Abstract
Objective
Alcohol use is prevalent among HIV-infected people and is associated with lower antiretroviral adherence and high-risk sexual and injection behaviors. We sought to determine factors associated with alcohol use among HIV-infected women engaged in clinical care and if baseline alcohol use was associated with time to combination antiretroviral therapy (cART) and death in this population.
Methods
In an observational clinical cohort, alcohol consumption at the initial medical visit was examined and categorized as heavy, occasional, past, or no use. We used multinomial logistic regression to test preselected covariates and their association with baseline alcohol consumption. We then examined the association between alcohol use and time to cART and time to death using Kaplan-Meier statistics and Cox proportional hazards regression.
Results
Between 1997 and 2006, 1030 HIV-infected women enrolled in the cohort. Assessment of alcohol use revealed occasional and hazardous consumption in 29% and 17% of the cohort, respectively; 13% were past drinkers. In multivariate regression, heavy drinkers were more likely to be infected with hepatitis C than nondrinkers (relative risk ratios [RRR] 2.06, 95% confidence interval [CI] 1.29-3.44) and endorse current drug (RRR 3.51, 95% CI 2.09-5.91) and tobacco use (RRR 3.85 95% CI 1.81-8.19). Multivariable Cox regression adjusting for all clinical covariates demonstrated an increased mortality risk (hazard ratio [HR] 1.40, 95% CI 1.00-1.97, p < 0.05) among heavy drinkers compared to nondrinkers but no delays in cART initiation (1.04 95% CI 0.81-1.34)
Conclusions
Among this cohort of HIV-infected women, heavy alcohol consumption was independently associated with earlier death. Baseline factors associated with heavy alcohol use included tobacco use, hepatitis C, and illicit drug use. Alcohol is a modifiable risk factor for adverse HIV-related outcomes. Providers should consistently screen for alcohol consumption and refer HIV-infected women with heavy alcohol use for treatment.
Introduction
In the United States, women are at risk for HIV infection and account for more than one quarter of all new HIV/AIDS diagnoses.1 High-risk heterosexual contact is the source of 80% of newly diagnosed HIV/AIDS infections among women.2 Women of color are especially affected by the HIV epidemic. Although African American women comprise only 12% of the female population in the United States, they account for 66% of new HIV infections.3
In the United States, approximately 50% of adults are current, regular drinkers.4 Men are more likely than women to be current drinkers. Among women, white women are more likely to be current drinkers compared to African American women (48% of non-Hispanic white women vs. 27% of non-Hispanic black women).4 Alcohol use is also prevalent among HIV-infected individuals5,6 and is associated with HIV acquisition and transmission through high-risk sexual and injection-related behaviors. Hutton et al.7 found that women binge drinkers engaged in anal sex at three times the rate of women who abstained from alcohol and were more than twice as likely to have multiple sexual partners. Among HIV-infected women in particular, alcohol consumption has also been found to be strongly associated with unsafe sexual practices, including inconsistent condom use and multiple male sexual partners.8 Furthermore, alcohol consumption may cause more rapid disease progression and HIV-related complications.9–12 Studies have demonstrated that HIV-infected individuals with heavy alcohol use are less likely to adhere to combination antiretroviral therapy (cART) and achieve viral suppression.9,13–16 In addition, one study found that heavy alcohol consumption had a negative impact on the CD4 cell count of HIV-infected people not on combined antiretroviral therapy.5
At present, little is known about factors associated with varying levels of alcohol consumption among HIV-infected women. Identifying and treating alcohol use disorders among HIV-infected women may improve their treatment outcomes. Thus, we sought to determine demographic and clinical factors associated with different levels of alcohol use in a sample of HIV-infected women entering clinical care and to evaluate the association between baseline alcohol consumption and time to cART and death among HIV-infected women.
Materials and Methods
Study design and population
We performed a retrospective cohort study of HIV-infected women enrolled in the Johns Hopkins HIV Clinical Cohort (JHHCC), a clinic-based observational longitudinal cohort of patients who receive primary HIV care in the Johns Hopkins HIV Clinic. Details of the cohort design have been described previously.17 Data collection is comprehensive and includes extensive demographic, socioeconomic, laboratory, therapeutic, and diagnostic information that is collected longitudinally starting with the first visit to the Johns Hopkins HIV Clinic. Maintenance of the database and use of its contents for analysis of patient outcomes are approved by the Institutional Review Board of the Johns Hopkins University School of Medicine, and participants provided written informed consent. Women were included in this study if they enrolled in the clinic between January 1997 and December 2006 and they were not currently on ART at the time of enrollment.
