Abstract
Scott syndrome is a rare hereditary bleeding disorder associated with an inability of stimulated platelets to externalize the negatively charged phospholipid, phosphatidylserine (PS). Canine Scott syndrome (CSS) is the only naturally-occurring animal model of this defect and therefore represents a unique tool to discover a disease gene capable of producing this platelet phenotype. We undertook platelet function studies and linkage analyses in a pedigree of CSS-affected German shepherd dogs. Based on residual serum prothrombin and flow cytometric assays, CSS segregates as an autosomal recessive trait. An initial genome scan, performed by genotyping 48 dogs for 280 microsatellite markers, suggested linkage with markers on chromosome 27. Genotypes ultimately obtained for a total of 56 dogs at 11 markers on chromosome 27 revealed significant LOD scores for 2 markers near the centromere, with multipoint linkage indicating a CSS trait locus spanning approximately 14 cM. These results provide the basis for fine mapping studies to narrow the disease interval and target the evaluation of putative disease genes.
Keywords: Dog genetic disease, Disease model, Blood phospholipids, Platelet activation
1. Introduction
The domestic dog has become a valuable animal model for the study of genetic disease.(Castelhano et al. 2009; Ostrander et al. 2000) The large litter size and well-documented parentage of purebred dogs, combined with recent developments in canine genomic resources provide the tools to perform linkage and association studies in this species. Distinct breed populations facilitate the discovery of disease genes and mutations that cause or contribute to noted “breed predispositions” to various disorders. Moreover, dogs in developed countries receive sophisticated veterinary care that includes diagnostic techniques capable of specifically defining disease phenotypes.
Numerous naturally occurring bleeding disorders have been identified in dogs.(Boudreaux 2008; Brooks 1999) Hemophilia and von Willebrand disease are the most common of these defects in dogs and people. Molecular genetic analyses indicate that these disorders are true homologs, caused by a variety of mutations within the corresponding genes of each species. Although hereditary platelet function defects are relatively uncommon in both species, discovery of their molecular basis has proven valuable in unraveling the complex pathways of normal platelet activation.(Salles et al. 2008)
Platelet procoagulant activity (PCA) refers to the ability of stimulated platelets to promote fibrin clot formation. Although not fully-defined, some mechanisms of PCA include engagement of platelet membrane receptors with coagulation factors and a distinct phospholipid and calcium-dependent property that facilitates assembly of catalytic factor complexes.(Bevers et al. 1991; Heemskerk et al. 2000; Zwaal et al. 1992) In response to certain stimuli, the activated platelet membrane rapidly externalizes a negatively charged phospholipid, phosphatidylserine (PS), and sheds membrane microparticles that express PS. Membrane-bound PS provides a surface for activation of coagulation complexes that generate a localized burst of thrombin sufficient to cleave soluble fibrinogen and produce a nascent fibrin clot. (Dachary-Prigent et al. 1995; Sims et al. 2001; van der Planken et al. 2000)
A variety of physiologic and pharmacologic agents are known to evoke platelet PCA, however the signaling pathways and molecular processes involved in rapid platelet membrane PS externalization remain to be elucidated. The rare hereditary hemostatic defect Scott syndrome (OMIM: 262890) is characterized by an inability of stimulated platelets to externalize PS.(Toti et al. 1996; Weiss 2009; Zwaal et al. 2004) Scott platelets, therefore, represent a model system to explore the biologic basis of the platelet procoagulant response. Unfortunately, the paucity of human cases has hindered genetic analyses of the trait and a disease gene capable of producing the Scott platelet phenotype has yet to be identified. A canine counterpart of Scott syndrome (CSS) has been identified that displays all the characteristic features of the human disease i.e. platelets with normal aggregation and secretion, yet specific impairment of stimulated prothrombinase activity, PS externalization, and microvesiculation.(Brooks et al. 2002) In order to further develop this model and define its hereditary basis, we undertook pedigree studies in a kindred of German shepherd dogs affected with CSS. Functional studies of platelet PCA indicated that the mode of inheritance of CSS is compatible with an autosomal recessive trait. Mapping studies, using microsatellite markers, revealed linkage of the CSS phenotype to a locus on canine chromosome 27 (CFA 27), a region demonstrating shared synteny with the centromeric region of human chromosome 12.
2. Materials and Methods
2.1 Study Subjects
All dogs originated from a single large colony, established from 61 foundation breeders (20 males and 41 females). The youngest study dogs represented generations 7 and 8 from colony inception. The initial diagnosis of CSS in the colony was based on a combination of abnormalities in platelet PCA assays (e.g. high residual serum prothrombin, low prothrombinase activity) and flow cytometric findings of abnormal PS externalization and lack of microvesiculation.(Brooks et al. 2002) Pedigree samples for phenotype and genotype analyses were collected from affected dogs and their relatives over a 3-year time interval (2005–2007).
