Abstract
Background
Given previous reports of ethnic differences in breast cancer survival among Hawaii's population, we investigated the role of adherence to treatment standards, treatment toxicity, preexisting chronic conditions, and obesity in the survival of 382 prospectively studied breast cancer patients representing six ethnic groups.
Methods
Participants were recruited from several hospitals in Honolulu. Information on tumor characteristics and treatment was abstracted from medical records. Based on the Physicians Data Query (PDQ®), we assessed compliance with recommended treatment guidelines. Vital status and cause of death data were obtained through linkage with the Hawaii Tumor Registry. Cox proportional hazard models were used to compute hazard ratios for predictors of survival.
Results
After a median follow-up time of 13.2 ± 3.7 years, 115 deaths had occurred, 43 from breast cancer and 72 from other causes. After adjustment, we observed only small differences in survival by ethnicity that were not statistically significant. In addition to advanced disease stage, obesity at diagnosis was a significant independent predictor of worse and receiving PDQ-recommended treatment of better breast cancer-specific and all-cause survival. Developing high-grade toxicity was associated with worse breast cancer survival, whereas comorbidity and older age at diagnosis were associated with higher all-cause mortality. Hormone receptor status, menopausal status, and type of health insurance were not associated with survival.
Conclusions
These findings suggest that given access to healthcare, breast cancer patients experience similar survival rates. Although more information about mechanisms of action would be useful, it appears reasonable to recommend weight control to breast cancer survivors.
Introduction
In earlier years, population-based studies with limited information on treatment and medical conditions reported that breast cancer survival differed among ethnic groups in Hawaii.1,2 In particular, the poorer breast cancer survival among Native Hawaiian and Filipino women compared with Caucasians and Japanese was only partially explained by stage at diagnosis.3–6 Differences in treatment, preexisting chronic diseases, and tolerance to chemotherapy were proposed as possible factors for the remaining differences. Compliance with consensus recommendations for breast cancer treatment has been associated with improved survival,7 and comorbidity has been associated with lower survival rates.8–10 For example, during 10 years of follow-up, 59% of breast cancer patients with severe comorbidity died of causes other than cancer compared with only 8% of women without comorbidity.10 Survival may be influenced by variable chemotherapy response due to ethnic-related genetic polymorphisms of chemotherapy metabolizing enzymes,11 but this type of information is mainly available from clinical trials rather than population-based studies.12 When we combined insurance claims data with tumor registry information, comorbidity and treatment patterns were significant predictors of breast cancer survival among women in Hawaii, whereas ethnic differences were minimal and not significant anymore.5 Our study, Patterns of Care and Outcomes for Patients with Breast Cancer (POCO), explored ethnic variations in breast cancer treatment using detailed information from medical charts, but no significant differences in adherence to Physicians Data Query (PDQ®)-recommended treatments were observed across ethnic groups.13 The current analysis reports on survival for the 382 POCO participants after >10 years of follow-up. Our goal was to investigate the impact of treatment given, comorbidity, tolerance to chemotherapy, and possible prognostic factors, such as obesity, on breast cancer and overall survival.
Materials and Methods
Study design
As described elsewhere, women diagnosed with breast cancer during March 1995–October 1996 on the Island of Oahu were identified through the Hawaii Tumor Registry (HTR) rapid reporting system.13 Of 843 eligible women, 406 (48.2%) participants were recruited; 147 women were members of Kaiser Permanente Hawaii, a health maintenance organization (HMO), and 259 women were recruited through several large community hospitals and fee-for-service (FFS) practice. The current analysis included only the 382 incident primary histologically confirmed cases. Data on ethnicity, age at diagnosis, menopausal status, height, weight, stage, hormone receptor status, carcinoma histological type, comorbidity, treatment received, adverse effects of treatment, and other characteristics were collected from medical charts and pathology reports. In 2009, we linked the POCO database with HTR, a statewide surveillance, Epidemiology, and End-Result (SEER) registry,14 to obtain vital status, date of last active follow-up, and primary cause and date of death for deceased subjects. The study was approved by the Committee on Human Studies of the University of Hawaii and by the Institutional Review Boards (IRBs) of the participating hospitals. All women recruited through the community hospitals signed an informed consent form. For the HMO participants, the two responsible IRBs approved the protocol as a medical records review study that involved no personal contact with the study subjects.
