Abstract
Objective
No studies have addressed the use of electronic personal health records (e-PHRs) for self-management in complex neurological disorders. We assessed and tested an Internet-based self-management system that utilized the e-PHR and determined its impact on self-assessed well-being, clinician-assessed well-being, and healthcare utilization in patients with multiple sclerosis (MS).
Materials and Methods
Subjects were randomized to usual care (a secure Web-based messaging system) or active intervention, which included secure messaging, self-monitoring, self-management of MS symptoms, and communication about upcoming clinic visits. Computers and Internet access were provided. Subjects were included if they had MS, lived within the county or region surrounding our MS center, had at least two appointments at our center in the previous 12 months, and demonstrated basic typing and computer skills. Study duration was 12 months.
Results
Of 220 subjects completing informed consent, 206 met the inclusion criteria. At the study's end, 83 subjects remained in the usual care group and 84 in the enhanced care group. Both groups used the available system components. The groups did not significantly differ on the primary endpoints or healthcare utilization.
Conclusions
Self-management support is an emerging aspect of chronic care management. We established the feasibility of conducting a randomized, controlled trial using e-PHRs for patient self-management. We did not find that e-PHR–enabled self-management augmented multidisciplinary MS center-based care, possibly because the differences between interventions were not great enough.
Key words: clinical trials randomized controlled (CONSORT agreement), multiple sclerosis, outcome research
Introduction
Individuals living with chronic illness are responsible for much of their disease management.1 Although patients have many options for documenting and monitoring their self-management, the use of electronic personal health records (e-PHRs) is growing.2 The benefits of patient self-management using e-PHRs have been well documented in diabetes and cardiovascular disease cases: subjects with both conditions improved on seven health status variables,3 and diabetics had improved hemoglobin A1c and low-density lipoprotein as well as systolic and diastolic blood pressure.4 No comparable published studies address the use of e-PHRs for neurological disorders. Patients with multiple sclerosis (MS) are largely responsible for administering their disease-modifying therapies and managing symptoms. Although feasibility and pilot studies have assessed the benefit of telehealth interventions to guide patients with MS through home rehabilitation programs,5–7 little is known about the effectiveness of patient self-management for MS.
Therefore, we investigated the effectiveness of self-management in MS. We expanded a secure Web-based electronic messaging system and then conducted a practical, randomized clinical trial8 to assess differences in patient outcomes between the original and expanded systems. The expanded system permitted access to all features of the original system and also allowed patients to monitor MS-related symptoms, make decisions about seeking help for symptoms, and notify their clinicians of issues to discuss at upcoming appointments. We hypothesized that patients using the enhanced system would experience better clinician- and self-assessed MS health status, improved sense of self-efficacy for disease self-management, greater satisfaction with care, and lower healthcare utilization.
Methods
Mellen Center Care Online System Development
In 1998, we developed Mellen Center Care Online (MCCO), a secure, Internet-based system of asynchronous electronic messaging between our clinicians and established MS patients at the Cleveland Clinic's Mellen Center for Multiple Sclerosis Treatment and Research. The Mellen Center, a multidisciplinary MS center, provides ongoing care for ∼7,000 MS patients annually. Key MCCO system components were designed to be integrated with the routine processes of neurological care. A pilot study demonstrated that patients used the system to initiate electronic communication with their healthcare providers to manage their disease.9 Patients communicated with staff using structured screens that guided them in providing clinical information, thereby enhancing the staff's ability to provide asynchronous consultation about the patients' changing healthcare concerns.
For the present project, the original MCCO system was expanded and then tested in a randomized trial. The expanded MCCO functionality included a self-monitoring and self-management system that allowed users to longitudinally assess their MS symptoms using the MS Quality of Life Inventory10 and receive graphical feedback about their current, most recent, and baseline scores for each MS Quality of Life Inventory scale. This component also permitted them to evaluate symptom changes and make decisions about responding to changes in their symptoms. Patients were prompted to conduct this self-monitoring in its entirety on a quarterly basis, could select individual scales for self-monitoring on a monthly basis, and could review their scores at any time. For the quarterly self-monitoring, the system sent a prompt to a patient-designated e-mail address and included a link to the secure MCCO site.
