Abstract
Our purpose was to quantify the effects of head pitch on muscle activity patterns of the decerebrate cat hindlimb during walking. Five decerebrate cats walked at 0.7 m/s on a treadmill positioned level with the head pitch either parallel to the treadmill, 50% nose down or 50% nose up. We collected electromyography data from six hindlimb muscles. During level walking, after we manipulated head pitch, our results were surprisingly equivalent to the research on slope walking. For instance, muscle activity during level walking with a 50% head pitch nose down mimicked uphill walking. The muscle activity of the iliopsoas and semitendinosus significantly increased. Muscle activity during level walking with a 50% head pitch nose up mimicked downhill walking. Specifically, the biceps femoris and semimembranosus were inactive during the entire step. These alterations in muscle activity occurred within one step of altering head pitch but dissipated as level walking continued. In conclusion, the time course of muscle activity patterns due to modifications in head pitch is immediate and transitory.
Keywords: Locomotion, Electromyography, Proprioception
Introduction
Humans and animals traverse a variety of terrains in their natural surroundings. For example, they walk on level ground as well as up or down hills, and each of these environments requires a different muscular, locomotor pattern. In short, during level walking of the cat, there is alternation of flexor muscles during swing and extensor muscles during stance. At the onset of swing, all joints are in flexion to elevate the paw. The iliopsoas (ILIO), a hip flexor, shows the greatest level of activation during the majority of the swing phase. Semitendinosus (ST), a knee flexor and hip extensor, is involved in the early portion of swing when the paw lifts off the ground and occasionally in the later portion of swing in preparation for paw contact. Prior to this paw contact, extensor muscles at the hip, knee, and ankle are activated. These extensors, anterior biceps femoris (aBF), anterior semimembranosus (aSM), vastus lateralis (VL), and lateral gastrocnemius (LG) continue to be active for most of the stance phase.
Carlson-Kuhta et al. (1998) and Smith et al. (1998) collected electromyography in cats walking uphill and downhill at grades from 25 to 100% (14°–45°). In general, during uphill walking, flexor muscle activity magnitude increased during swing and extensor muscle activity magnitude increased during stance (Matsuyama and Drew 2000b). During downhill walking, hip and knee extensor muscle activity decreased during stance. Interestingly, in addition to these predicted changes, uphill and downhill walking resulted in unexpected electromyography patterns. In particular, during uphill walking, semitendinosus, typically active only during swing, was active during the initial portion of stance (Carlson-Kuhta et al. 1998). During downhill walking, the aBF and aSM, typically active during stance, were inactive during the entire step (Smith et al. 1998). In addition, iliopsoas, primarily active during swing, was active during stance. Smith and colleagues also completed a kinematic analysis and noted that during slope walking, cats assume a crouched posture, decrease stride length, and tilt the head down during uphill conditions and up during downhill conditions (Carlson-Kuhta et al. 1998; Smith et al. 1998).
Similar to these examinations of kinematics and electromyography, other investigators have contributed important insights regarding neural mechanisms for the control of locomotion. While there is agreement that sensory feedback is important in order to initiate and maintain a consistent walking motor program, there continues to be disagreement about how these various programs are initiated or maintained. Recent studies suggest that locomotion is a centrally generated pattern that is likely modified through sensory feedback: proprioceptive (muscle spindles, Golgi tendon organs) or exteroreceptive (optic, vestibular, cutaneous). The transition between speeds and terrains during locomotion may be controlled by one of these mechanisms or a combination (Dietz and Duysens 2000; Bent et al. 2004). For instance, decerebrate cats match the speed of the treadmill by altering the duration of the stance phase. During this condition, both proprioceptive and exteroreceptive inputs aid in regulating the step cycle during locomotion (Duysens and Pearson 1976). Unfortunately, how sensory information initiates diverse locomotor patterns in a variety of environments, such as uphill and downhill walking, is not well understood.
