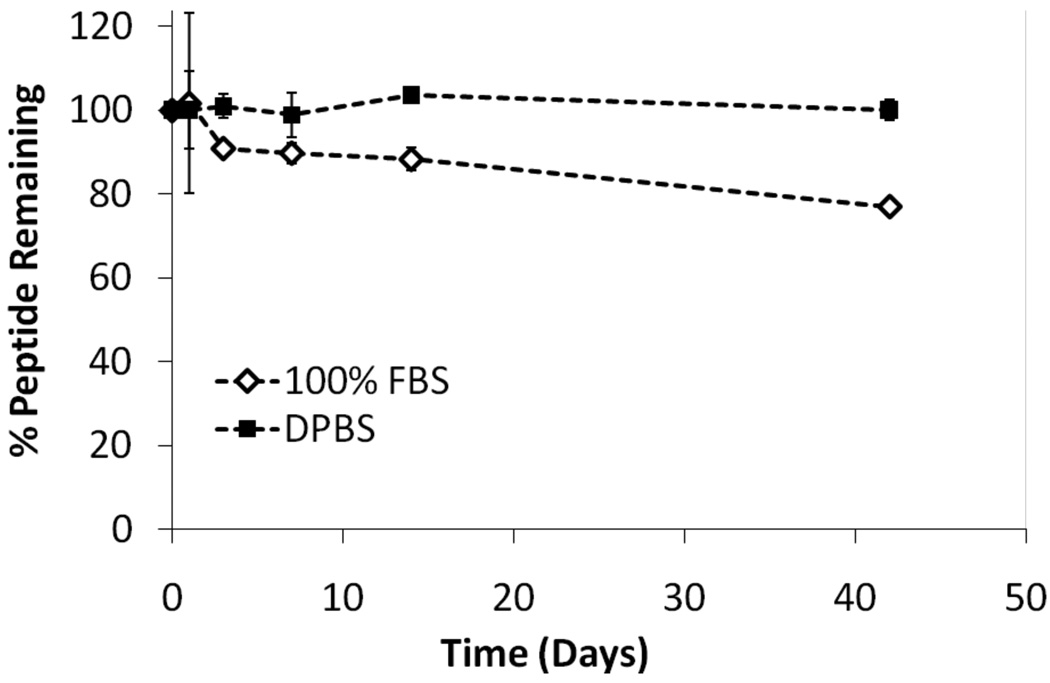
Before progressing to an evaluation of the biological activity of the newly synthesized peptide, we first determined the binding affinity of the linear peptide, 1, to a polystyrene substrate. Because the coating’s longevity relies on its adsorption strength, any weakening of the physisorptive interaction between the coating and material would reduce its efficacy. From previous studies we knew the base FFSFFFPASAWGS sequence had the requisite affinity, but we wanted to ensure that the introduction of the CRRETAWAC sequence did not significantly interfere with the underlying non-covalent adsorptive interactions. To test this, a modified ELISA was performed where the PS-CRRETAWAC peptide was introduced to the wells of a PS plate and, using a chromogenic pNPP readout, the apparent dissociation constant, or “relative Kd”, was determined to be the concentration corresponding to 50% of the maximal absorbance signal (EC50) (Figure 2). This value is typically within a factor of 2 to dissociation constants from a direct assay. These results were compared to binding data for the underlying PS-binding base sequence FFSFFFPASAWGSSGSSGK(biotin), 2 (PS-biotin). As shown, the inclusion of the CRRETAWAC sequence did not significantly reduce the overall binding strength of the underlying domain as both are within the same order-of-magnitude (1.1 µM for 1, 6.3 µM for 2). In fact, the modified binder showed slightly improved affinity, which we hypothesize is due to its increased amphiphilic properties allowing for increased presentation of the biotin. Further studies and modeling are planned to test this hypothesis. Because these peptide coatings are ultimately for use in vivo, their desorption rate is also an important consideration when examining their overall efficacy. Thus, we performed an experiment where the CRRETAWAC coating on a polystyrene surface was challenged with either 100% fetal bovine serum (FBS) or buffered saline (DPBS) for up to 6 weeks of incubation with continuous shaking and fluid replacement as necessary (Figure 3). The peptide coating proved to be resilient with undetectable desorption in the saline solution, and only 23% desorption over the 6 weeks in the more physiologically relevant FBS solution.