Four response options were available after patients conducted any self-monitoring: they could contact the clinical team about symptom changes, seek information about that symptom by linking to Web sites vetted for the study, take both actions, or take no action.
The second added component permitted users to conduct appointment preparation. Patients received an automated e-mail at 2 weeks in advance of an appointment for a routine physician or advanced practice clinician (APC)—advanced practice nurse or physician assistant—visit. If they did not respond to this prompt, a second e-mail was sent 1 week later. After logging onto the MCCO system, patients were prompted to identify questions or issues they wanted to address during the upcoming appointment; these were then sent directly to the clinician. Users who completed the appointment preparation were later contacted electronically to determine the extent to which those issues were addressed during the appointment.
Usability Assessment of the Enhanced System
Feedback from users of the original messaging system was considered during the design phase of the enhanced system. The enhanced system was further evaluated and modified based on feedback from focus groups who tested and reviewed the new system.11
Practical, Randomized Clinical Trial
Standard protocol approval, registration, and patient consent
The Cleveland Clinic IRB approved this trial, and all enrolled participants completed written informed consent. The trial is registered at the National Institutes of Health and can be accessed at http://projectreporter.nih.gov/project_info_details.cfm?aid=7125093&icde=3218116.
We compared the clinical impact of the enhanced system to the original system in a randomized, controlled trial using practical clinical trial methodology.8 Practical clinical trials select clinically relevant alternative interventions to compare, include a diverse population of study participants, recruit participants from heterogeneous practice settings, and collect data on a broad range of health outcomes. We hypothesized that the subjects randomized to having access to all three system components would have better outcomes on the measures described below than the subjects who had access to the original system, which employed only secure electronic messaging. Subjects were enrolled for 12 months. The protocol also allowed for a 6-week window on either side of the scheduled exit date. For example, if a subject had a scheduled clinical appointment at 3 weeks before the scheduled exit and could not return for a separate exit visit, he or she would complete the study at that clinical visit.
Subjects
Patients were included if they had clinically definite MS, resided in the county where the Mellen Center is located or in one of the five surrounding counties, and had completed at least two appointments with a physician or an APC at our center in the 12 months previous to enrollment. The geographic restriction was necessary as we installed computers and established Internet access for participants who did not have them. The prior appointment restriction helped to ensure that patients were included only if they received their primary neurological care at our center. Individuals also had to demonstrate that they could turn a computer on and off, send an e-mail message, and pass a typing test.
The automated recruitment process that generated a study population representative of the potential participants has been described elsewhere.12 Briefly, the MCCO system regularly queried our electronic health record system (EpicCare; Epic Systems Corporation, Verona, WI) during the study period. The medical record numbers of identified patients were placed in a secure electronic “dynamic potential patient pool,” meaning that eligibility could change depending on the number of physician or APC visits a patient completed in the preceding 12 months and whether he or she moved into or out of the designated geographic area. Patients were randomly assigned to the original (MCCO-original) or enhanced system (MCCO-enhanced) after collection of all baseline data. The study coordinator requested a randomization assignment from the automated randomization program after patients were stratified so that the two groups were balanced with respect to computer ability and number of subjects (1:1 allocation). Given the nature of this study, neither subjects nor study personnel were blinded to study assignment; study personnel were not blinded to data collection of outcome variables.
Intervention
Both groups had access to the secure asynchronous electronic messaging component of the system and could generate messages. Because this messaging component was routinely available to and utilized by Mellen Center patients, it was considered standard care. Patients in the MCCO-enhanced group had access to the secure electronic messaging plus the new MCCO components.
Measures
All measures were assessed at baseline and month 12 under the study coordinator's supervision. Consistent with practical trial methodology, a number of primary outcome measures were assessed: the Sickness Impact Profile (SIP),13 the MS Functional Composite,14 and the Control Subscale of the MS Self-Efficacy Scale.15 The control subscale measures confidence in one's ability to manage disease symptoms, reactions to disease-related symptoms, and disease impact on life activities. The Seniors' General Satisfaction and Physician Quality of Care16 was modified so that the questions referred to their MS care at the Mellen Center over the previous 3 months. The Euro-Quality of Life 517 was used to quantify general health-related quality of life. Self-reported healthcare utilization was assessed as a secondary measure.