The rationale for this study is that evaluating the muscle activity of decerebrate walking cats while head pitch is manipulated will indicate, which sensory systems potentially regulate the walking motor pattern when there is a change in terrain. Since the transition from level to slope walking involves a reorientation of the body with respect to gravity, signals representing body orientation could contribute to the altered motor patterns. This signal can in principle be derived from information about orientation of the body with respect to the head from neck proprioceptors and orientation of the head with respect to gravity from vestibular apparatus (Suzuki et al. 1985; Manzoni et al. 2004). We compared the muscle activity of decerebrate cats during level walking with the head pitch parallel to the treadmill to level walking with the head pitch nose down or nose up. In general, we tested the hypothesis that neck proprioceptors and vestibular apparatus aid in the initiation of appropriate muscle activity patterns during walking.
Materials and methods
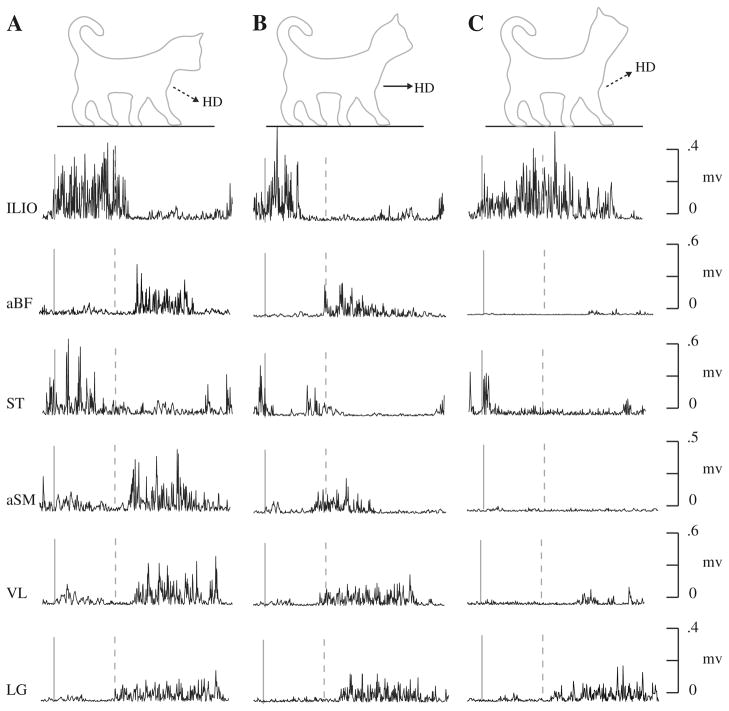
Five decerebrate cats walked at 0.7 m/s on a level treadmill while the head was parallel to the treadmill, 50% nose down, and 50% nose up (50% = 26.6°). We randomized these three conditions for each testing session (Fig. 1a–c). The Emory University Institutional Animal Care and Use Committee approved all the procedures utilized in this study.
Fig. 1.
Typical electromyography records from select hind-limb muscles during the first step when the treadmill was level with the head pitch 50% up (a) parallel (b) and 50% down (c). The solid line indicates paw-lift-off while the dashed line indicates paw-contact
For each experiment, we started by anesthetizing the cat with isoflurane and inserting EMG electrodes. The electrodes were 38 gauge braided and coated, stainless steel wire with approximately 0.5 mm of the Teflon insulation removed. We tied the wires in staggered fashion to prevent contact of the de-insulated ends, and inserted the wires into each muscle using a needle and the long end of the suture. We implanted these wires in select hindlimb muscles: ILIO (hip flexor), aBF (hip extensor), aSM (hip extensor), ST (hip extensor and knee flexor), VL (knee extensor), and LG (knee flexor and ankle extensor). The EMG electrodes were connected to a custom preamplification stage using miniature gold connectors.