Statistical analysis
Using the SAS software package, release 9.2 (SAS Institute, Cary, NC), univariate and multivariate Cox proportional hazard models examined the relation between the variables of interest and survival time and provided predictions to plot 10-year survival curves.15 In models of breast cancer and all-cause mortality, survival time was calculated from the date of diagnosis until death or, if censored, the last active date of a follow-up. When modeling breast cancer mortality, participants who either died of causes other than breast cancer or who were alive at the last active follow-up were censored.
All participants were categorized into six ethnic groups based on self-declared ethnic background. Women with any Native Hawaiian ancestry were considered Native Hawaiian, whereas women were classified as Caucasian, Japanese, Filipino, and Chinese if they reported only one ethnic background. Women of mixed ethnicity, other than Hawaiian, were included in the other ethnicity category. Japanese were used as the reference category in the survival analysis because they were the largest ethnic group. Body mass index (BMI) was calculated as the ratio of weight in kilograms divided by the square of the height in meters and categorized as underweight (<18.5), normal (18.5–<25), overweight (25–<30), and obese (≥30). Tumor, node, metastasis (TNM) stages were assigned according to the 1995–1996 version the American Joint Committee on Cancer Staging Manual and collapsed into three categories.16 Hormone receptor status was classified by a three-level variable: (1) estrogen receptor+/progesterone receptor + (ER+/PR+), (2) ER−/PR+ or ER+/PR−, (3) ER−/PR−. Comorbidity recorded from medical charts included cardiovascular disease (CVD), pulmonary disease, liver disease, neuromuscular/skeletal disorders, and kidney disease and was classified into one dichotomous variable.5 A three-level variable was created to describe PDQ adherence based on medical charts: (1) adhered to PDQ® recommendations, (2) received extra treatment in addition to what was recommended, e.g., chemotherapy for tumors of stage TIA–TIB, (3) minimum required treatment was not received,13,17 but we did not have information on compliance or reasons why less or additional treatment was administered. We evaluated the effect of high-grade (levels 3 and 4) toxicity based on the National Cancer Institute (NCI) common toxicity criteria using a dichotomous variable. Type of medical insurance was classified as HMO membership vs. FFS setting.
Results
The mean age at diagnosis for the 382 patients was 59.3 ± 12.9 years (Table 1). Two thirds of women were diagnosed at stage 0 or I. In 2009, 115 (30.1%) women had died and 267 (69.9%) women were reported alive (Table 1). The median follow-up time for all subjects was 13.2 ± 3.7 years. Among the deceased, 43 died of breast cancer, 21 of other malignancies, and 51 of causes other than cancer. Overall survival was high: 340 (89%) patients were alive 5 years after diagnosis, and 291 (78%) were known to be alive after 10 years (Fig. 1).
Table 1.