We also measured potential mediating variables: sex, age, marital status, years of education, ethnicity, work status, having a computer in the home prior to the study, and MS disability as assessed with the Incapacity Status Scale.18 Cognitive status was assessed via computer, using the MicroCog program.19 Process measures of system utilization, including number and type of messages initiated by all subjects, were automatically captured. Scheduled self-monitoring and appointment preparation activities were collected electronically for the MCCO-enhanced group. At the end of the study, satisfaction with the MCCO system was assessed according to group assignment.
Statistical analysis
Only those subjects who completed the study were included in the analysis. Descriptive analyses were conducted to summarize patient baseline characteristics, which were compared between MCCO-original and MCCO-enhanced groups. Chi-square tests were used for categorical variables and t-tests for continuous variables. Analysis of covariance for continuous outcomes and logistic regression analysis for dichotomous outcomes were used to compare between the groups, with adjustment for covariates. A sample size of 112 subjects for each group was estimated. Based on Table 1 of the SIP User's Manual and Interpretation Guide,13 we estimated the mean (±standard deviation) SIP score in our patients to be ∼21.0 ± 12.0. A realistic and clinically meaningful improvement would be a three-point reduction in this score. Under these assumptions and using a one-tailed, 0.05 analysis of covariance t-test, a sample size of 224 gives powers from 0.85 to 0.93. All tests were two-tailed, and all data are reported as least square means with standard error of the mean. A p-value of ≤0.05 was considered significant. All analyses were conducted using SAS version 8.2 (SAS Institute, Cary, NC).
Table 1.
Baseline Characteristics in the Mellen Center Care Online-Original and Mellen Center Care Online-Enhanced Patient Self-Management Groupsa
| Characteristic | MCCO-Original (N = 104) | MCCO-Enhanced (N = 102) | P-Value |
|---|---|---|---|
| Demographic features | |||
| Female (%) | 85% | 72% | 0.02 |
| Age (mean ± SD) | 48.1 (9.7) | 48.1 (9.1) | 0.95 |
| Married (%) | 63% | 61% | 0.80 |
| Years of education (mean ± SD) | 14.6 (2.6) | 14.7 (2.6) | 0.80 |
| White (%) | 75% | 78% | 0.56 |
| % employed | 67% | 55% | 0.07 |
| Computer at home prior to study (%) | 65% | 66% | 0.96 |
| Measures of clinician-assessed functioning | |||
| Incapacity status scale (mean ± SD) | 12.3 (9.2) | 12.7 (8.2) | 0.71 |
| Measures of cognitive function | |||
| MicroCog—mean general functioning19 | 81.3 (22.3) | 82.8 (21.4) | 0.63 |
| MicroCog—mean general proficiency19 | 91.3 (18.1) | 92.0 (14.7) | 0.76 |
| Measures of primary outcomes | |||
| SIP13 | 20.4 (15.3) | 21.7 (11.9) | 0.50 |
| MSFC14 | −0.67 (2.0) | −0.56 (1.7) | 0.68 |
| MSSE15 | 64.5 (17.2) | 59.7 (17.3) | 0.046 |
| SGSPQ-GSMC16 | 23.3 (4.5) | 23.4 (4.1) | 0.82 |
| SGSPQ-PPQ16 | 32.8 (3.1) | 32.9 (3.5) | 0.84 |
| EQ-5D index score | 0.75 (0.18) | 0.75 (0.17) | 0.98 |
| EQ-5D Visual Analog Scale | 73.4 (15.7) | 75.5 (18.7) | 0.38 |
| Measures of healthcare utilization | |||
| Hospitalized within prior 3 months | 6.9% | 4.8% | 0.57 |
| Admitted to ED within prior 3 months | 12.8% | 7.7% | 0.26 |
| Missed paid employment within prior 3 months (only those employed) | 17.1% (n = 70) | 16.1% (n = 56) | 0.87 |
| Ever paid for someone to help with daily activities within prior 3 months | 10.8% | 17.3% | 0.23 |
| Number of medical office visits within prior 3 months | 6.55 (8.08) | 6.17 (8.52) | 0.70 |
| Number of home health visits within prior 3 months | 0.45 (1.99) | 2.21 (14.2) | 0.21 |
| Number of prescriptions within prior 3 months | 6.42 (4.39) | 6.93 (5.39) | 0.45 |
All data shown are least square means and standard error, unless otherwise indicated.