Following EMG electrode insertion, the cat was positioned over a treadmill in natural stance with the head fixed in a sterotaxic frame and hip height controlled with a clamp attached to the base of the tail. Next, we performed a pre-mammillary decerebration. The brainstem was transected rostral to the superior colliculus, at approximately a 45° angle, in order to preserve the mammillary bodies and sub-thalamic nucleus. All brain matter rostral and lateral to the transection was removed. We then reduced the anesthesia for approximately 1 h and subsequently eliminated the anesthesia. Finally, once the animal initiated spontaneous stepping movements, we imposed a rhythmic pattern by turning on the treadmill. In order to manipulate head pitch, the stereotaxic frame was mounted on a pivot located at the base of the skull at the atlanto-occipital joint.
We processed the EMG data in two stages. First, we determined when in a stride the muscles were active and second, we quantified the magnitude of activation. After data collection at a rate of 2,000 Hz, the data were bandpass filtered to retain frequencies between 10 and 500 Hz. A MatLab program was created to determine the difference between the baseline and periods of muscle activity. We defined a period as active if the EMG activity exceeded a threshold of two standard deviations above the baseline mean for at least 50 ms. For the amplitude analysis of the active periods, we full-wave rectified the band-pass filtered signals and calculated the mean EMG amplitude (mEMG).
We analyzed electromyographic data across all conditions using a 3-level one-way repeated measures analysis of variance (ANOVA). Additionally, we performed a New-man-Keuls post hoc tests to analyze the differences between each experimental condition. Significance was defined as p < 0.05.
Results
To begin with, we sought to evaluate how our decerebrate preparation compared to intact cats walking on the level. Opportunely, the EMG of each decerebrate cat was nearly identical to previous data on intact cats walking on the level (Fig. 1b). We observed alternating muscle activity between flexor muscles during swing and extensor muscles during stance. In addition, the timing and magnitude was similar to the previous work on cats (Smith and Carlson-Kuhta 1995; Carlson-Kuhta et al. 1998; Smith et al. 1998; Matsuyama and Drew 2000a, b), as we matched the speed of the treadmill to their velocity average. Specifically, both the ILIO and ST were primarily active during swing, while the aBF, aSM, VL, and LG were active during the majority of stance.
Intriguingly, immediately after we manipulated head pitch, our level EMG data during the first step were equivalent to the results on slope walking (Table 1). For instance, within one step, muscle activity during level walking with a 50% head pitch nose down mimicked uphill walking (Figs. 1a, 2a). During this condition, muscle activity of the hip and knee extensor muscles significantly increased compared to level walking with the head parallel to the treadmill. With the head pitch nose down, the aBF, aSM, and VL muscle activity started just after paw contact and remained active throughout stance with a higher magnitude than with the head parallel. In detail, the aBF increased by almost half where both the aSM and VL more than doubled compared to level walking with the head parallel (p < 0.01). The hip flexor muscles showed similar slope related patterns during level walking with a 50% head pitch nose down. The ILIO and ST, associated with swing activity increased in magnitude by 39 and 127% respectively compared to level walking with the head parallel to the treadmill (p < 0.05).
Table 1.
Values are mean ± SD for the active periods of the first step of three conditions, with three trials for each animal
| 50% nose down | Head parallel | 50% nose up | |
|---|---|---|---|
| Iliopsoas-swing | 0.39 ± 0.09* | 0.28 ± 0.06 | 0.26 ± 0.08 |
| Iliopsoas-stance | 0.03 ± 0.02 | 0.05 ± 0.06 | 0.40 ± 0.09* |
| Anterior biceps femoris | 0.32 ± 0.05* | 0.22 ± 0.05 | 0.01 ± 0.01* |
| Semitendinosus-swing | 0.50 ± 0.06* | 0.22 ± 0.07 | 0.15 ± 0.11* |
| Semitendinosus-stance | 0.20 ± 0.09 | 0.16 ± 0.07 | 0.15 ± 0.02 |
| Anterior semimembranosus | 0.34 ± 0.08* | 0.12 ± 0.03 | 0.01 ± 0.01* |
| Vasus lateralis | 0.28 ± 0.17* | 0.13 ± 0.05 | 0.09 ± 0.02 |
| Lateral gastrocnemius | 0.17 ± 0.07 | 0.16 ± 0.04 | 0.15 ± 0.06 |
Significant difference between experimental condition and control (treadmill level with head pitch parallel)
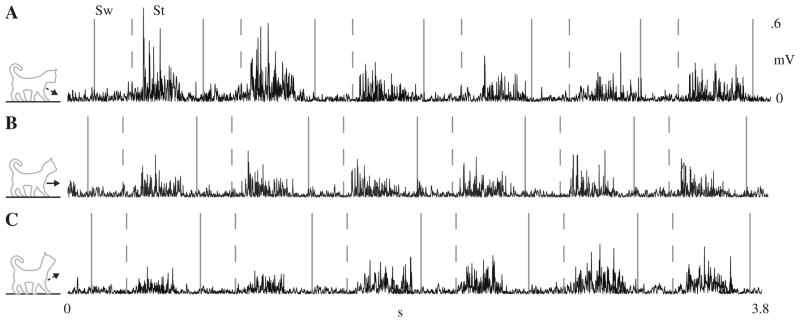
Fig. 2.