Characteristics of 382 Study Participants by Ethnicity
| Characteristic | Caucasian | Chinese | Filipino | Hawaiian | Japanese | Other | p |
|---|---|---|---|---|---|---|---|
| Number | 93 | 45 | 27 | 49 | 137 | 31 | NA |
| Vital status | |||||||
| Alive | 68.8 | 62.2 | 70.4 | 65.3 | 75.2 | 67.7 | |
| Breast cancer death | 6.5 | 20.0 | 18.5 | 16.3 | 8.0 | 12.9 | |
| Death from other cause | 24.7 | 17.8 | 11.1 | 18.4 | 16.8 | 19.4 | 0.27 |
| Follow-up (years) | |||||||
| 0–<10 | 22.6 | 35.6 | 25.9 | 28.6 | 19.7 | 19.4 | |
| 10–<13 | 19.4 | 20.0 | 11.1 | 26.5 | 17.5 | 29.0 | |
| 13–<14 | 47.3 | 37.8 | 55.6 | 26.5 | 49.6 | 38.7 | |
| 14–17 | 10.8 | 6.7 | 7.4 | 18.4 | 13.1 | 12.9 | 0.27 |
| Age at diagnosis (years) | |||||||
| 25–<50 | 22.6 | 26.7 | 37.0 | 36.7 | 20.4 | 45.2 | |
| 50–<60 | 29.0 | 26.7 | 33.3 | 36.7 | 18.2 | 16.1 | |
| 60–<70 | 20.4 | 13.3 | 18.5 | 22.4 | 35.0 | 19.4 | |
| 70–95 | 28.0 | 33.3 | 11.1 | 4.1 | 26.3 | 19.4 | 0.0001 |
| Menopausal status | |||||||
| Premenopausal | 26.9 | 26.7 | 40.7 | 42.9 | 26.3 | 41.9 | |
| Postmenopausal | 73.1 | 73.3 | 59.3 | 57.1 | 73.7 | 58.1 | 0.13 |
| Body mass index (kg/m2) | |||||||
| <18.5 | 19.4 | 20.0 | 11.1 | 8.2 | 19.0 | 2 6.5 | |
| 18.5–<25 | 37.6 | 42.2 | 59.3 | 18.4 | 38.7 | 41.9 | |
| 25–<30 | 25.8 | 24.4 | 18.5 | 26.5 | 32.1 | 22.6 | |
| ≥30 | 17.2 | 13.3 | 11.1 | 46.9 | 10.2 | 29.0 | 0.0002 |
| Tumor stage | |||||||
| 0–I | 68.8 | 57.8 | 66.7 | 46.9 | 73.7 | 58.1 | |
| IIA | 19.4 | 15.6 | 14.8 | 26.5 | 15.3 | 25.8 | |
| IIB–IV | 11.8 | 26.7 | 18.5 | 26.5 | 10.9 | 16.1 | 0.06 |
| Hormonal status | |||||||
| ER+/PR+ | 66.7 | 57.8 | 55.6 | 67.3 | 67.2 | 64.5 | |
| ER+/PR−&ER−/PR+ | 19.4 | 15.6 | 14.8 | 10.2 | 12.4 | 16.1 | |
| ER−/PR− | 14.0 | 26.7 | 29.6 | 22.4 | 20.4 | 19.4 | 0.67 |
| PDQa | |||||||
| No | 39.8 | 24.4 | 25.9 | 30.6 | 30.7 | 19.4 | |
| Yes | 34.4 | 46.7 | 48.1 | 34.7 | 40.9 | 51.6 | |
| Yes+ | 25.8 | 28.9 | 25.9 | 34.7 | 28.5 | 29.0 | 0.58 |
| High toxicity | |||||||
| No | 86.0 | 86.7 | 88.9 | 85.7 | 85.4 | 90.3 | |
| Yes | 14.0 | 13.3 | 11.1 | 14.3 | 14.6 | 9.7 | 0.98 |
| Comorbidity | |||||||
| No | 73.1 | 80.0 | 70.4 | 61.2 | 65.7 | 83.9 | |
| Yes | 26.9 | 20.0 | 29.6 | 38.8 | 34.3 | 16.1 | 0.12 |
| Health insurance | |||||||
| Fee for service | 57.0 | 62.2 | 55.6 | 38.8 | 79.6 | 45.2 | |
| HMO | 43.0 | 37.8 | 44.4 | 61.2 | 20.4 | 54.8 | <0.0001 |
All numbers in table except first line are percentages.
Adherence to treatment guidelines according to Physicians Data Query (PDQ); Yes+ indicates additional treatments.
HMO, health maintenance organization.