ED, emergency department; EQ-5D, Euro-Quality of Life 5; MCCO, Mellen Center Care Online; MSFC, Multiple Sclerosis Functional Composite; MSSE, Multiple Sclerosis Self-Efficacy Control Scale; SD, standard deviation; SGSPQ-GSMC, Seniors' General Satisfaction and Physician Quality of Care–General Satisfaction with Medical Care; SGSPQ-PPQ, Seniors' General Satisfaction and Physician Quality of Care–Perception of Physician Quality; SIP, Sickness Impact Profile.
Results
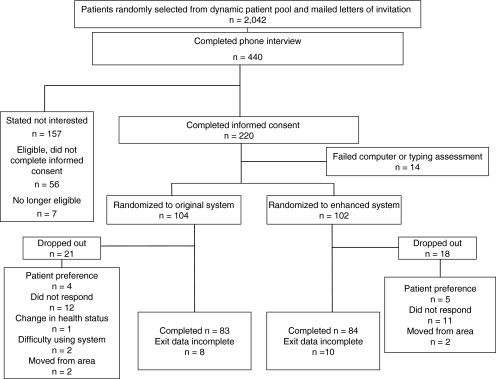
Of 2,042 patients who received letters of invitation, 440 requested additional information and 220 patients completed informed consent, but 14 of the latter did not meet the typing and computer skills criteria. Thus, 206 subjects were enrolled. At the study's end, 83 subjects remained in the MCCO-original group and 84 remained in the MCCO-enhanced group (Fig. 1). Data were incomplete for 18 subjects, but available data were included in the analysis. Patients were recruited from November 2004 through October 2007; follow-up occurred for 12 months after enrollment.
Fig. 1.
Flowchart of subject disposition throughout the study. At the study's end, 83 subjects remained in the Mellen Center Care Online (MCCO)-original group and 84 remained in the MCCO-enhanced group.
Group Characteristics at Baseline
The MCCO-original and MCCO-enhanced groups were similar on most demographic characteristics and outcome measures (Table 1): only the percentage of women differed (85% MCCO-original vs. 72% MCCO-enhanced; p = 0.02). In comparison with the 167 subjects who completed the study, the 39 who did not finish had higher (worse) scores on the SIP (19.9 ± 13.6 completers vs. 25.7 ± 13.2 noncompleters; p = 0.02) (Table 2).
Table 2.
Comparison of Subjects Who Did Complete with Those Who Did Not Complete the Studya
| Demographic Feature or Scale | Completers (N = 167) | Noncompleters (N = 39) | P-Value |
|---|---|---|---|
| Female (%) | 79.64% | 70.73% | 0.22 |
| Age (SD) | 45.86 (7.23) | 48.69 (10.13) | 0.14 |
| % married | 64.67% | 51.22% | 0.11 |
| Years of education (SD) | 14.8 (2.6) | 14.2 (2.6) | 0.19 |
| White (%) | 77.84% | 73.17% | 0.32 |
| Employed (%) | 107 (64%) | 19 (49%) | 0.08 |
| Computer at home prior to study (%) | 114 (68%) | 21 (54%) | 0.09 |
| Incapacity status scale (mean ± SD) | 12.6 (9.0) | 12.3 (7.3) | 0.85 |
| MicroCog—mean general functioning19 | 82.0 (21.9) | 82.1 (21.8) | 0.98 |
| MicroCog—mean general proficiency19 | 92.5 (16.4) | 87.8 (16.2) | 0.12 |
| SIP13 | 19.9 (13.6) | 25.7 (13.2) | 0.02 |
| MSFC14 | −0.60 (1.86) | −0.71 (1.78) | 0.73 |
| MSSE15 | 62.1 (17.4) | 62.2 (17.6) | 0.97 |
| SGSPQ-GSMC16 | 23.5 (4.2) | 22.6 (4.6) | 0.28 |
| SGSPQ-PPQ16 | 33.1 (3.0) | 32.1 (4.1) | 0.18 |
| EQ-5D index score17 | 0.76 (0.17) | 0.73 (0.18) | 0.46 |
| EQ-5D Visual Analog Scale17 | 75.8 (16.1) | 68.8 (20.8) | 0.057 |
| Ever hospitalized | 6.9% | 4.8% | 0.57 |
| Ever admitted to ED | 12.8% | 7.7% | 0.26 |
| Ever missed paid employment in previous 3 months (only those employed) | 17.1% (n = 70) | 16.1% (n = 56) | 0.87 |
| Ever paid for someone to help with daily activities | 10.8% | 17.3% | 0.18 |
| Number of medical office visits | 6.55 (8.08) | 6.17 (8.52) | 0.70 |
| Number of home health visits | 0.45 (1.99) | 2.21 (14.2) | 0.21 |
| Number of prescriptions | 6.42 (4.39) | 6.93 (5.39) | 0.45 |
All data shown are least square means and standard error.