Definitions
Alcohol, illicit drug, and cigarette use
At a participant's first clinic visit, the medical provider completed a standard history and physical form. Trained coders then abstracted baseline alcohol and illicit drug (heroin/cocaine) use, defining current use as any use within the 6 months before enrollment and past use as use >6 months before enrollment but none in the past 6 months. Current alcohol use was categorized as occasional or heavy. Occasional drinking included occasional or light use; heavy drinking included binge drinking or daily use. Because abstraction relied on provider documentation, strict quantity/frequency definitions were not used for the drinking categories. If the provider documented social use, occasional or light use, this would fall into the occasional category, as would lower quantities of alcohol use, such as one or fewer drinks per day. Heavy drinking included binge drinking, heavy alcohol use as well as quantities of ≥4 drinks per occasion, or daily drinking of >1 drink per day (National Institute on Alcohol Abuse and Alcoholism [NIAAA]).18 Illicit drug use included use of heroin or cocaine within 6 months of enrollment in the cohort. Participants who did not use alcohol or drugs were defined as none. Cigarette use was abstracted from the baseline visit and coded as either smoking or nonsmoking.
Combination antiretroviral therapy
cART was defined as the use of an antiretroviral regimen containing a protease inhibitor, a nonnucleoside reverse transcriptase inhibitor, three nucleoside reverse transcriptase inhibitors, or a fusion inhibitor. We defined baseline cART as documented cART within the first 90 days of clinic enrollment. Any ART before enrollment in the clinic was ascertained from the baseline history and physical form and clinical records, where this information is recorded.
Death
Information on death was obtained from a death registry maintained by the clinic that receives reports from families, funeral homes, other medical institutions, and local coroners. In addition, death records of the Maryland Bureau of Vital Records and the Social Security Death Index were regularly searched.
HIV risk factor
For our analyses, HIV transmission risk factors included injection drug use (IDU) and heterosexual transmission (defined as either heterosexual activity with a partner at high risk for HIV or sex with an HIV-infected individual). Risk factor information was provided by patient self-report and documented by the clinician. Risk factor assignment was not mutually exclusive.
Hepatitis C status
Hepatitis C status was defined as the presence of antibody to the hepatitis C virus. This information was electronically captured through laboratory data, and individuals were categorized as hepatitis C antibody positive, negative, or unknown.
Depression
Depression was determined by manual abstraction of psychiatric and medical records by trained coders. The Johns Hopkins HIV Clinic has on-site psychiatric services. If a diagnosis of depression was recorded in the clinical record within 6 months of clinic enrollment, the participant was designated as having depression.
Statistical analysis
All statistical analysis was performed using Stata version 9.1 for windows (StataCorp, College Station, TX). We compared participant characteristics by drinking categories using Kruskal Wallace test for continuous variables and Pearson's chi-square test for categorical variables. We used multinomial logistic regression to examine bivariate and multivariable associations between preselected covariates (age, race, cART, illicit drug use, enrollment HIV RNA, CD4 lymphocyte count, and HIV transmission risk) and drinking category. We report relative risk ratios (RRR) for increased alcohol use with 95% confidence intervals (CI). We then examined the association between alcohol use and time to cART and time to death using Kaplan-Meier statistics and Cox proportional hazards regression. Our time origin for analyses examining time to cART was the date of clinic enrollment and for time to death was 90 days after date of enrollment to account for the 90-day window for which we defined baseline cART. We used the log rank test to compare survival curves. In multivariable analysis, we adjusted for baseline characteristics, including age, race, HIV risk factor, CD4 cell count, active drug use, and hepatitis C status. We tested the interactive effect of drug and alcohol use on our outcomes of interest and the interaction between alcohol use and hepatitis C status on our outcomes.
A subsample (n = 114) of individuals within our cohort participated in an audio computer-assisted self-interview (ACASI) within 6 months of their enrollment into the cohort. For this ACASI, individuals provide information on the number of drinks per week they consumed within the past 6 months. With this information, we used a multiple imputation for measurement-error correction to provide a sensitivity analysis for the association between alcohol use and our outcomes of time to cART and time to death.19
Results
A total of 1030 HIV-infected women who enrolled in care between January 1997 and December 2006 were assessed. The median follow-up time was 1571 days (interquartile ratio [IQR] 821–2466), and 3% of women per year were lost to follow-up. Baseline characteristics for the cohort, stratified by level of alcohol consumption, are listed in Table 1. The cohort was predominantly African American (83.5%) and had heterosexual contact as the principal mode of HIV transmission (76%) and a mean age of 38.4 years. Assessment of alcohol use revealed heavy consumption in 17% and occasional consumption in 29%; 13% were past drinkers. Active illicit drug use (heroin or cocaine) was present in 42.3% of the cohort (70% of heavy drinkers). Approximately 45% of the cohort was hepatitis C antibody positive.