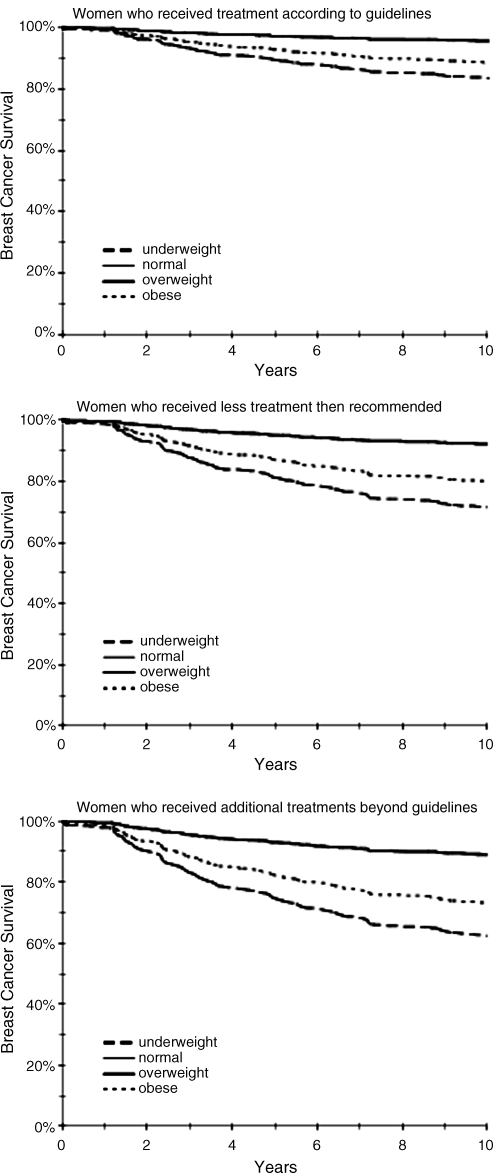
FIG. 1.
Survival curves by adherence to treatment guidelines and weight status (based on Cox regression models).
More than half of the study subjects (n = 209) reported Asian ancestry; Japanese constituted the largest ethnic group, with 137 women. Statistically significant ethnic differences were observed for age at diagnosis, BMI, and type of health insurance. The difference in tumor stage was borderline significant, with relatively more early-stage disease among Japanese and more late-stage disease in Native Hawaiian and Chinese. Close to half of all women were overweight or obese; the proportion was highest among Native Hawaiians and lowest among women with Asian ancestry. There was no ethnic difference in hormone receptor status, PDQ treatment adherence, comorbidities, and toxicity. Tumor characteristics, such as stage and hormone receptor status, did not differ by type of medical insurance.
Close to 30% of women had at least one concomitant chronic condition. When adjusted for age, obese women were more likely to report a comorbid condition, with an odds ratio (OR) of 2.3 (95% confidence interval [CI] 1.2-4.3). However, comorbidity was not associated with stage at diagnosis, adherence to PDQ recommendations, or high-grade toxicity. The majority of women (69.1%) received all treatment as recommended by PDQ, although 109 of these women (28.5%) received additional treatment. A younger age, more advanced stage at diagnosis, and positive hormone receptor status were predictors of receiving additional treatment. Older age was the only predictor of receiving less treatment than recommended by PDQ. All 104 women who received chemotherapy developed at least one low-grade toxicity, and close to half of all women experienced at least one grade 3 or 4 toxicity, cytopenia being the most frequent.
In univariate models, women of Chinese ancestry had a higher risk of dying of breast cancer compared with Japanese women, with a hazard ratio (HR) of 2.80 (95% CI 1.16-6.76). In the multivariate model (Table 2), however, ethnicity was no longer a significant predictor of breast cancer survival; the slightly lower risk in Caucasians and the higher risk in Chinese were not significant. As expected, advanced tumor stage predicted significantly higher breast cancer mortality (HR 11.2; 95% CI 4.97-25.3). Receiving recommended treatment lowered the risk of dying from breast cancer by 74%, but receiving additional treatments was only related to a nonsignificant 25% risk reduction. Obesity predicted significantly higher breast cancer mortality (HR 2.99, 95% CI 1.22-7.33). This relation was observed independent of adherence to treatment (Fig. 1). Normal and overweight women experienced similar survival, whereas underweight women had the highest risk of mortality. The association of high toxicity with worse survival in the univariate analysis (HR 3.06, 95% CI 1.59-5.86) was attenuated after adjustment for covariates, primarily by stage at diagnosis (HR 2.13, 95% CI 0.94-4.87). For patients who received chemotherapy, obese but not overweight women were less likely to experience treatment toxicity than normal weight women (OR 0.24, p = 0.03), but the interaction was not significant. Age, menopausal status, comorbidity, hormone receptor status, and type of health insurance were not associated with breast cancer-specific survival.
