Abstract
Background
We present here a case of haemorrhagic brain infarction in a middle-aged and physically active male, who had never smoked. This case report aims to remind the internist and neurologist to bear in mind unusual aetiologies of brain infarcts in patients without classical cardiovascular risk factors.
Case Description
A 49-year-old male with pulmonary asthma and a prior history of nasal polyps had a wake-up stroke with left-sided symptoms and speech disturbance. A head MRI and MR angiography revealed a recent haemorrhagic infarct in the right putamen and corona radiata. The left hemiparesis progressed to sensory-motor hemiplegia on the 4th day. In the head CT, it was shown that the haemorrhagic infarct had progressed to a large haematoma. A pansinusitis was also diagnosed. The aetiological investigations revealed a minor atrial septal defect (ASD) with shunting and a heterozygotic clotting factor V R506Q mutation. A remarkable blood eosinophilia of 9.80 E9/l (42%) together with fever, sinusitis, wide-spread bilateral nodular pulmonary infiltrates that did not respond to wide-spectrum antimicrobial treatment, positive anti-neutrophilic cytoplasmic antibodies, a high myeloperoxidase antibody level and slightly positive anti-proteinase 3 antibodies suggested the diagnosis of Churg-Strauss syndrome. These inflammatory symptoms and findings promptly responded to treatment with corticosteroids and cyclophosphamide.
Conclusions
Even after the concomitant findings of the low risk factors, i.e. small ASD and heterozygotic clotting factor mutation, continued search for the final aetiology of stroke revealed Churg-Strauss syndrome, which was the key to the treatment.
Key Words: Small vessel vasculitis, Churg-Strauss syndrome, Eosinophilia, Factor V Leiden, mutation, Atrial septal defect, Patent foramen ovale, Haemorrhagic stroke
Case Report
In mid-January 2010, a 49-year-old male with pulmonary asthma and a prior history of nasal polyps woke up at 00.30 at night with a feeling of tongue swollenness. Upon standing up, he noticed paraesthesia of the left hand and a left facial droop. When the emergency medical staff arrived, he was sitting, his speech was blurred, and the muscle strength of his extremities was unaffected. Due to the wake-up nature of the mild hemiparesis, he was not considered a candidate for thrombolysis treatment when he was pre-notified to the neurological emergency by the emergency medical staff.
The patient had never smoked, and his previous medical history was free of cardiac symptoms, hypertension, hyperlipidaemia, diabetes, or migraine. He was in excellent physical condition and had done a 10-km cross-country skiing trip 2 days before. In addition, he had previously run several marathons. Physical examination at the emergency department revealed a slight pronation of the left arm and a mild slowness of the diadochokinesia of the left hand together with a residual paresis and paraesthesia of the lower facial area. The head CT scan showed no findings, and neither did the plain chest X-ray. The patient was admitted to a neurological ward with a working hypothesis of brain stem infarction, and acetylsalicylic acid was administered at a loading dose of 250 mg orally, which was continued at a dose of 100 mg daily.
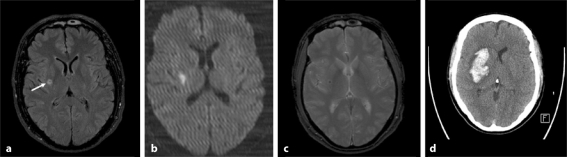
The next day, a head MRI and MR angiography (MRA) revealed a fresh minor infarction at the corona radiata on a FLAIR sequence (fig. 1a), with a diffusion-weighted image showing restricted diffusion of the lesion, consistent with an infarct (fig. 1b), as well as a 3-mm hemosiderin ring at the right putamen (fig. 1c). The MRA of the intracranial and neck vessels was unremarkable. The left hemiparesis progressed to sensory-motor hemiplegia on the 4th day. The head CT scan showed an expansive right-sided intracerebral haemorrhage in the basal ganglia extending to the corona radiata (fig. 1d).
Fig. 1.
Head MRI on admission (a–c) and control head CT scan after symptom progression on day 4 (d). a An axial FLAIR image showing an acute infarct in the right putamen (arrow). b A diffusion-weighted image demonstrating restricted diffusion of the lesion, consistent with an infarct. c A small haemorrhage (black dot) is noted within the infarcted area in a T2∗-weighted image. d Progression of the haemorrhagic transformation in the putamen on day 4 in CT.
The ancillary aetiological studies of the haemorrhagic infarction included transcranial Doppler sonography, which showed right-sided microemboli that were increased after a Valsalva manoeuvre. Correspondingly, a minor atrial septal defect (ASD) with shunting was suspected at transthoracic echocardiography. The patient had a heterozygotic clotting factor V R506Q mutation, i.e. activated protein C (APC) resistance.