Table 1.
Baseline Demographic and Clinical Characteristics Among HIV-Infected Women, Stratified by Levels of Alcohol Consumption
| Variable n (%) | Total sample n = 1030 | No alcohol n = 422 (41%) | Occasional consumption n = 296 (28.7%) | Heavy consumption n = 177 (17.2%) | Past consumption n = 135 (13.1%) |
|---|---|---|---|---|---|
| Mean age (SD) | 38.4 (9.3) | 38.1 (10.0) | 37.8 (8.5) | 38.4 (9.0) | 40.3 (8.7) |
| Race | |||||
| African American | 860 (83.5) | 363 (86.0) | 234 (79.1) | 154 (87.0) | 109 (80.7) |
| Other | 170 (16.5) | 59 (14.0) | 62 (20.9) | 23 (13.0) | 26 (19.3) |
| HIV risk factor | |||||
| IVDU | 391 (38.0) | 124 (29.4) | 86 (29.1) | 108 (61.0) | 73 (54.1) |
| HET | 787 (76.4) | 323 (76.5) | 244 (82.4) | 130 (73.5) | 90 (66.7) |
| Heroin or cocaine use*** | |||||
| Never | 457 (44.4) | 253 (60) | 141 (47.6) | 32 (18.1) | 31 (23) |
| Active | 436 (42.3) | 133 (31.5) | 116 (39.2) | 123 (69.5) | 64 (47.4) |
| Past | 137 (13.3) | 36 (8.5) | 39 (13.2) | 22 (12.4) | 40 (29.6) |
| Tobacco use*** | 760 (74.4) | 256 (33.7) | 220 (28.9) | 167 (22.0) | 117 (15.4) |
| Depression*** | 329 (31.9) | 112 (34.0) | 88 (26.8) | 70 (21.3) | 59 (17.9) |
| Hepatitis C status*** | |||||
| Positive | 461 (44.8) | 153 (36.3) | 112 (37.8) | 120 (67.8) | 76 (34.1) |
| Negative | 459 (44.6) | 223 (52.8) | 145 (49.0) | 45 (25.4) | 46 (56.3) |
| Unknown | 110 (10.7) | 46 (10.9) | 39 (13.2) | 12 (6.8) | 13 (9.6) |
| cART** | |||||
| Yes | 243 (23.6) | 107 (25.4) | 85 (28.7) | 25 (14.1) | 26 (19.3) |
| No | 787 (76.4) | 315 (74.6) | 211 (71.3) | 152 (85.9) | 109 (80.7) |
| Prior h/o cART | 155 (15.1) | 74 (47.7) | 43 (27.7) | 17 (11.0) | 21 13.6 |
| CD4 count | |||||
| ≤200 | 368 (35.7) | 162 (38.4) | 91 (30.7) | 63 (35.6) | 52 (38.5) |
| >200 | 662 (64.3) | 260 (61.6) | 205 (69.3) | 114 (64.4) | 83 (61.5) |
| HIV RNA* | |||||
| <10,000 | 396 (38.5) | 177 (41.9) | 116 (39.2) | 52 (29.4) | 51 (37.8) |
| 10,000–100,000 | 362 (35.2) | 126 (29.9) | 115 (38.9) | 75 (42.4) | 46 (34.1) |
| >100,000 | 272 (26.4) | 119 (28.2) | 65 (22.0) | 50 (28.2) | 38 (28.1) |
p < 0.05; **p < 0.01; ***p < 0.001.
cART, combination antiretroviral therapy; Prior h/o cART, prior history of cART prior to enrollment in clinic, HET, heterosexual; IVDU, intravenous drug use; SD, standard deviation.
Factors associated with level of alcohol consumption
The results of bivariate and multivariable analyses are presented in Table 2. In multivariable regression, heavy drinkers were 3 times more likely to report active illicit drug use (RRR 3.51, p < 0.001) and twice as likely to endorse past heroin or cocaine use (RRR 2.71, p < 0. 01) as nondrinkers. Heavy drinkers were more likely to have hepatitis C. Women with past alcohol consumption were older and significantly more likely to be diagnosed with depression and report illicit drug use compared to nondrinkers. Occasional and heavy alcohol consumption were both associated with cigarette smoking.
Table 2.