Typical electromyography records from a representative hind-limb extensor muscle (vastus lateralis) when the treadmill was level with the head pitch 50% up (a) parallel (b) and 50% down (c). The area
Similarly, within one step, muscle activity during level walking with a 50% head pitch nose up mimicked downhill walking (Figs. 1c, 2c). The ILIO was active during stance and the aBF and aSM were inactive during the entire step. In fact, the ILIO increased by approximately eight times the value during level walking with the head parallel. The ST activity during swing with the head pitch up decreased by 47% (p < 0.05). However, the most significant change during the nose up condition was the reduction of activity to nearly zero for both the aBF and aSM during stance (p < 0.01). In contrast, with the head pitch nose up, the LG muscle activity did not differ significantly compared to when the head pitch was parallel (p = 0.40).
Lastly, a critical detail during both the nose down and nose up conditions, is that the effect of head pitch was transitory (Fig. 2a, c). As an example, the VL activity, during level walking, originated after paw contact and terminated prior to paw liftoff (Fig. 2b). When the head was tilted down, the mean stance activity increased over two times compared to when the head was parallel (p < 0.01). Similarly, when the head was tilted up, the mean stance activity decreased by 31% compared to when the head was parallel (p = 0.07). These alterations only lasted, on average, for 2 step cycles with a range of 1–4 step cycles.
Discussion
In summary, modifying head pitch during decerebrate cat level walking causes significant muscle activity pattern changes that resemble intact cat slope walking patterns. In following the solid line and prior to the dashed line is swing (Sw), while the area following the dashed line is stance (St) terms of timing, muscle activity patterns, due to modifications in head pitch is immediate and transitory. It is possible that the vestibular apparatus or neck proprioceptors are responsible for the initiation of changes in terrain.
Early studies have demonstrated how vestibular input aids in the control of posture in various environments. Magnus and deKleijn discovered that a number of postural reflexes exist in the labyrinth that affect extensor muscles in the limbs (Magnus and de Kleijn 1912). They showed that changes in head pitch cause the greatest increase in extensor muscle activation, while changes in head roll cause little alteration in muscle activation. Additionally, they destroyed the labyrinth to examine the influence of head pitch on limb posture during standing. A nose down orientation triggered increased muscle activity in the hind limbs. In contrast, a nose up orientation triggered increased activity in the forelimbs. These results illustrate the influence of neck reflexes on limb muscle activity and ultimately locomotion.
These changes in head and neck position stimulate neck proprioceptive reflexes or cervicocollic reflexes that stimulate neck muscles in response to perturbations. Vestibulocervical and vestibulospinal reflexes utilize vestibular signals to stabilize the posture of the head and the neck. Roberts (1967) studied the interaction of these sensory systems by changing the pitch of the head and the slope of the surface. When he tilted the surface while the head remained parallel to the surface, the uphill condition caused forelimb flexion and the downhill condition caused hindlimb flexion. When he tilted the surface while the head remained parallel to the ground, the uphill and downhill conditions caused the same response (Roberts 1967). In the former example, the vestibular system initiated the modification in posture, while in the later example the neck proprioceptors were responsible for the change. Overall, it appears that sensory feedback contributes to the control of posture. Our locomotion data also suggests that neck proprioceptors and/or the vestibular organs play a key role in the initiation of appropriate muscular activity patterns during walking.