SGSPQ-GSMC, Seniors' General Satisfaction and Physician Quality of Care—General Satisfaction with Medical Care; SGSPQ-PPQ, Seniors' General Satisfaction and Physician Quality of Care—Perception of Physical Quality.
System Utilization
Messaging
The types of messages sent and the number of individuals sending the messages generally were evenly distributed between the two groups, with 96 (92%) of the MCCO-original subjects and 86 (84%) of the MCCO-enhanced group having sent at least one message (Table 3). The type of message sent most often in each group was to report a change in symptoms (135 of all 439 messages sent; 31%). The fewest number of messages sent were those for prescription refills or requests for forms.
Table 3.
Type and Number of Secure Electronic Messages Initiated by MCCO-Original and MCCO-Enhanced Self-Management Groups
| Type of Message | Total Messages, N (Subjects, N) | MCCO-Original Messages N (Subjects, N) | MCCO-Enhanced Messages, N (Subjects, N) |
|---|---|---|---|
| Report change in symptoms | 135 (50) | 57 (26) | 78 (24) |
| Question about medication or treatment | 95 (37) | 42 (17) | 53 (20) |
| Prescription refill request | 61 (30) | 32 (16) | 29 (14) |
| Appointment request | 75 (35) | 41 (17) | 34 (18) |
| Request completion of forms, letters, equipment authorization | 73 (30) | 42 (20) | 31 (10) |
| Total | 439 (182)a | 214 (96) | 225 (86) |
The results represent messages from subjects who did and did not complete the study.
MCCO-enhanced self-monitoring
The MCCO-enhanced group received quarterly automated notifications to complete a scheduled self-monitoring. The number of prompts each subject received varied depending on when they exited the study. A total of 395 unique notifications to complete self-monitoring were sent to the 102 patients in the MCCO-enhanced group. Of these patients, 67 (66%) responded to 165 (42%) prompts. Twelve (12%) patients completed all their scheduled self-monitoring sessions. Forty (39%) patients conducted at least one unscheduled self-monitoring, and they evaluated themselves on at least two scales. The scales most commonly used for unscheduled self-monitoring were the SF-36 Physical and Mental Component Summary Scales (40 individuals conducted 82 self-monitorings) and the Modified Fatigue Impact Scale (39 individuals conducted 82 self-monitorings). The scale least often selected for self-monitoring was the Sexual Satisfaction Scale (34 individuals conducted 70 self-monitorings). In response to conducting or reviewing their self-monitoring results, subjects were more likely to search Web sites (130 links/31 patients) than to contact their clinician (30 messages/13 patients).
MCCO-enhanced appointment preparation
Of the 102 patients, 21 did not have a scheduled physician or APC visit during their 12-month study enrollment. The remaining 81 patients had 381 scheduled appointments, of whom 37 completed 89 appointment preparations.
Practical, Randomized, Controlled Trial Endpoints
At the end of the study, the MCCO-original group had higher general health-related quality of life as measured by the Euro-Quality of Life 5 Visual Analog Scale (p = 0.04). No other between-group differences were found. After adjustment for age, gender, ethnicity, work status, and cognitive ability, the SIP score in the MCCO-original group was 21.7 ± 2.0 and 22.4 ± 1.8 in the MCCO-enhanced group (p = 0.77). Similarly, neither the MS functional composite score (−0.80 ± 0.24 vs. −0.63 ± 0.22, MCCO-original vs. MCCO-enhanced, p = 0.51) nor the MS Self-Efficacy Control Scale (64.5 ± 2.8 vs. 62.5 ± 2.6, MCCO-original vs. MCCO-enhanced, p = 0.50) differed. In both groups, change from baseline scores on these measures was very limited (Table 4).