Table 2.
Predictors of Breast Cancer and All-Cause Mortality in Women with Breast Cancer
| |
Breast cancer mortality |
All-cause mortality |
||||
|---|---|---|---|---|---|---|
| Characteristic | HR | CI | p | HR | CI | p |
| Ethnicity | ||||||
| Japanese | 1.00 | 1.00 | ||||
| Caucasian | 0.39 | 0.13-1.16 | 0.09 | 1.03 | 0.60-1.75 | 0.92 |
| Chinese | 2.28 | 0.86-6.03 | 0.10 | 1.49 | 0.81-2.76 | 0.20 |
| Filipino | 1.68 | 0.50-5.67 | 0.40 | 1.76 | 0.79-3.95 | 0.17 |
| Hawaiian | 0.60 | 0.20-1.86 | 0.38 | 1.29 | 0.66-2.53 | 0.45 |
| Other | 1.57 | 0.45-5.44 | 0.48 | 1.52 | 0.72-3.20 | 0.27 |
| Age at diagnosis (years) | ||||||
| 25–<50 | 1.00 | 1.00 | ||||
| 50–<60 | 2.48 | 0.83-7.42 | 0.10 | 1.07 | 0.47-2.48 | 0.87 |
| 60–<70 | 1.60 | 0.41-6.32 | 0.50 | 1.19 | 0.46-3.07 | 0.72 |
| 70–95 | 1.08 | 0.26-4.51 | 0.92 | 3.19 | 1.28-7.93 | 0.01 |
| Menopausal status | ||||||
| Premenopausal | 1.00 | 1.00 | ||||
| Postmenopausal | 0.80 | 0.28-2.33 | 0.69 | 1.19 | 0.52-2.72 | 0.69 |
| Body mass index (kg/m2) | ||||||
| <18.5 | 2.16 | 0.64-7.35 | 0.22 | 0.99 | 0.41-2.38 | 0.99 |
| 18.5–<25 | 1.00 | 1.00 | ||||
| 25–<30 | 0.92 | 0.38-2.23 | 0.85 | 0.98 | 0.60-1.60 | 0.94 |
| ≥30 | 2.99 | 1.22-7.33 | 0.02 | 2.06 | 1.23-3.44 | 0.006 |
| Tumor stage | ||||||
| 0–I | 1.00 | 1.00 | ||||
| IIA | 3.08 | 1.14-8.33 | 0.03 | 1.84 | 1.05-3.22 | 0.03 |
| IIB–IV | 11.2 | 4.97-25.3 | <0.0001 | 3.72 | 2.27-6.09 | <0.0001 |
| Hormonal status | ||||||
| ER+/PR+ | 1.00 | 1.00 | ||||
| ER+/PR− and ER−/PR+ | 1.08 | 0.35-3.32 | 0.90 | 1.58 | 0.80-3.12 | 0.19 |
| ER−/PR− | 0.88 | 0.39-2.01 | 0.76 | 1.07 | 0.62-1.86 | 0.80 |
| PDQa | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.26 | 0.10-0.65 | 0.004 | 0.50 | 0.32-0.79 | 0.003 |
| Yes+ | 0.75 | 0.32-1.74 | 0.51 | 0.72 | 0.42-1.21 | 0.21 |
| High toxicity | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.13 | 0.94-4.84 | 0.07 | 1.27 | 0.69-2.33 | 0.44 |
| Comorbidity | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.10 | 0.50-2.41 | 0.82 | 2.19 | 1.46-3.28 | 0.0002 |
| Health insurance | ||||||
| Fee for service | 1.00 | 1.00 | ||||
| HMO | 1.66 | 0.85-3.25 | 0.14 | 1.23 | 0.81-1.87 | 0.32 |
Multivariate Cox's model adjusted for all other variables (hazard ratios and 95% confidence intervals).