The sources of gait disorders are diverse, however, the initiation of terrain transitions is a common reoccurring impediment for locomotion. There are conflicting results regarding the importance of the proprioceptive and vestibular systems for these transitions during walking. Dietz et al. (1988) induced sudden head tilts during gait and concluded that this perturbation has little influence on muscle activity during gait. In contrast, Sasaki et al. (2001) measured the kinetics of gait initiation in patients with bilateral vestibular loss. They discovered that the distance traveled in the initial steps was shorter and the velocity of the initial steps was slower compared to control patients.
The present study demonstrates that the vestibular apparatus or neck proprioceptors potentially initiate modifications in muscle activity patterns for uphill and downhill walking, and will thereby possibly aid future rehabilitation methods. For instance, if tilting the head up causes a decrease in muscle activity, this would be a beneficial manipulation for Parkinson’s disease patients who have difficult ceasing movement. On the other hand, if tilting the head down causes an increase in muscle activity, this would be a helpful addition to incomplete spinal cord locomotion therapy to initiate movement. In the future, we plan to scrutinize these phenomena by analyzing diverse conditions where both the treadmill slope and the head pitch are manipulated. These combinations will be used to test potential interactions of the feedback from both vestibular and neck afferents. The apparently transient effects of tilting the head on motor patterns could in fact be due to a delayed influence of vestibular input that cancels the afferent feedback from the neck. In the end, we aim to gain a more clear understanding of which sensory systems initiate transitions in terrain and which systems maintain these locomotor patterns.
Acknowledgments
This work was supported by a National Institute of Health Grant NS054542 (Postdoctoral NRSA) and NS020855 (ROI). We thank past and present members of the Emory University Neurophysiology Laboratory for help with data collection.
References
- Bent LR, Inglis JT, McFadyen BJ. When is vestibular information important during walking? J Neurophysiol. 2004;92:1269–1275. doi: 10.1152/jn.01260.2003. [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol. 1998;79:1687–1701. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann GA, Berger W. Fast head tilt has only a minor effect on quick compensatory reactions during the regulation of stance and gait. Exp Brain Res. 1988;73:470–476. doi: 10.1007/BF00406603. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Magnus R, de Kleijn A. Pflugers Arch. 1912. p. 145. [Google Scholar]
- Manzoni D, Andre P, Pompeiano O. Proprioceptive neck influences modify the information about tilt direction coded by the cerebellar anterior vermis. Acta Otolaryngol. 2004;124:475–480. doi: 10.1080/00016480410016595. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol. 2000a;84:2237–2256. doi: 10.1152/jn.2000.84.5.2237. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined plane. J Neurophysiol. 2000b;84:2257–2276. doi: 10.1152/jn.2000.84.5.2257. [DOI] [PubMed] [Google Scholar]
- Roberts T. Neurophysiology of postural mechanisms. Plenum; New York: 1967. [Google Scholar]
- Sasaki O, Asawa S, Katsuno S, Usami S, Taguchi K. Gait initiation in bilateral vestibular loss. Auris Nasus Larynx. 2001;28:295–299. doi: 10.1016/s0385-8146(01)00094-3. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P. Unexpected motor patterns for hindlimb muscles during slope walking in the cat. J Neurophysiol. 1995;74:221–2215. doi: 10.1152/jn.1995.74.5.2211. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol. 1998;79:1702–1716. doi: 10.1152/jn.1998.79.4.1702. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Timerick SJ, Wilson VJ. Body position with respect to the head or body position in space is coded by lumbar interneurons. J Neurophysiol. 1985;54:123–133. doi: 10.1152/jn.1985.54.1.123. [DOI] [PubMed] [Google Scholar]