Table 4.
Comparison of Primary Endpoints and Healthcare Utilization Between Groups in the Practical, Randomized, Controlled Triala
| Measure | MCCO-Original | MCCO-Enhanced | P-Value |
|---|---|---|---|
| SIP13 | 21.7 (2.0) (n = 76) |
22.4 (1.8) (n = 75) |
0.77 |
| MSFC14 | −0.80 (0.24) (n = 81) |
−0.63 (0.22) (n = 84) |
0.51 |
| MSSE15 | 64.5 (2.8) (n = 77) |
62.5 (2.6) (n = 77) |
0.50 |
| SGSM-GSC16 | 23.3 (0.72) (n = 77) |
23.2 (0.67) (n = 77) |
0.96 |
| SGSM-PC16 | 33.2 (0.47) (n = 77) |
33.7 (0.43) (n = 77) |
0.30 |
| EQ-5D index score17 | 0.757 (0.025) (n = 75) |
0.756 (0.023) (n = 75) |
0.96 |
| EQ-5D Visual Analog Scale17 | 76.3 (2.6) (n = 75) |
70.2 (2.4) (n = 74) |
0.04 |
| Ever hospitalized | 1.24% (2.04%) (n = 80) |
2.86% (1.87%) (n = 83) |
0.46 |
| Ever admitted to ED | 3.4% (4.1%) (n = 80) |
12.4% (3.8%) (n = 83) |
0.08 |
| Ever missed paid employment in previous 3 months (only those employed) | 3.7% (7.1%) (n = 55) |
14.1% (6.8%) (n = 48) |
0.17 |
| Days paid for someone to help with daily activities | 0.25 (0.058) (n = 80) |
0.15 (0.052) (n = 83) |
0.10 |
| Number of medical office visits | 7.36 (1.59) (n = 70) |
8.53 (1.31) (n = 77) |
0.46 |
| Number of home health visits | 1.58 (0.42) (n = 70) |
0.79 (0.34) (n = 77) |
0.058 |
| Number of prescriptions | 11.0 (1.10) (n = 80) |
10.5 (1.00) (n = 82) |
0.68 |
Results are adjusted for age, gender, marital status, race, years of education, work status, and MicroCog general proficiency. All data shown are least square means and standard error.
Discussion
We report the first randomized clinical trial of a Web-based patient self-management intervention integrated with an e-PHR for any neurological disease. We demonstrated that such a trial is feasible to construct, enroll patients, and implement. Using a novel randomized, automated recruitment system that included broad inclusion criteria and actively solicited participation, we recruited a sample of patients that is typical of the general MS clinical population and specifically representative of the patients at our MS center. Thus, we consider the study sample and findings to be generalizable to other MS clinic patients.12
The enrolled patients were predominantly female, white, and ∼50 years old, with a mean educational level beyond high school. Measures of physical and cognitive abilities suggest that subjects were minimally to moderately impaired. The two groups were comparable on most pertinent variables.
Although the disease-specific system components and content make it difficult to compare our system utilization to that reported for other Web-based self-management programs, each component was utilized, with subjects (88%) from both groups using the messaging function and 64% (66/102) of the MCCO-enhanced group responding to prompts to complete scheduled self-monitoring. The retention rate (81%) from this 12-month intervention is comparable (79%) to that reported by Lorig et al.3 at 1 year after a 6-week Internet-based chronic disease intervention for patients with a variety of conditions and is greater than that for a large 5-year (n = 985, retention rate: 52%) Internet-based intervention for diabetes management.4 These comparisons must be viewed cautiously, given the nature and duration of the interventions.