Adherence to treatment guidelines according to Physicians Data Query; Yes+ indicates additional treatments.
The multivariate model for all-cause mortality also indicated no differences by ethnicity, and obesity, tumor stage, and PDQ adherence showed similar associations as for breast cancer-specific survival. However, all-cause mortality was significantly elevated for women ≥70 years and for those with comorbidity; the respective HRs were 3.19 (95% CI 1.28-7.93) and 2.19 (95% CI 1.46-3.28).
Discussion
In this breast cancer investigation with >13 years of follow-up, 43 deaths from breast cancer and 72 from other causes were analyzed. As expected, tumor stage at diagnosis was the most important predictor for survival. Although the small ethnic differences in survival were not significant and probably because of the limited number of events in each ethnic group, our findings do not exclude ethnic disparities as observed elsewhere, in particular among African American women.18,19 Noteworthy findings were that obesity at diagnosis was a significant independent predictor of worse and receiving recommended treatments of better breast cancer-specific and all-cause survival. High-grade toxicity was associated with worse breast cancer survival, whereas comorbidity and older age at diagnosis were associated with higher all-cause mortality. Hormone receptor status, menopausal status, and type of health insurance were not associated with survival.
Because access to care was uniform in this study and known prognostic factors were included in the analysis, the nonsignificant ethnic differences indicate that differences in genetic susceptibility by ethnic group do not affect response to treatment to a significant degree. From the limited comparative data, there is also little indication that, unlike in African Americans, Her2/neu expression and the triple negative type are more common in Asians than Caucasians.20,21 Although associated with survival in other studies, ER/PR status was not significant in predicting survival, possibly because of the lack of standardized assays and missing values.22,23 In contrast to our study, age was an independent adverse predictor of breast cancer survival in a report that did not adjust for comorbidities.24 The protective effect of recommended treatment agrees with other reports, although guidelines vary by time and location,7,25 but receiving additional treatments over the recommended ones did not improve survival. As we have no information on treatment decisions, we cannot explain why 30% of patients did not receive all recommended treatments. As was shown in our previous report, the differences in adherence to treatment guidelines were mainly explained by age at diagnosis.13 According to our data, comorbidity did not influence adherence to treatment guidelines, but in agreement with previous data, comorbidity was an independent predictor of dying from all causes.8
Accumulating evidence indicates that obesity and lack of physical activity are linked with breast cancer incidence and survival in women who have had breast cancer.26–28 A large nutritional trial observed better survival among women who lost weight, but only with ER−/PR− tumors.22 Our findings support the idea that obesity around the time of diagnosis constitutes a risk factor for breast cancer-specific and overall survival (Fig. 1). Several mechanisms to explain the association between obesity and reduced prognosis have been proposed.29 Obese women may be given lower doses of chemotherapy because the ideal body surface area rather than true body surface area is used to estimate the dose of chemotherapy.30–32 The fact that obese women experienced less toxicity than normal weight women supports that hypothesis, but we did not detect an interaction between obesity and toxicity on breast cancer-specific survival. Other proposed biological mechanisms include higher estrogen levels among obese women that counter the efficacy of tamoxifen and increase cell proliferation, elevated adipokine levels caused by obesity that may accelerate cancer progression, independent effects of insulin, altered immune responses, and oxidative stress.33,34
The main limitations of this study were a small number of deaths by ethnic group, the absence of longitudinal weight information, the lack of tumor markers not evaluated in 1995–1996, (e.g., Her2/neu), and the nonstandardized assessments for ER and PR. Better control for these factors may have diminished the importance of obesity or comorbidity, further reduced the ethnic differences, or explained the differences due to adherence to treatment standards. We also note that with significant differences in stage at diagnosis and a large number of subjects who were diagnosed with early-stage disease, we cannot exclude residual effects of screening even after controlling for stage at diagnosis.35 Given that treatment guidelines change rapidly, the findings of a study conducted at a later time may differ.