Multinomial Regression of Factors Associated with Occasional and Heavy and Past Alcohol Consumption Compared to None Among HIV-Infected Women
| |
Occasional consumption |
Heavy consumption |
Past consumption |
|||
|---|---|---|---|---|---|---|
| Variable | Bivariate | Multivariate | Bivariate | Multivariate | Bivariate | Multivariate |
| Age | 1.00 (0.98-1.01)a | 1.00 (0.98-1.02) | 1.00 (0.99-1.02) | 1.00 (0.97-1.02) | 1.02 (1.00-1.05) | 1.03* (1.00-1.05) |
| Black race | 0.61 (0.41-0.91) | 0.65* (0.43-0.97) | 1.09 (0.65-1.83) | 1.20 (0.69-2.09) | 0.68 (0.41-1.13) | 0.68 (0.39-1.16) |
| Heroin or cocaine | ||||||
| Never | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| Active | 1.56 (1.13-2.16) | 1.42 (0.95-2.13) | 7.31 (4.70-11.38) | 3.51*** (2.09-5.91) | 3.93 (2.44-6.33) | 2.87*** (1.61-5.11) |
| Past | 1.94 (1.18-3.20) | 1.79* (1.04-3.08) | 4.83 (2.53-9.21) | 2.71** (1.34-5.46) | 9.07 (5.05-16.27) | 7.14*** (3.71-13.77) |
| Tobacco use | 1.88 (1.36-2.60) | 1.75** (1.17-2.61) | 10.83 (5.56-21.10) | 3.85*** (1.81-8.19) | 4.21 (2.47-7.18) | 1.89 (0.98-3.62) |
| Depression | 1.17 (0.84-1.63) | 0.96 (0.68-1.37) | 1.81 (1.25-2.62) | 1.22 (0.81-1.82) | 2.15 (1.44-3.21) | 1.60* (1.04-2.48) |
| HET | 1.44 (0.99-2.09) | 1.46 (0.98-2.18) | 0.85 (0.57-1.27) | 1.35 (0.86-2.12) | 0.61 (0.40-0.94) | 0.83 (0.51-1.33) |
| Prior cART | 0.80 (0.53-1.20) | 0.90 (0.59-1.38) | 0.50 (0.29-0.87) | 0.60 (0.33-1.08) | 0.87 (0.51-1.47) | 0.92 (.53-1.61) |
| CD4 | ||||||
| ≤200 | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| >200 | 1.40 (1.02-1.92) | 1.27 (0.889-1.82) | 1.13 (0.78-1.62) | 1.04 (0.67-1.60) | 0.99 (0.67-1.48) | 0.98 (0.61-1.57) |
| HIV RNA | ||||||
| >100,000 | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| 10,000–100,000 | 1.67 (1.13-2.48) | 1.39 (0.91-2.14) | 1.42 (0.92-2.19) | 1.29 (0.78-2.11) | 1.14 (0.69-1.88) | 0.97 (0.56-1.68) |
| <10,000 | 1.20 (0.82-1.76) | 1.00 (0.65-1.55) | 0.70 (0.44-1.10) | 0.72 (0.42-1.23) | 0.90 (0.56-1.46) | 0.74 (0.42-1.31) |
| Hepatitis C | ||||||
| Negative | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| Positive | 1.13 (0.81-1.55) | 0.77 (0.52-1.14) | 3.89 (2.61-5.80) | 1.65* (1.03-2.65) | 2.41 (1.58-3.66) | 0.89 (0.53-1.48) |
| Unknown | 1.30 (0.81-2.10) | 1.33 (0.81-2.17) | 1.29 (0.63-2.63) | 1.59 (0.75-3.40) | 1.37 (0.69-2.74) | 1.38 (0.66-2.88) |
Alcohol: never is the base outcome.
Relative risk ratio (RRR) and 95% confidence interval (95% CI).
p < 0.05; **p < 0.01; ***p < 0.001.
Factors associated with time to cART and time to death
In our cohort, there were 630 women (61%) initiated on cART during the time of observation. Cox proportional hazards model for factors associated with time to cART and survival are presented in Table 3. Current illicit drug use was associated with delayed cART initiation (hazard ratio [HR] 0.73 p < 0.01) after adjusting for age, race, CD4, HIV RNA, and hepatitis C status. In bivariate analysis, heavy alcohol use was associated with delayed cART; however, this association did not persist after multivariable analysis.
Table 3.