However, our hypotheses were not confirmed. We found no significant differences for the primary outcome measures. Several reasons may account for this lack of effect. First, the anticipated three-point change in SIP, which was the basis of our sample size calculation, did not occur. In fact, clinical change was minimal from baseline to study month 12 for the majority of outcome measures. Most MS clinical trials designed to test medications involve a study period of 2 years or more, whereas we looked for differences in endpoints over 12 months. Although we did not determine the number of participants with relapsing-remitting or primary progressive MS, a significant proportion of subjects were more likely in the relapsing-remitting stage of the disease. Given the slowly progressive nature of relapsing-remitting MS, this may account for a minimal change; if patients do not experience a change in symptoms, they may be less likely to use the system. Second, the number of individuals who did not complete the study was larger than estimated in the sample size calculation, thus influencing the power to detect differences. Finally, this intervention was patient-driven and designed to allow the participant to determine the amount of intervention. Although many features of the enhanced system were used, it is possible they were not utilized often enough to make a difference in outcomes. Since this study was initiated, several reports have suggested that there are barriers to patients with MS actively engaging in health promotion20 and self-care decision making.21 Additionally, several recent studies indicate interactive self-management interventions that involve clinical staff as active partners who engage participants in goal-setting, monitoring, and education produce better results22,23; the Agency for Healthcare Research and Quality has suggested such an approach.24 As our system does not involve direct patient–clinician interaction, our findings support these studies. Although the utilization of the enhanced MCCO system suggests that these features may be useful components to include in future systems, perhaps a more active intervention that permits subjects to determine which aspects of self-management they want to address (e.g., fatigue management or increased exercise tolerance) and includes self-management support as described above would be more effective. Finally, the amount of intervention between the two groups may have been insufficient to produce between-group differences; in particular, this is suggested by the findings that only a minority of patients used the appointment preparation function and fewer than expected used the self-monitoring function.
This study has several limitations. This intervention was designed to assist patients with a wide variety of symptoms to improve self-management. More targeted, goal-directed interventions may have greater benefit. The duration of the intervention may have also limited its impact.
In conclusion, we found that individuals in both groups used the assigned components of our self-management system, but that the enhanced system did not lead to the expected improvements in patient- and clinician-reported outcomes. Nonetheless, this study demonstrates the feasibility of conducting practical trials using Internet-based systems that interface with e-PHRs. This trial serves as a possible model for using practical, controlled trials in comparative effectiveness research in neurological diseases.
Acknowledgments
This study was supported by an NIH grant (5RO1LM8-154-4 to D.M.M.).
Disclosure Statement
D.M. Miller, Ph.D., has served on a scientific advisory board for the Research Triangle Institute and, in 2009, received research funding from Teva NeuroScience. S.M. Moore, Ph.D., has several NIH grants related to health behavior change and has a grant from the Robert Wood Johnson Foundation related to quality improvement. R.J. Fox, M.D., has served as a consultant and speaker for Biogen Idec, EMD Serono, and Genentech; receives research grant support from the National Institutes of Health, National Multiple Sclerosis Society, and Biogen Idec; and serves on the Editorial Board of Neurology. A. Atreja, M.D., has received unrestricted funding from AstraZeneca for outcomes research in diabetic patients. A.Z. Fu, Ph.D., has received a research grant from Merck & Co., Inc., in 2009. J.-C. Lee, M.S., W. Saupe, B.S., M. Stadtler, and S. Chakraborty, M.E., have nothing to disclose. C.M. Harris, M.D., serves on the Board of Invacare, a publicly traded, durable medical equipment company, and is currently the Chairman of the HIMSS Board, an association of information technology professionals and vendors. R.A. Rudick, M.D., conducts research on interferon and MS funded by the NIH (NINDS and NCRR) and National MS Society and has accepted consulting fees from Biogen Idec, Wyeth, and Novartis.