Conclusions
The most noteworthy observations in this survival analysis within a relatively small population of breast cancer patients are related to ethnicity, adherence to treatment guidelines, and obesity. The present data suggest that given similar access to healthcare, breast cancer patients of different ethnicity experience similar survival rates. Adherence to recommended treatment standards was effective in improving survival outcomes in women with breast cancer. Possible reasons for deviating from PDQ guidelines, however, such as the physician's judgment about biologically more serious disease or treatment tolerance due to ill health or comorbidities, were not known. Finally, it is encouraging to know that there appears to be a modifiable lifestyle factor that may improve breast cancer survival. Although more information about mechanisms of action would be useful, as well as the amount of weight loss needed to improve outcomes, it appears reasonable to recommend weight control to breast cancer survivors. Although the literature and our findings agree that a healthy weight is important for breast cancer survivors, it is important to note that many times, behavior, such as diet and exercise, cluster together.36 Thus, looking at these behaviors in a larger context is crucial. A critical direction for the future is to understand what leads to the adoption of health behaviors by breast cancer survivors, in particular Native Hawaiian women with high rates of obesity and comorbidity,37 because interventions may lead to a healthier weight.38,39
Acknowledgments
This project was supported by a grant (R01CA64045) from the National Cancer Institute and the Hawaii Tumor Registry and by contract N01PC35137 from the National Cancer Institute. We are grateful to the women and their physicians who participated in this study. We also acknowledge the POCO study staff for collecting data and Hawaii Tumor Registry for providing us with the follow-up information on all study participants.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Meng L. Maskarinec G. Wilkens L. Ethnic differences and factors related to breast cancer survival in Hawaii. Int J Epidemiol. 1997;26:1151–1158. doi: 10.1093/ije/26.6.1151. [DOI] [PubMed] [Google Scholar]
- 2.LeMarchand L. Kolonel LN. Nomura AM. Relationship of ethnicity and other prognostic factors to breast cancer survival patterns in Hawaii. J Natl Cancer Inst. 1984;73:1259–1265. [PubMed] [Google Scholar]
- 3.Hinds MW. Kolonel LN. Nomura AMY. Lee J. Stage-specific breast cancer incidence rates by age among Japanese and Caucasian women in Hawaii. Br J Cancer. 1982:118–123. doi: 10.1038/bjc.1982.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeMarchand L. Kolonel LN. Nomura AM. Breast cancer survival among Hawaii Japanese and Caucasian women. Ten-year rates and survival by place of birth. Am J Epidemiol. 1985;122:571–578. doi: 10.1093/oxfordjournals.aje.a114136. [DOI] [PubMed] [Google Scholar]
- 5.Maskarinec G. Pagano IS. Yamashiro G. Issell BF. Influences of ethnicity, treatment, and comorbidity on breast cancer survival in Hawaii. J Clin Epidemiol. 2003;56:678–685. doi: 10.1016/s0895-4356(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 6.Braun KL. Fong M. Gotay C. Pagano IS. Chong C. Ethnicity and breast cancer in Hawaii: Increased survival but continued disparity. Ethn Dis. 2005;15:453–460. [PubMed] [Google Scholar]
- 7.Hebert-Croteau N. Brisson J. Latreille J. Rivard M. Abdelaziz N. Martin G. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol. 2004;22:3685–3693. doi: 10.1200/JCO.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Satariano WA. Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 9.West DW. Satariano WA. Ragland DR. Hiatt RA. Comorbidity and breast cancer survival: A comparison between black and white women. Ann Epidemiol. 1996;6:413–419. doi: 10.1016/s1047-2797(96)00096-8. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME. Pompei P. Ales KL. MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Neber DW. Roe AL. Ethnic and genetic differences in metabolism genes and risk of toxicity and cancer. Sci Total Environ. 2001;274:93–102. doi: 10.1016/s0048-9697(01)00732-x. [DOI] [PubMed] [Google Scholar]