Figure 2.
Binding curves for FFSFFFPASAWGSSGSSGK(biotin)-CRRETAWAC, 1, and FFSFFFPASAWGSSGSSGK(biotin), 2, peptides. The EC50 for 1 and 2 are 1.1 µM and 6.3 µM with a 4-parameter model fit of R2 = 0.98 and 0.99, respectively. (n=3)
Figure 3.
Peptide desorption curves for FFSFFFPASAWGSSGSSGK(biotin)-CRRETAWAC, 1, for up to 6 weeks in 100% FBS or DPBS. (n=3; mean ± SD)
Next, to determine which conformation possessed superior biological properties, solutions containing molar equivalents of the linear or cyclic CRRETAWAC versions were coated onto a PS plate in triplicate followed by the addition of 10,000 total human umbilical vein endothelial cells (HUVECs) per well (3.1×104 cells/cm2). After a 2 hour incubation and subsequent wash, an MTS proliferation assay was then used to count the number of remaining cells. The linear version was found to retain ~51% of the incident cells (5068 ± 859) while the cyclic version only retained ~7% (747 ± 247). We suspect that the cyclic peptide, with the loss of its two hydrophilic thiols, might have buried the RRETAWA motif too close to the surface to be displayed properly. We have observed similar results with our previous RGD peptides and found the linear version to work better with that motif as well. All subsequent experiments therefore used the linear version.
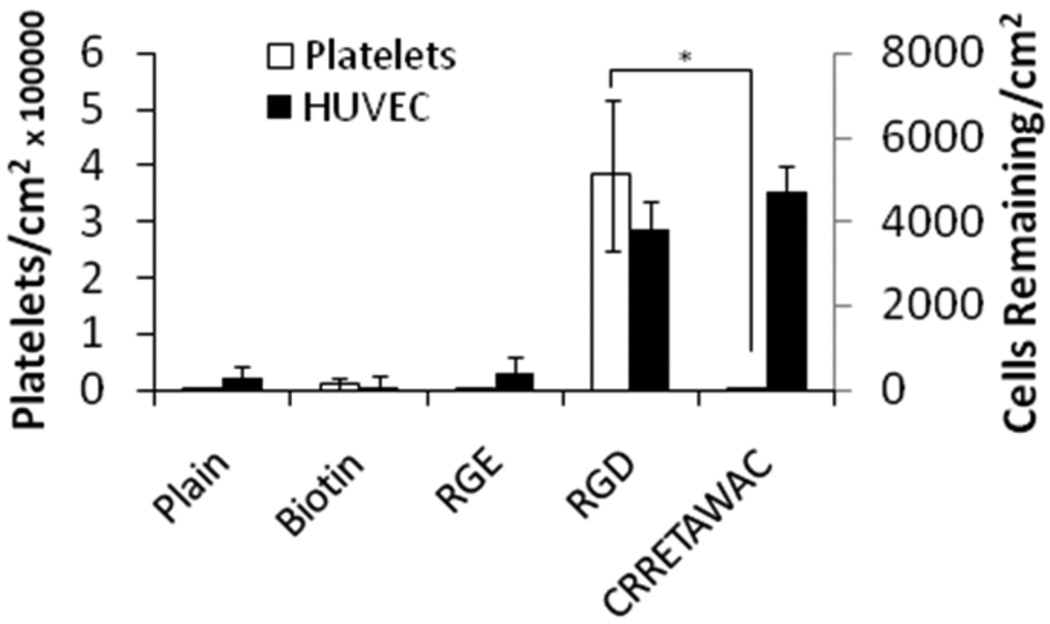
In general, an RGD surface coating works well for integration of implanted devices with surrounding tissues, but as mentioned, is non-ideal for cardiovascular use. To ensure that the proposed selective peptide, 1, continues to interact appropriately with ECs, the following experiment was devised. Wells of a PS plate were coated in sextuplicate with equal molarities of FFSFFFPASAWGSSGSSGK(biotin)CRRETAWAC, 1, FFSFFFPASAWGSSGSSGK(Biotin), 2, FFSFFFPASAWGSSGSSGRGD, 3, or FFSFFFPASAWGSSGSSGRGE, 4, for 2 hours, with the latter two serving as a positive and negative control, respectively (Table 1). Another set of wells were left untreated. The plates were washed to remove any non-specific peptide adsorption and 5000 total endothelial cells (1.6×104 cells/cm2) were added to the coated well variants in the presence of serum and left to interact for a 2 hour period. After washing, an MTS readout was utilized to count the amount of cells remaining on the surface (Figure 4). The CRRETAWAC motif functioned slightly better than the ubiquitous RGD for binding the endothelial cells (p=0.03). Additionally, and as expected, both modalities significantly outperformed the negative controls of an uncoated plate, biotinylated PS-binder, and non-functional RGE terminated PS-binder which all only showed minimal background binding. The CRRETAWAC motif therefore functions similar in regard to cell capture and adherence over the prototypical RGD and so is suitable for interfacial use to encourage biological integration of an implanted medical device. Marchant et al have also investigated the binding of endothelial cells to a