On admission, the blood chemistry showed mild leucocytosis of 9.2 E9/l and the C-reactive protein (CRP) level was 7 mg/l. Head CT scan revealed asymptomatic pansinusitis of the frontal, ethmoidal, and left sphenoidal cavities, and the patient was given oral amoxycillin. However, during the first 10 days, the CRP level rose up to 185 mg/l, and the leucocyte count up to 20.7 E9/l. Chest X-ray remained normal. Maxillary puncture was performed, and the bacterial cultures of the pus grew coliforms and local normal flora, susceptible to the antimicrobials being used. The empiric antimicrobial therapy was changed to cefuroxime and metronidazole and then piperacillin plus tazobactam, without any response in the inflammatory parameters. The patient had high fever (up to 38.8°C) and intense muscle pain, but blood cultures were negative. Consequently, contrast-enhanced body CT was performed and showed wide-spread bilateral nodular chest infiltrates in all pulmonary lobes and reactive lymph nodes in the upper mediastinal area. Bronchoalveolar lavage was not possible due to the haemorrhagic brain infarction.
An infectious diseases specialist was consulted, and further diagnostic work-up showed a remarkable blood eosinophilia of 4.89–9.8 E9/l (30–42%) (fig. 2), positive anti-neutrophilic cytoplasmic antibodies (P-ANCA) antibody titer (200; reference value <20), a high serum myeloperoxidase antibody level (63.4 IU/ml; reference value <6) and slightly positive serum proteinase 3 antibodies (9.9 IU/ml; reference value <4), slightly decreased levels of serum IgA (0.74 g/l; reference value 0.88–4.84), normal IgG, but elevated IgG4 (4.45 g/l; reference value 0.08–1.4) (table 1). The patient had never visited the tropics. Also, stool examinations were negative for parasites including amoeba staining and Strongyloides culture, and the patient did not show serum antibodies against Trypanosoma, Toxocara, Echinococcus, Fasciola, schistosoma, Filaria, Strongyloides or Toxoplasma. Additionally, serologic tests for HIV, cytomegalovirus, Epstein-Barr virus, human herpes virus 1 and 2, and for hepatitis A, B and C were all negative. The patient did not have anti-phospholipid antibodies. HLA typing showed HLA-B*35 allele (heterozygous mutation).
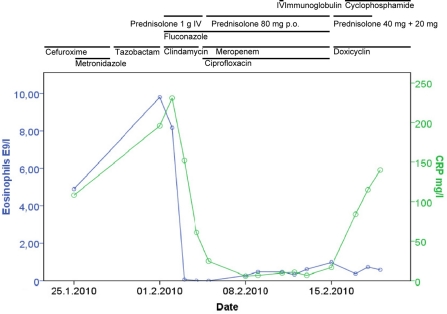
Fig. 2.
Blood eosinophil count (E9/l) and CRP level (mg/l) in relation to the treatments.
Table 1.
The autoantibodies at the time of diagnosis of Churg-Strauss syndrome and after high-dose IV steroid treatment
| Antoantibodies | Value at 1 week | Reference | Value at 1 month |
|---|---|---|---|
| S-ANCA | |||
| S-Pr3AbG | 9.9 IU/ml | <4 | <2 IU/ml |
| S-MPOAbG | 63.4 IU/ml | <6 | <2 IU/ml |
| S-C-ANCIF | <20 titer | <20 | <20 titer |
| S-P-ANCIF | 200 titer | <20 | <20 titer |
| S-ANAAb | 80 | <80 | <80 |
The bone marrow aspirate revealed normal morphology and relatively strong eosinophilia, and the chromosomal investigation was unremarkable. Interferon gamma release assay for M. tuberculosis was negative.
The combination of asthma, sinusitis, history of nasal polyps, and eosinophilia, together with the detection of antinuclear and anti-neutrophilic antibodies evoked the suspicion of Churg-Strauss syndrome, leading to the treatment decision of a 3-day therapy with intravenous pulse methylprednisolone (1 g/day). The blood eosinophils and ANCA antibodies were undetectable 3 days and 1 month after treatment onset, respectively (fig. 2). Beta-lactam antimicrobials were changed to clindamycin together with ciprofloxacin. There were no skin or joint manifestations, cardiac dysfunction or signs of peripheral neuropathy at the time of the diagnosis, nor did the head MRA demonstrate findings that would have confirmed the CNS vasculitis as aetiology of the haemorrhagic brain infarction. The cerebrospinal fluid was not investigated, nor was a brain biopsy taken to confirm the CNS vasculitis, because of the urgent need to start immunomodulatory therapy due to the suspicion of Churg-Strauss syndrome with a cytotoxically high eosinophil level. Nasal mucous membrane biopsy did not confirm vasculitis either. No renal biopsy was performed; however, the CT scan showed wedge-like lesions, suggesting renal infarcts. In addition, urine examination showed slight microscopic haematuria and proteinuria (834 mg/l). Alanine aminotransferase temporarily increased to 509 U/l.