Multivariate Cox Proportional Hazard Analyses for Predictors of Time to Initiation of cART and Mortality
| |
Time to cART |
Time to Death |
||
|---|---|---|---|---|
| Variable | Bivariate | Multivariate | Bivariate | Multivariate |
| Age | 1.01 (1.00-1.02)a | 1.01* (1.00-1.02) | 1.03 (1.02-1.04) | 1.03*** (1.01-1.04) |
| Black race | 0.97 (0.78-1.19) | 0.86 (0.70-1.07) | 1.49 (1.01-2.20) | 1.22 (0.82-1.81) |
| Heroin or cocaine use | ||||
| Never | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| Active | 0.63 (0.53-0.75) | 0.73** (0.59-0.91) | 1.94 (1.47-2.55) | 1.62** (1.13-2.31) |
| Past | 0.83 (0.66-1.06) | 0.91 (0.69-1.20) | 1.85 (1.26-2.71) | 1.69* (1.09-2.64) |
| Tobacco use | 0.67 (0.56-0.80) | 0.75** (0.60-0.93) | 1.86 (1.32-2.62) | 1.22 (0.81-1.85) |
| Depression | 0.93 (0.79-1.10) | 1.10 (0.92-1.32) | 1.10 (0.85-1.43) | 1.07 (0.81-1.40) |
| HET | 0.96 (0.80-1.16) | 0.91 (0.75-1.11) | 0.64 (0.49-0.83) | 0.74* (0.55-0.98) |
| cART | 0.58 (0.41-0.80) | 0.45*** (0.32-0.64) | ||
| Prior cART | 2.22 (1.84-2.68) | 2.04*** (1.69-2.48) | 0.88 (0.63-1.23) | 1.02 (0.73-1.43) |
| CD4 | ||||
| ≤200 | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| >200 | 0.39 (0.33-0.46) | 0.45*** (0.37-0.54) | 0.49 (0.38-0.63) | 0.54*** (0.41-0.72) |
| HIV RNA | ||||
| >100,000 | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| 10,000–100,000 | 0.57 (0.47-0.69) | 0.73** (0.59-0.90) | 0.51 (0.38-0.68) | 0.59*** (0.43-0.80) |
| <10,000 | 0.48 (0.39-0.59) | 0.64*** (0.51-0.79) | 0.38 (0.28-0.53) | 0.47*** (0.34-0.67) |
| ETOH use | ||||
| Never | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| Occasional | 1.03 (0.85-1.24) | 1.18 (0.97-1.43) | 0.71 (0.50-1.00) | 0.71 (0.51-1.02) |
| Heavy | 0.79 (0.63-1.00) | 1.04 (0.81-1.34) | 1.84 (1.34-2.51) | 1.40* (1.00-1.97) |
| Past | 0.98 (0.76-1.26) | 1.09 (0.83-1.42) | 1.71 (1.18-2.46) | 1.21 (0.81-1.79) |
| Hepatitis C | ||||
| Negative | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) | 1.0 (Ref ) |
| Positive | 0.76 (0.64-0.89) | 0.91 (0.74-1.12) | 1.48 (1.14-1.91) | 0.86 (0.62-1.18) |
| Unknown | 0.97 (0.75-1.27) | 1.03 (0.78-1.35) | 1.00 (0.60-1.66) | 0.98 (0.58-1.66) |
All variables included in the final model except baseline cART in Time to cART analysis.
Relative hazard (95% CI).
p < 0.05; **p < 0.01; ***p < 0.001.
ETOH, ethyl alcohol.
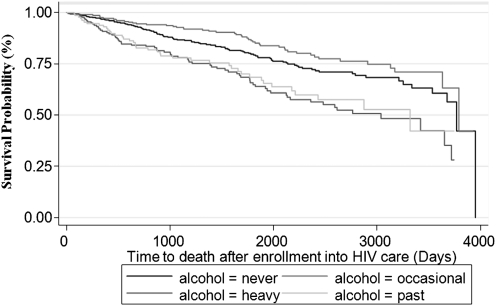
Overall, there were 254 deaths in our sample. The median time to death was 1065 days (IQR 482–1849 days). Factors associated with more rapid time to death in bivariate analysis include older age, black race, active and past heroin or cocaine use, heavy or past alcohol use, and being hepatitis C positive. Multivariable Cox regression adjusting for all other covariates demonstrated an increased mortality risk (HR 1.40, 95% CI 1.00-1.97 p < 0.05) among heavy drinkers compared to nondrinkers. Kaplan-Meier statistics assessing time to death by alcohol consumption level are shown in Figure 1. The survival curves demonstrate improved survival among social drinkers and nondrinkers compared to HIV-infected women who are past or heavy drinkers. There was no interactive effect between alcohol and drug use or alcohol use and hepatitis C status on outcomes of time to cART initiation or time to death.
FIG. 1.
Survival analysis stratified by level of alcohol consumption using the Kaplan-Meier method (p < 0.001 by log-rank test).
When our sample was limited to the 114 women who completed an ACASI within 6 months of clinic enrollment, point estimates of the association between occasional alcohol use and death (adjusted hazard ratio [AHR] 95% CI 0.63, 0.09-4.18) and heavy alcohol use and death (AHR 1.70, 95% CI 0.25-11.47) were similar in magnitude and direction to the overall cohort. Because the validation sample was small, the standard errors after applying multiple imputation for measurement error were greatly inflated, resulting in wide confidence intervals.