CRRETAWAC coating (on PTFE), and they observed normal cell attachment and proliferation over 96 hours without issues.25 These data suggest that a CRRETAWAC modified surface can support initial attachment and proliferation of HUVEC. The benefit of the non-covalent coating technique of the interfacial peptides can readily be observed in this experiment where a surface such as native polystyrene, which lacks a mechanism for directing biology, was rendered active by incubating with a solution containing the appropriate peptides. Additionally, we have used the bifunctional coating technology, with an RGD terminator, to show normal endothelial cell attachment, growth and spreading on titanium,29 polystyrene,30 and polyglycolic acid,33 as well as proliferation until a confluent monolayer was achieved on polystyrene.30
Table 1.
Sequences used to modify PS surfaces. The underlying sequence FFSFFFPASAWGS was identified previously for use with PS.
| No. | Sequence | Nomenclature |
|---|---|---|
| 1 | FFSFFFPASAWGSSGSSGK (biotin) CRRETAWAC | PS-CRRETAWAC |
| 2 | FFSFFFPASAWGSSGSSGK (biotin) | PS-biotin |
| 3 | FFSFFFPASAWGSSGSSGRGD | PS-RGD |
| 4 | FFSFFFPASAWGSSGSSGRGE | PS-RGE |
Figure 4.
(black bars) HUVEC binding to a variety of surface coatings of equal molar concentrations. (n=6; mean ± SD) (white bards) Count of human platelets adhered to a variety of surface coatings. Platelets were counted from a 40× magnification brightfield image over an area of 443×332 µm and were verified using an anti-CD41a stain. (n=3; mean ± SD; *p<0.01).
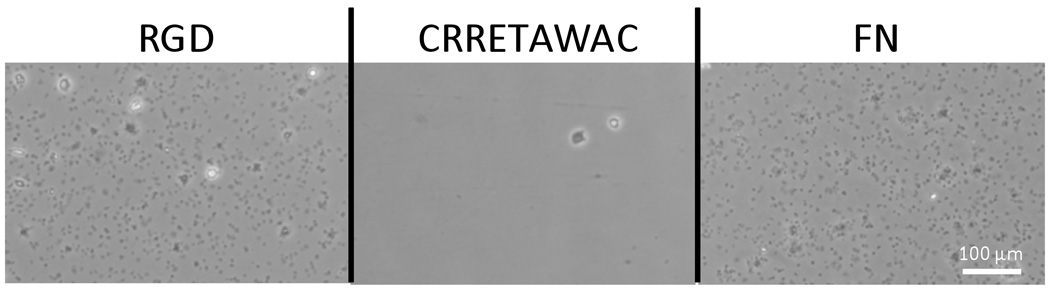
A significant concern with implanted cardiovascular devices is the binding of platelets to a surface which can induce both inflammation and potentially a life-threatening thrombus.37–38 To examine whether CRRETAWAC when bound to the PS peptide did prevent platelet adherence we collected fresh platelet rich plasma (PRP), containing approximately 274 million platelets per mL, and introduced 500 µL of the fluid (100 million platelets/cm2) into wells of a polystyrene plate pre-treated in the same manner as outlined previously. The platelets were left to interact for 30 minutes at 37 °C before a wash was used to remove any unbound and weakly bound platelets. Those that remained were fixed, imaged, and counted to determine how effective the surface treatment was at preventing the adhesion of the platelets (Figure 4, 5). Counts were done by hand using the brightfield phase contrast images over a 443 µm by 332 µm area and agreed within a 10 % error with counts obtained using custom image analysis software. To verify that the spots in the images were platelets and not another component of plasma, a mouse anti-CD41a antibody was used as a primary stain, followed by a goat anti-mouse Alexa Fluor 594 conjugate. The results from all three counting methods (hand-count brightfield, immunostaining, computerized analysis) were in agreement and verified the presence and placement of the platelets.