After treatment with high-dose corticosteroids i.v. for 3 days, the patient was first given cyclophosphamide for 2 weeks together with oral prednisolone, until he developed prolonged neutropenia and fever. During that time, he also developed ileus, which was managed conservatively. Later, a colonoscopy was performed which showed a rectal tubular polyp but no ischaemia or inflammatory lesions. At that time, he was started on immunoglobulins (0.4 g/kg i.v.) given every 3 weeks as an additive immunomodulatory treatment together with oral corticosteroids (fig. 2).
Three months after the start of the immunosuppressive treatment, while on oral corticosteroids (prednisolone 20 + 10 mg) and azathioprine (100 mg), the patient suddenly and unfortunately developed peritonitis. A laparoscopy showed an ileal perforation, but there were no histological changes suggesting vasculitis. Though taken during immunosuppressive treatment, electroneuromyography (ENMG) suggested vasculitis-like neuropathic changes, but an undiagnostic muscle biopsy from the right vastus lateralis muscle contained only subcutaneous tissue with no signs of necrotizing vasculitis or eosinophils.
The left-sided sensory-motor hemiplegia and the neuropsychological symptoms indicated physio- and ergotherapeutic as well as neuropsychological rehabilitation, which was actively continued after the initial complications. The patient was still in a neurological rehabilitation institution almost 7 months after the ictus.
Discussion
Here, we report a case of haemorrhagic brain infarction in a middle-aged male, who had no typical risk factors. Intense investigations, however, revealed a few but rare putative predisposing factors. He had an ASD and a heterozygotic clotting factor V R506Q mutation, i.e. APC resistance, which could well have been the sole underlying cause of the infarct. However, high eosinophilic leucocytosis, fever, muscle pain, resistant sinusitis and pneumonic infiltrates led to the suspicion of a small vessel vasculitis, which was supported by the presence of P-ANCA. The diagnosis could not be confirmed by biopsies from nasal mucosa, gut or subcutaneous tissue taken during immunosuppressive treatment. The patient's inflammatory disease responded rapidly to treatment, but he still needed neurological rehabilitation 7 months afterwards.
There has been an increasing emphasis on the role of patent foramen ovale (PFO) in the genesis of ischaemic stroke during the last decade. A recent meta-analysis summarized that PFO is significantly associated with stroke in patients younger than 60 years of age, in particular in the subgroup of young patients with cryptogenic stroke. Several factors have been suggested to increase the risk for stroke or recurrent stroke in patients with a PFO: these include a younger age, the association with atrial septal aneurysm, the presence of a right-to-left shunt at rest, the size of the PFO, and the association with thrombophilic conditions [1]. Our patient had 3 out of these 5 factors. The most frequently encountered hereditary hypercoagulable states are APC resistance due to factor V Leiden mutation, prothrombin 20210A mutation, protein C deficiency, protein S deficiency, and AT III deficiency. Patients with a hypercoagulable state typically present with a venous thrombosis and, less commonly, arterial thrombosis including ischaemic stroke may occur. Paradoxical cerebral arterial emboli may be due to deep venous/pelvic thrombi in patients with an ASD or a patent foramen ovale [2]. The inherited thrombophilia has often been suggested to be coincidental rather than causal in stroke patients [3]. The investigations in our patient did not include a search for deep vein thrombosis or pelvic thrombosis, which leaves the aetiological role of heterozygotic factor V Leiden mutation and ASD uncertain. In addition, the initially small-sized ischaemic brain infarct which occurred at rest was not typical for cardioembolism. The high eosinophil count most obviously also contributed to thrombophilia in our patient.
The spectrum of small vessel vasculitides includes Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, Henoch-Schönlein purpura, cutaneous leucocytoclastic vasculitis and essential cryoglobulinaemic vasculitis [4]. The first 3 conditions are characterized by the presence of circulating ANCAs [5]. The ANCA autoantibodies have been suggested to play an in vivo pathogenetic role by having an effect on neutrophil function. In patients with necrotizing vasculitis, ANCA-induced release of toxic oxygen radicals and noxious granule enzymes from cytokine-primed neutrophils has been proposed to mediate vascular inflammation [6]. Also, the HLA-B*35 allele positivity detected in our patient has been associated for example with endothelial dysfunction, increased mononuclear cell apoptosis and rapid progression in HIV infection [7, 8, 9], which may have played a role in the inflammatory cascade.