Discussion
Among this cohort of HIV-infected women, heavy alcohol consumption was independently associated with earlier death. Active illicit drug use was also associated with increased risk of mortality. In contrast to active drug use, however, heavy alcohol consumption was not associated with delayed time to cART initiation. Baseline factors associated with heavy alcohol use included tobacco use, hepatitis C, and illicit drug use. These findings underscore the importance of identifying alcohol use among HIV-infected women entering care and counseling on the hazards associated with alcohol consumption in general and when coinfected with hepatitis C.
Our finding of earlier death among heavy drinking HIV-infected women is consistent with the literature about women without HIV. Several large prospective cohort studies have evaluated the association between alcohol use and mortality among women. One study, examining data from the Nurses' Health Study, found a U-shaped relationship; light-to-moderate alcohol consumption (1.5–29.9 g of alcohol per day) was associated with decreased mortality, whereas heavy drinking (≥30 g of alcohol per day) was associated with increased mortality.20 An earlier study found that among heavy drinkers, women were at much greater relative risk than men for noncardiovascular death.21 Braithwaite et al.22 estimated the impact of alcohol use on survival among a cohort of HIV-infected individuals, the majority of whom were male. They examined 2702 HIV-positive people and found that hazardous alcohol consumption decreased overall survival by >3 years if frequency of alcohol use was once per week or greater and by 6.4 years with daily alcohol use. Decreased survival was also found among nonhazardous drinkers. Our finding that heavy alcohol consumption was associated with decreased survival among HIV-infected women, independent of such clinical factors as hepatitis C, cART, or CD4 count at baseline, suggests that the association between alcohol and mortality may be extended to HIV-infected women. Of interest, before adjustment for potential confounders, occasional alcohol use was associated with delayed death. Although this would be consistent with data from the Nurses' Health Study, this is an area that requires further investigation.
We found no differences in time to cART by level of alcohol consumption, which lends support to recent findings in the Swiss HIV Cohort Study, which examined treatment-naïve individuals with CD4 count <200 cells/μL across alcohol consumption levels and found no association between delayed cART initiation and alcohol consumption.23 However, our finding that active drug use was associated with delayed cART initiation in our cohort supports well-established evidence that physicians may be highly reluctant to prescribe cART to HIV-infected IDUs.24
Although we found no delay in cART initiation among heavy drinkers, alcohol use was associated with earlier death. One possible explanation is that although women with heavy alcohol consumption are receiving cART, their increased mortality may be secondary to lower adherence and, consequently, poorer response to cART. Hazardous alcohol consumption is a well-established risk factor for cART nonadherence.25–29 Given that our study population was exclusively HIV-infected women, it is important to note that gender-specific differences in alcohol use and antiretroviral adherence may exist. Evaluation of two longitudinal prospective cohort studies found a dose-response relationship between alcohol use and decreasing adherence among women only.30 Even small reductions in antiretroviral adherence may compromise its effectiveness and have important survival implications.31 It is also possible that increased mortality among women with heavy alcohol use may be irrespective of cART, which would be consistent with the literature from the Nurses' Health Study and the study by Klatsky et al.21 which have described a general association between heavy alcohol use and mortality. In addition, among HIV-infected individuals, alcohol abuse is one of the most important risk factors for bacterial community-acquired pneumonia.32 One study conducted among veterans with HIV infection found a strong linear association between bacterial pneumonia prevalence and level of alcohol use.33 The association between alcohol use and death among HIV-infected women is likely multifactorial.
We did not find a baseline diagnosis of depression to be associated with delayed CART or earlier death. This is in contrast to other cohorts of HIV-infected women, where depression and depressive symptoms have been associated with decreased medication adherence,34 cART discontinuation,35 virological failure,36 and death.37,38 In our cohort, if depression was recorded in the clinical record within 6 months of clinic enrollment, the participant was designated as having depression. This may have missed women who had undiagnosed or undocumented depression, which may explain why our findings differ from those of other cohort studies conducted among HIV-infected women that demonstrated that depression was associated with death.39 In addition, there is literature about HIV-infected individuals suggesting that those with mental health disorders engaged in mental healthcare have similar or even better outcomes than those without mental health disorders.40
In this study, factors associated with heavy alcohol consumption included hepatitis C and illicit drug use. Alcohol and illicit drug use frequently co-occur,41 and illicit drug users constitute the majority of new hepatitis C infections.42 Excess alcohol consumption is known to hasten the progression of liver disease related to hepatitis C and can reduce the effectiveness of treatment for hepatitis C infection.42,43 Women coinfected with HIV and hepatitis C must be screened for both alcohol and drug use and counseled on the hazards associated with alcohol use and progression of hepatitis C disease.