Figure 5.
Example micrographs of platelets adhered to both the RGD, CRRETAWAC, and fibronectin (FN) treated surfaces exemplifying the difference between their platelet adherent abilities.
The results showed that CRRETAWAC outperforms the RGD motif in regards to hindrance of platelet binding with more than a 94-fold reduction observed (Figure 4; p < 0.01). Additionally, CRRETAWAC preformed quantitatively as well as all three negative controls (plain PS, 2, and 4), which all had minimal background platelet binding. A positive control of fibronectin treated wells showed equivalent platelet adherence compared to the RGD treatment (p = 0.59). Examining both the HUVEC and platelet data together reveals the clear benefit to using a CRRETAWAC terminated peptide which had high HUVEC adherence and low platelet adherence while RGD had both high HUVEC and high platelet adherence. A brief experiment where the platelets added to the experimental surfaces were pre-incubated with soluble tetrapeptide sequences of RGDS and RGES was also conducted in parallel. The results of this experiment were as expected with the RGES having no effect on the platelet binding, producing similar results to those in Figure 4, while the RGDS prevented a majority of the platelet adherence to the RGD and FN surfaces; confirming the interaction mechanism between the platelets and peptides.
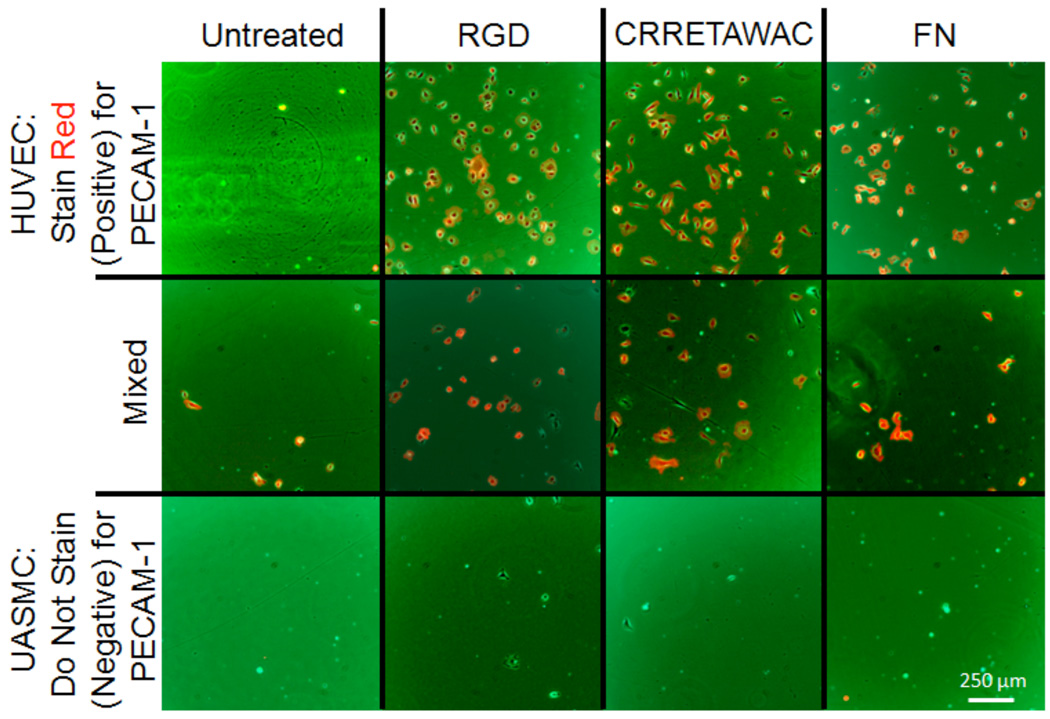
While reduction of platelet binding was the primary benefit of the peptide coating, a reduction in smooth muscle cell (SMC) binding would potentially improve clinical outcomes by lessening the incidence of restenosis—a narrowing of the lumen due to SMC overproliferation—and by reducing the need for revision surgeries. To determine whether such a preference for ECs over SMCs was obtainable using the peptide coating, a staining technique was utilized to visualize the cells interacting with the surfaces. Wells of a polystyrene plate were again treated with molar equivalents of peptide variants for 2 hours. After a wash, 3×104 total (1.5×104 cells/cm2) HUVEC, UASMC, or a 50/50 mix of both were added to an untreated, PS-RGD treated, PSCRRETAWAC treated, or fibronectin (FN) treated PS plate. After 1 hour of incubation, a wash was performed and any remaining cells were fixed and stained with a mouse anti-PECAM-1 antibody and then a secondary staining with goat anti-mouse-Alexa Fluor 594 (ECs stain red, SMCs do not) (Figure 6). As observed, the untreated surface showed minimal