The Churg-Strauss syndrome is the most infrequent of the ANCA vasculitides. ANCA vasculitis mostly present at middle age, as in the present case. The primary systemic vasculitis damages the vessel walls, which leads either to obstruction, stenosis, or dilatation, resulting in ischaemic or bleeding consequences [4]. In the Churg-Strauss syndrome, a granulomatous inflammation may also be present in addition to vasculitis. The diagnosis of small vessel vasculitis should preferably rely on both clinical findings and histopathological examination of the organ involved. In the present case, the clinical combination of asthma, sinusitis, history of nasal polyps, eosinophilia, and pulmonary findings together with the detection of antinuclear and anti-neutrophilic antibodies served as a substitute for a diagnostic biopsy from the affected organ. In addition, ENMG, kidney imaging and urine sediment findings were slightly suggestive of vasculitis. A previous case series included brain infarcts as a manifestation or complication of Churg-Strauss syndrome in 3 patients [10]. One case report described intracerebral haemorrhage as a rare sequel of Churg-Strauss syndrome [11], and another reported an elderly woman with multiple cerebral infarctions and hypereosinophilia with assumed cardiac involvement and multiple cerebral infarctions due to cardiac embolism [12].
Cyclophosphamide combined with glucocorticoids is the standard therapy for remission induction in generalized ANCA-associated vasculitis [13, 14]. These treatments were indicated also in our patient due to the severe CNS manifestation and systemic multi-organ findings (including pulmonary infiltrates). The fact that the high levels of eosinophilia and autoantibodies promptly responded to immunosuppressive treatment is also supportive for the Churg-Strauss syndrome diagnosis. Ileal perforation may have been an iatrogenic complication, but due to the absence of any other known predisposing factors, gut vasculitis was suspected as an underlying cause despite normal histology of the gut biopsy during immunosuppressive treatment.
Disclosure Statement
The authors have nothing to disclose.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Giacalone G, Abbas MA, Corea F. Prevention strategies for cardioembolic stroke: present and future perspectives. Open Neurol J. 2010;4:56–63. doi: 10.2174/1874205X01004020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr. 2005;10:567–578. doi: 10.1017/s109285290001021x. [DOI] [PubMed] [Google Scholar]
- 3.Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke. 2001;32:1793–1799. doi: 10.1161/01.str.32.8.1793. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder CG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 5.Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 6.Falk JR, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santaniello A, Salazar G, Lenna S, Antonioli R, Colombo G, Beretta L, Scorza R. HLA-B35 upregulates the production of endothelin-1 in HLA-transfected cells: a possible pathogenetic role in pulmonary hypertension. Tissue Antigens. 2006;68:239–244. doi: 10.1111/j.1399-0039.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- 8.Salazar G, Colombo G, Lenna S, Antonioli R, Beretta L, Santaniello A, Scorza R. HLA-B35 influences the apoptosis rate in human peripheral blood mononucleated cells and HLA-transfected cells. Hum Immunol. 2007;68:181–191. doi: 10.1016/j.humimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, Goedert JJ, Buschbinder S, Hoots K, Vlahov D, O'Brien SJ, Carrington M. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med. 2001;344:1668–1675. doi: 10.1056/NEJM200105313442203. [DOI] [PubMed] [Google Scholar]
- 10.Wolf J, Bergner R, Mutallib S, Buggle F, Grau AJ. Neurologic complications of Churg-Strauss syndrome – a prospective monocentric study. Eur J Neurol. 2010;17:582–588. doi: 10.1111/j.1468-1331.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- 11.Winek J, Zych J, Wiatr E, Oniszh K, Roskowski-Sliz K. Stroke as a predominant symptom at Churg-Strauss syndrome. Pneumonol Alergol Pol. 2007;75:191–196. [PubMed] [Google Scholar]
- 12.Ghaeni L, Siebert E, Ostendorf F, Endres M, Reuter M. J Multiple cerebral infarctions in a patient with Churg-Strauss syndrome. J Neurol. 2010;257:678–680. doi: 10.1007/s00415-009-5439-1. [DOI] [PubMed] [Google Scholar]
- 13.Bosch X, Guilabert A, Espinosa G, Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: a systematic review. JAMA. 2007;298:655–669. doi: 10.1001/jama.298.6.655. [DOI] [PubMed] [Google Scholar]
- 14.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallneberg CGM, Merkel PA, Raspe H, Salvarani C, Scott DGI, Stegeman C, Watts R, Westman K, Witter J, Yazici H, Luqmani R, for the European Vasculitis Study Group EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68:310–317. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]