There are several limitations to our study. First, this is an observational cohort study; thus, there may have been residual confounding. In addition, our alcohol variable categorization was based on provider interview, resulting in a crude categorization of alcohol use. Although trained abstractors retrieved the data, provider documentation may have been variable, patients may have underreported alcohol use, and providers may not have adequately assessed alcohol use, resulting in some misclassification of alcohol use. Should the level of alcohol use have been underreported, however, it is likely that our estimates are conservative and actual associations are stronger. Notably, our sensitivity analysis revealed associations that were similar in direction and of slightly greater magnitude, although the confidence intervals were wide because of the small validation sample. Although the sensitivity analysis using ACASI data is reassuring, it is not in itself a gold standard. Thus, additional studies using validated alcohol assessments, such as the Alcohol Use Disorders Identification Test or the Time Line Follow Back, are needed to confirm our findings.44–47 In addition, both illicit drug use and depression were obtained from provider documentation, and, thus, it is possible that these variables may not have been adequately assessed or documented. Further, variables, including housing stability and baseline medication adherence, were not collected in our cohort and were unable to be assessed. Finally, our cohort was urban and predominantly African American, which may limit generalizability.
Despite these limitations, this study evaluating the association between baseline alcohol consumption and time to cART and death among HIV-infected women has important strengths. Our study is one of few to directly assess the impact of alcohol consumption on mortality among HIV-infected women. In addition our cohort consists of a large sample of women engaged in care, the majority of whom are urban African American women acquiring HIV via heterosexual contact, thus largely mirroring the epidemic of HIV among women in the United States.
In summary, alcohol use is common among HIV-infected women, and our results suggest that heavy alcohol consumption is associated with decreased survival. Future longitudinal studies should continue to examine alcohol use and HIV survival, as well as specific causes of death. In addition, given that alcohol is a modifiable risk factor for adverse HIV-related outcomes, providers should consistently screen for alcohol consumption and refer HIV-infected women with heavy alcohol use for treatment.
Acknowledgments
This work was supported by National Institute on Alcoholism and Alcohol Abuse (NIAAA) R01 AA016893-02 and NIAAA RO1-AA014500. R.D.M. was supported by the National Institute on Drug Abuse K24DA000432-10. B.L. was supported by K01-AI071754, and G.C. was supported by NIAAA K23 AA015313.
Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. HIV/AIDS among women fact sheet (revised 2008) www.cdc.gov/hiv/topics/women/resources/factsheets/pdf/women.pdf. [Dec 9;2009 ]. www.cdc.gov/hiv/topics/women/resources/factsheets/pdf/women.pdf
- 2.Centers for Disease Control and Prevention. HIV/AIDS surveillance report, 2005, Vol. 17 (revised 2007) Atlanta. www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/pdf/2005SurveillanceReport.pdf. [Dec 9;2009 ]. www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/pdf/2005SurveillanceReport.pdf
- 3.Centers for Disease Control and Prevention. HIV/AIDS and African American women consultation: Meeting report, 2007. www.cdc.gov/hiv/topics/aa/resources/reports/women_consult/pdf/womens_consult.pdf. [Dec 9;2009 ]. www.cdc.gov/hiv/topics/aa/resources/reports/women_consult/pdf/womens_consult.pdf
- 4.Centers for Disease Control and Prevention. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Atlanta. www.cdc.gov/nchs/data/series/sr_10/sr10_242.pdf. [Jun 27;2010 ]. www.cdc.gov/nchs/data/series/sr_10/sr10_242.pdf
- 5.Samet JH. Cheng DM. Libman H. Nunes DP. Alperen JK. Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chander G. Josephs J. Fleishman JA, et al. Alcohol use among HIV-infected persons in care: Results of a multisite survey. HIV Med. 2008;9:196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton H. McCaul ME. Santora PB. Erbelding EJ. The relationship between recent alcohol use and sexual behaviors: Gender differences among sexually transmitted disease clinic patients. Alcohol Clin Exp Res. 2008;32:2008–2015. doi: 10.1111/j.1530-0277.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theall KP. Clark RA. Powell A. Smith H. Kissinger P. Alcohol consumption, ART usage and high-risk sex among women infected with HIV. AIDS Behav. 2007;11:205–215. doi: 10.1007/s10461-006-9159-6. [DOI] [PubMed] [Google Scholar]
- 9.Strauss S. Tiburcio N. Munoz-Plaza C, et al. HIV care providers' implementation of routine alcohol reduction support for their patients. Aids Patient Care STDS. 2009;23:211–218. doi: 10.1089/apc.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conigliaro J. Madenwald T. Bryant K, et al. The Veterans Aging Cohort Study: Observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28:313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- 11.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system: A review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 12.Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Res Health. 2001;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- 13.Braithwaite RS. McGinnis KA. Conigliaro J, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 14.Heckman BD. Catz SL. Heckman TG. Miller JG. Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 15.Samet JH. Horton NJ. Meli S. Freedberg KA. Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 16.Chander G. Lau B. Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: The Johns Hopkins HIV Clinical Practice Cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 18.Helping patients who drink too much. Clinician's guide. 2005 DHHS. NIH. NIAAA. pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. [Feb 16;2010 ]. pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf
- 19.Cole SR. Chu H. Greenland S. Multiple imputation for measurement-error correction. Int J Epidemiol. 2006;35:1074–1081. doi: 10.1093/ije/dyl097. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs C. Stampfer M. Colditz G, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 21.Klatsky A. Armstrong M. Friedman G. Alcohol and mortality. Ann Intern Med. 1992;117:646–654. doi: 10.7326/0003-4819-117-8-646. [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite R. Conigliaro J. Roberts M, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conen A. Fehr J. Glass TR, et al. Self-reported alcohol consumption and its association with adherence and outcome of antiretroviral therapy, in the Swiss HIV Cohort Study. Antivir Then. 2009;14:349–357. [PubMed] [Google Scholar]
- 24.Spire B. Lucas G. Carrieri M. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–270. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox R. Alcohol and HIV: A serious cocktail for transmission and medication adherence. HIV Clinician. 2009;21:1–4. [PubMed] [Google Scholar]
- 26.Parsons J. Rosof E. Mustanski B. The temporal relationship between alcohol consumption and HIV medication adherence: A multilevel model of direct and moderating effects. Health Psychol. 2008;27:628–637. doi: 10.1037/a0012664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braithwaite RS. Conigliaro J. McGinnis KA. Maisto SA. Bryant K. Justice AC. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcohol Clin Exp Res. 2008;32:1645–1651. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg KM. Demas PA. Howard AA. Schoenbaum EE. Gourevitch MN. Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004;19:1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Applebaum AJ. Richardson MA. Brady SM. Brief DJ. Keane TM. Gender and other psychosocial factors as predictors of adherence to highly active antiretroviral therapy (HAART) in adults with comorbid HIV/AIDS, psychiatric and substance-related disorder. AIDS Behav. 2009;13:60–65. doi: 10.1007/s10461-008-9441-x. [DOI] [PubMed] [Google Scholar]
- 30.Lazo M. Gange SJ. Wilson TE, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: Longitudinal study of men and women. Clin Infect Dis. 2007;45:1377–1385. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 31.Karon JM. Fleming PL. Steketee RW. De Cock KM. HIV in the United States at the turn of the century: An epidemic in transition. Am J Public Health. 2001;91:1060–1068. doi: 10.2105/ajph.91.7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madeddu G. Porqueddu E. Cambosu F, et al. Bacterial community acquired pneumonia in HIV-infected inpatients in the highly active antiretroviral therapy era. Infection. 2008;36:231–236. doi: 10.1007/s15010-007-7162-0. [DOI] [PubMed] [Google Scholar]
- 33.Justice C. Lasky E. McGinnis K, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care. 2006;44(Suppl 2):552–560. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 34.Spire B. Duran S. Souville M. Leport C. Raffi F. Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: From a predictive to a dynamic approach. Soc Sci Med. 2002;54:1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 35.Li X. Margolick JB. Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 36.Parienti JJ. Massari V. Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. Epub April 14, 2004. [DOI] [PubMed] [Google Scholar]
- 37.French A. Gawel S. Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen M. French A. Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ickovics JR. Hamburger ME. Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 40.Himelhoch S. Moore RD. Treisman G. Gebo KA. Does the presence of a current psychiatric disorder in AIDS patients affect the initiation of antiretroviral treatment and duration of therapy? J Acquir Immune Defic Syndr. 2004;37:1457–1463. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 41.Chander G. Josephs J. Fleishman JA, et al. Alcohol use among HIV-infected persons in care: Results of a multi-site survey. HIV Med. 2008;9:196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell J. Hagan H. Latka M, et al. High prevalence of alcohol use among hepatitis C virus antibody-positive injection drug users in three U.S. cities. Drug Alcohol Depend. 2006;81:259–265. doi: 10.1016/j.drugalcdep.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters M. Terrault N. Alcohol use and hepatitis C. Hepatology. 2002;36(Suppl 1):S220–225. doi: 10.1053/jhep.2002.36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohn MJ. Babor TF. Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 45.Bradley KA. Bush KR. Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 46.Sobell LC. Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, editor; Litten RZ, editor. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 47.Sobell LC. Maisto SA. Sobell MB. Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]