cell adhesion in all cases. However, the functional CRRETAWAC and RGD coatings both adhered incident cells with a preference for HUVEC over umbilical artery smooth muscle cells (UASMCs). Some UASMCs are observed exhibiting the spindle morphology typical of the cell type, but the majority of the adhered cells in the mix case are ECs. Both the RGD and CRRETAWAC surfaces performed equally well in this regard and compared favorably to results obtained using the FN positive control. Therefore, solely in terms of EC/SMC interactions, CRRETAWAC and RGD are both acceptable with no likely advantage existing to using one over the other. As can be observed from the images, SMCs (bottom row) lacked the ability to rapidly interact with the surfaces regardless of which integrin binder was used. In a separate experiment, HUVEC and UASMC binding studies were performed independently on the CRRETAWAC modified PS surface, and analyzed using an MTS assay. After two hours, 40% of the 5000 seeded HUVECs attached to the surface whereas only a few of the 5000 seeded UASMCs attached in the same time period and the resulting MTS signal was not statistically different than the untreated and negative controls. A similar cell binding preference of HUVEC over UASMC to the RGD coating was observed. This is not to say that UASMC cells do not bind the surface at all, but the timescale for such a binding appears to be slower, or the interaction is weaker and so the cells are readily removed by washing. Other experiments that used a long term culture of the cells did show that the smooth muscle cell line will adhere to an interfacial peptide coated surface but the attachment interaction is not as rapid as with the endothelial cell phenotype. In this particular short-time case, human endothelial cells are more readily able to adhere to the peptide coating over SMCs. However, the exact benefit such a short temporal selectivity would have in vivo where a device is introduced into a biological system with extra-cellular matrix adhered (as opposed to suspended) surrounding cells remains to be observed. What is clear is that the PS-CRRETAWAC coating has a pronounced benefit in lowering the binding and surface recognition ability of platelets to near background levels while maintaining the ability of endothelial cells to bind to the modified surface.
Figure 6.
Fluorescent micrographs of HUVEC and UASMC cells placed onto a variety of coated surfaces. HUVEC stain positively (Red) for PECAM-1.