- 12.Du XL. Osborne C. Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol. 2002;20:4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issell BF. Maskarinec G. Pagano I. Gotay CC. Breast cancer treatment among women of different ethnicity in Hawaii. Cancer Invest. 2005;23:497–504. doi: 10.1080/07357900500201442. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society, Cancer Research Center of Hawaii, Hawaii Department of Health. Hawaii cancer facts & figures 2003–2004. American Cancer Society; 2004. [Google Scholar]
- 15.Allison PD. Survival analysis using the SAS system: A practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 16.American Joint Committee on Cancer. AJCC cancer staging manual. 5th. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 17.National Cancer Institute. PDQ®—NCI's comprehensive cancer database. 2004. www.cancer.gov/cancerinfo/pdq www.cancer.gov/cancerinfo/pdq
- 18.Wojcik BE. Spinks MK. Optenberg SA. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82:1310–1318. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.McKenzie F. Jeffreys M. Do lifestyle or social factors explain ethnic/racial inequalities in breast cancer survival? Epidemiol Rev. 2009;31:52–66. doi: 10.1093/epirev/mxp007. [DOI] [PubMed] [Google Scholar]
- 20.Kwan ML. Kushi LH. Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parise CA. Bauer KR. Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2010;76:44–52. doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Chlebowski RT. Blackburn GL. Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 23.Onitilo AA. Engel JM. Greenlee RT. Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggard MA. O'Connell JB. Lane KE. Liu JH. Etzioni DA. Ko CY. Do young breast cancer patients have worse outcomes? J Surg Res. 2003;113:109–113. doi: 10.1016/s0022-4804(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 25.Yun YH. Park SM. Noh DY, et al. Trends in breast cancer treatment in Korea and impact of compliance with consensus recommendations on survival. Breast Cancer Res Treat. 2007;106:245–253. doi: 10.1007/s10549-006-9490-7. [DOI] [PubMed] [Google Scholar]
- 26.Carmichael AR. Bendall S. Lockerbie L. Prescott RJ. Bates T. Does obesity compromise survival in women with breast cancer? Breast. 2004;13:93–96. doi: 10.1016/j.breast.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 27.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition and the prevention of cancer: A global perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 28.Patterson RE. Cadmus LA. Emond JA. Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: A review of the epidemiologic literature. Maturitas. 2010;66:5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.McTiernan A. Obesity and cancer: The risks, science, and potential management strategies. Oncology (Williston Park) 2005;19:871–881. [PubMed] [Google Scholar]
- 30.Modesitt SC. van Nagell JRJ. The impact of obesity on the incidence and treatment of gynecologic cancers: A review. Obstet Gynecol Surv. 2005;60:683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 31.Gurney H. How to calculate the dose of chemotherapy. Br J Cancer. 2002;86:1297–1302. doi: 10.1038/sj.bjc.6600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griggs JJ. Sorbero ME. Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin PJ. Ennis M. Fantus IG, et al. Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J Clin Oncol. 2005;23:6037–6042. doi: 10.1200/JCO.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 34.Renehan AG. Roberts DL. Dive C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A. Clegg LX. Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 36.Gritz ER. Demark-Wahnefried W. Health behaviors influence cancer survival. J Clin Oncol. 2009;27:1930–1932. doi: 10.1200/JCO.2008.21.3769. [DOI] [PubMed] [Google Scholar]
- 37.Mau MK. Sinclair K. Saito EP. Baumhofer KN. Kaholokula JK. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev. 2009;31:113–129. doi: 10.1093/ajerev/mxp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock CL. Demark-Wahnefried W. Can lifestyle modification increase survival in women diagnosed with breast cancer? J Nutr. 2002;132:3504S–3507S. doi: 10.1093/jn/132.11.3504S. [DOI] [PubMed] [Google Scholar]
- 39.Rock CL. Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]