References
- 1.Thorne S. Paterson B. Russell C. Schultz A. Complementary/alternative medicine in chronic illness as informed self-care decision making. Int J Nurs Stud. 2002;39:671–683. doi: 10.1016/s0020-7489(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 2.American Health Information Management Association, American Medical Informatics Association. The value of personal health records. A joint position statement for consumers of health care. Stud Health Technol Inform. 2008;137:402–405. [PubMed] [Google Scholar]
- 3.Lorig KR. Ritter PL. Laurent DD. Plant K. Internet-based chronic disease self-management: a randomized trial. Erratum appears in Med Care 2007;45:276. Med Care. 2006;44:964–971. doi: 10.1097/01.mlr.0000233678.80203.c1. [DOI] [PubMed] [Google Scholar]
- 4.Shea S. Weinstock RS. Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 Year results of the IDEATel study. J Am Med Inform Assoc. 2009;16:446–456. doi: 10.1197/jamia.M3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huijgen BCH. Vollenbroek-Hutten MMR. Zampolini M, et al. Feasibility of a home-based telerehabilitation system compared to usual care: Arm/hand function in patients with stroke, traumatic brain injury and multiple sclerosis. J Telemed Telecare. 2008;14:249–256. doi: 10.1258/jtt.2008.080104. [DOI] [PubMed] [Google Scholar]
- 6.Cha E. Castro HK. Provance P. Finkelstein J. Acceptance of home telemanagement is high in patients with multiple sclerosis. AMIA Annu Symp Proc. 2007:893. [PubMed] [Google Scholar]
- 7.Finkelstein J. Lapshin O. Castro H. Cha E. Provance PG. Home-based physical telerehabilitation in patients with multiple sclerosis: A pilot study. J Rehabil Res Dev. 2008;45:1361–1373. [PubMed] [Google Scholar]
- 8.Tunis SR. Stryer DB. Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 9.Miller DM. Rudick RA. Nichols K. Lee J-C. To use or not to use: Exploring factors that predict which patients who have registered for a Web-based communication system with their clinicians actually use it. Mult Scler. 2006;12:436. [Google Scholar]
- 10.National Multiple Sclerosis Society, Centers Consortium of MS Centers. Multiple Sclerosis Quality of Life Inventory: A user's manual. New York: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 11.Atreja A. Mehta N. Miller D, et al. One size does not fit all: Using qualitative methods to inform the development of an Internet portal for multiple sclerosis patients. AMIA Annu Symp Proc. 2005:16–20. [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DM. Fox RA. Atreja A. Moore SM. Lee J-C. Fu AZ. Jain A. Saupe W. Chakraborty S. Stadtle M. Rudick RA. Using an automated recruitment system to generate an unbiased study sample of multiple sclerosis patients. Telemed J E Health. 2010;16:63–68. doi: 10.1089/tmj.2009.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergner M. Bobbitt RA. Carter WB. Gilson BS. The Sickness Impact Profile: Development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Rudick RA. Cutter G. Reingold S. The multiple sclerosis functional composite: A new clinical outcome measure for multiple sclerosis trials. Mult Scler. 2002;8:359–365. doi: 10.1191/1352458502ms845oa. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz CE. Coulthard-Morris L. Zeng Q. Retzlaff P. Measuring self-efficacy in people with multiple sclerosis: A validation study. Arch Phys Med Rehabil. 1996;77:394–398. doi: 10.1016/s0003-9993(96)90091-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y. Kasper JD. Assessment of medical care by elderly people: General satisfaction and physician quality. Health Serv Res. 1998;32:741–758. [PMC free article] [PubMed] [Google Scholar]
- 17.Rabin R. de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 18.International Federation of Multiple Sclerosis Organizations. MRD: Minimal record of disability for multiple sclerosis. New York: National Multiple Sclerosis Society; 1985. [Google Scholar]
- 19.Powell DH. Kaplan EF. Whitla D. Weintraub S. Catlin R. Funkenstein HH. MicroCog assessment of cognitive functioning manual. San Antonio: The Psychological Corporation, Harcourt Brace; 1993. [Google Scholar]
- 20.Becker H. Stuifbergen A. What makes it so hard? Barriers to health promotion experienced by people with multiple sclerosis and polio. Fam Community Health. 2004;27:75–85. doi: 10.1097/00003727-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Paterson B. Thorne S. Russell C. Disease-specific influences on meaning and significance in self-care decision-making in chronic illness. Can J Nurs Res. 2002;34:61–74. [PubMed] [Google Scholar]
- 22.Garcia-Lizana F. Sarria-Santamera A. New technologies for chronic disease management and control: A systematic review. J Telemed Telecare. 2007;13:62–68. doi: 10.1258/135763307780096140. [DOI] [PubMed] [Google Scholar]
- 23.Dellifraine JL. Dansky KH. Home-based telehealth: A review and meta-analysis. J Telemed Telecare. 2008;14:62–66. doi: 10.1258/jtt.2007.070709. [DOI] [PubMed] [Google Scholar]
- 24.RAND Health MLP. Patient self-management support programs: An evaluation. www.ahrq.gov/qual/ptmgmt/ptmgmt.pdf. [Aug 10;2010 ]. www.ahrq.gov/qual/ptmgmt/ptmgmt.pdf