Future experiments are planned to further monitor the RRETAWA coating and its interactions with other cell types prevalent in the circulatory system such as leukocytes and other inflammatory/immune cells. These cells are also known to use a variety of integrins (αLβ2, α4β1, αMβ2, αVβ3, or α5β1) to extravasate and migrate through the stroma, with the main integrins generally from the α4β1 family.39–43 It is thought that extracellular chemo-attractants or chemical signals are necessitated for leukocyte adherence to a substrate through the α5β1 integrins—the integrin that binds RRETAWA—and that these signals come from activated platelets and ECs.44 Because we have shown that the RRETAWA coating reduces platelet adherence and provides a natural surface on which the ECs can reside, the release of the chemical signals would be deprecated in the location of the coating and we hypothesize that it would be unlikely that the immune cells would adhere using the α5β1 integrin as they would not become activated. An examination of leukocyte interaction with the RRETAWA peptide has not been reported to date, and we plan on further examining these issues as it would be important in the development of a RRETAWA based pro-healing stent.
Conclusion
This coating can be non-covalently applied to devices of any geometry through a simple dipcoating procedure and requires no harsh chemicals or energetic treatments. As shown, the FFSFFFPASAWGSSGSSGK(biotin)CRRETAWAC peptide possesses a number of properties that would be desirable for a stent coating. Importantly, experiments showed that this bi-functional peptide readily coated surfaces and bound endothelial cells but possessed minimal platelet adhesion. Specifically, only background platelet binding was observed with this coating, while an RGD terminated PS peptide performs as expected with significant adhesion. In regards to the interaction with HUVEC and UASMC, there is an apparent short-term difference in adherence, but again, how translatable this difference is to in vivo condition remains to be determined. All results were obtained in the presence of protein rich media, either plasma or serum, respectively, demonstrating that the peptide coating can function in a complex biological milieu. Continued in vitro and in vivo studies with such interfacial biomaterials may lead to the creation of next-generation pro-healing stent surfaces that promote the endothelialization of the device, while simultaneously inhibiting the adhesion and thrombus formation typical of platelet interactions.
Acknowledgements
MWG and DJK thank the National Institutes of Health for funding through grant EB-000501.
Footnotes
Supporting Information: None
COI Mark W. Grinstaff and Daniel J. Kenan are co-Founders of Affinergy Inc.
References
- 1.Williams DF. Biomaterials. 2008;29:2941. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials Science: An Introduction to Materials in Medicine. San Diego: Academic Press; 2000. [Google Scholar]
- 3.Anderson JM. Annual Review of Materials Research. 2001;31:81. [Google Scholar]
- 4.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJ. J Am Coll Cardiol. 2005;45:1574. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Healy KE. Current Opinion in Solid State & Materials Science. 1999;4:381. [Google Scholar]
- 6.Jordan SW, Chaikof EL. J Vasc Surg. 2007;45 Suppl A:A104. doi: 10.1016/j.jvs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Billinger M, Buddeberg F, Hubbell JA, Elbert DL, Schaffner T, Mettler D, Windecker S, Meier B, Hess OM. Journal of Invasive Cardiology. 2006;18:423. [PubMed] [Google Scholar]
- 8.Whelan DM, van der Giessen WJ, Krabbendam SC, van Vliet EA, Verdouw PD, Serruys PW, van Beusekom HM. Heart (British Cardiac Society) 2000;83:338. doi: 10.1136/heart.83.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Journal of the American College of Cardiology. 2006;48:193. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Letourneur D, Grainger DW. Biomacromolecules. 2007;8:3281. doi: 10.1021/bm700540p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Co M, Tay E, Lee CH, Poh KK, Low A, Lim J, Lim IH, Lim YT, Tan HC. American Heart Journal. 2008;155:128. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Duckers H, Onuma Y, Benit E, de Winter RJ, Wijns W, Grisold M, Verheye S, Silber S, Teiger E, Hill J, Serruys PW. Circulation. 2008;118:S1042. [Google Scholar]
- 13.Miglionico M, Patti G, D'Ambrosio A, Di Sciascio G. Catheter Cardiovasc Interv. 2008;71:600. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 14.Seth DA. Catheter Cardiovasc Interv. 2008;71:605. doi: 10.1002/ccd.21571. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Sata M, Hikichi Y, Sohma R, Fukuda D, Uchida T, Shimizu M, Komoda H, Node K. Circulation. 2007;115:553. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 16.Pierschbacher MD, Ruoslahti E. Nature. 1984;309:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 17.Ruoslahti E. Annual review of cell and developmental biology. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 18.Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Journal of Biomedical Materials Research Part A. 2007;80:306. doi: 10.1002/jbm.a.30927. [DOI] [PubMed] [Google Scholar]
- 19.Elmengaard B, Bechtold JE, Søballe K. Journal of Biomedical Materials Research Part A. 2005;75:249. doi: 10.1002/jbm.a.30301. [DOI] [PubMed] [Google Scholar]
- 20.Ho JE, Barber TA, Virdi AS, Sumner DR, Healy KE. Journal of Biomedical Materials Research Part A. 2007;81A:720. doi: 10.1002/jbm.a.31008. [DOI] [PubMed] [Google Scholar]
- 21.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Biomaterials. 2006;27:5561. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Clinical Oral Implants Research. 2006;17:251. doi: 10.1111/j.1600-0501.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. Cell. 2002;110:673. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 24.Koivunen E, Wang B, Ruoslahti E. Journal of Cell Biology. 1994;124:373. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen CC, Kligman F, Tang C, Kottke-Marchant K, Marchant RE. Biomaterials. 2007;28:3537. doi: 10.1016/j.biomaterials.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mould AP, Burrows L, Humphries MJ. The Journal of Biological Chemistry. 1998;273:25664. doi: 10.1074/jbc.273.40.25664. [DOI] [PubMed] [Google Scholar]
- 27.Mould AP, Koper EJ, Byron A, Zahn G, Humphries MJ. Biochem J. 2009;424:179. doi: 10.1042/BJ20090992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenan DJ, Walsh EB, Meyers SR, O’Toole GA, Carruthers EG, Lee WK, Zauscher S, Prata CAH, Grinstaff MW. Chemistry & Biology. 2006;13:695. doi: 10.1016/j.chembiol.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Meyers SR, Hamilton PA, Walsh EB, Kenan DJ, Grinstaff MW. Advanced Materials. 2007;19:2492. [Google Scholar]
- 30.Meyers SR, Khoo X, Huang X, Walsh EB, Grinstaff MW, Kenan DJ. Biomaterials. 2009;30:277. doi: 10.1016/j.biomaterials.2008.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers SR, Kenan DJ, Grinstaff MW. ChemMedChem. 2008;3:1645. doi: 10.1002/cmdc.200800205. [DOI] [PubMed] [Google Scholar]
- 32.Khoo X, Hamilton P, O'Toole GA, Snyder BD, Kenan DJ, Grinstaff MW. Journal of the American Chemical Society. 2009;131:10992. doi: 10.1021/ja9020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Zauscher S, Klitzman B, Truskey GA, Reichert WM, Kenan DJ, Grinstaff MW. Ann Biomed Eng. 2010;38:1965. doi: 10.1007/s10439-010-9986-5. [DOI] [PubMed] [Google Scholar]
- 34.Hoess RH. Chemical Reviews. 2001;101:3205. doi: 10.1021/cr000056b. [DOI] [PubMed] [Google Scholar]
- 35.Smith GP, Petrenko VA. Chemical Reviews. 1997;97:391. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 36.Newman PJ. J Clin Invest. 1997;100:S25. [PubMed] [Google Scholar]
- 37.Weyrich AS, Zimmerman GA. Trends in immunology. 2004;25:489. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Wagner DD, Burger PC. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:2131. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 39.Ulbrich H, Eriksson EE, Lindbom L. Trends Pharmacol Sci. 2003;24:640. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Springer TA. Nature. 1990;346:425. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 41.Hemler ME. Annu Rev Immunol. 1990;8:365. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 42.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Cell. 1995;80:413. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 43.van den Berg JM, Mul FP, Schippers E, Weening JJ, Roos D, Kuijpers TW. Eur J Immunol. 2001;31:276. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Blood. 1996;88:146. [PubMed] [Google Scholar]