2.2 Platelet Function-Phenotype Analyses
The residual serum prothrombin time (PT) assay was used as a screening test of platelet PCA, as previously described.(Brooks et al. 2002; Castaman et al. 1997) Assay results were expressed as residual PT%, representing a ratio of plasma and 2-hour serum PT values. In this assay, the CSS phenotype results in residual PT ≥ 90%, indicating minimal activation and depletion of factors in serum due to CSS platelets’ inability to support prothrombinase assembly. The dogs with abnormal screening test results available for referral were also evaluated using flow cytometric assays. Cytometry was performed with washed platelet suspensions as previously described.(Brooks et al. 2002) In brief, platelet suspensions (1.0 X 107 platelets/mL) were reacted with calcium ionophore (A23187, 3 uM final concentration) in a buffer containing 2mM calcium, and then dual-labeled to detect Annexin-V binding (denoting PS externalization) and CD61 (to define platelets and platelet-derived microparticles).
2.3 Microsatellite Genotyping
Genomic DNA was isolated from blood samples using the Puregene DNA Purification Kit (Gentra Systems, Minneapolis, MN), and adjusted to 50ng/uL concentration for use in amplification reactions. Multiplex PCR was performed using fluorescently-labeled primers for markers of the minimal screening set-2 (MSS-2).(Clark et al. 2004; Guyon et al. 2003) Amplification reactions (12.5 to 15 uL total volume) contained 0.08 U Taq polymerase (Biolase, Bioline, Randolph, MA) in the manufacturer’s buffer, adjusted to a final concentration of 2.4 mM MgCl2. Cycling conditions were: 95°C for 5 min; 4 cycles of 95°C for 30 s, 58°C for 20 s, 72°C for 20 s; 29 cycles of 95° C for 20 s, 56°C for 20 s, 72°C for 20s; 72°C for 5 min. Based on preliminary genome scan results, genotypes were subsequently obtained for 3 additional microsatellite markers located on CFA 27 (REN208N23, FH4079, FH4001) and 1 marker on CFA 30 (C02806). Products were resolved at a core facilitya using an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA), with internal size standards (GeneScan 500 LIZ, Applied Biosystems). Allele sizes were determined using Genemapper Software v4.0 (Applied Biosystems).
2.4 Data Analyses
Summary statistics (mean, sd, 95% confidence intervals) for residual PT values for CSS affected and unaffected dogs were compiled using a web-based application. (Kirkman, T.W. 1996. Statistics to Use. http://www.physics.csbsju.edu/stats/). The web-based application SUPERLINK (http://bioinfo.cs.technion.ac.il/superlink-online/) was used to check markers for Mendelian segregation and to calculate 2-point and multipoint LOD scores. For these analyses, all dogs with high (> 90%) residual serum PT were considered affected with CSS. The disease was modeled as an autosomal recessive trait, with a population allele frequency (q) of 0.25.
3. Results
3.1 Pedigree Samples and Platelet Function Studies
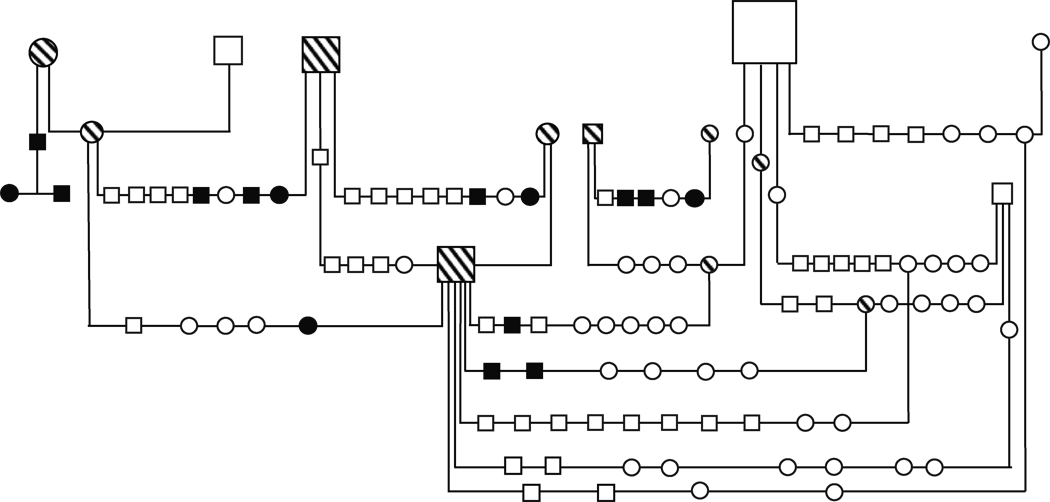
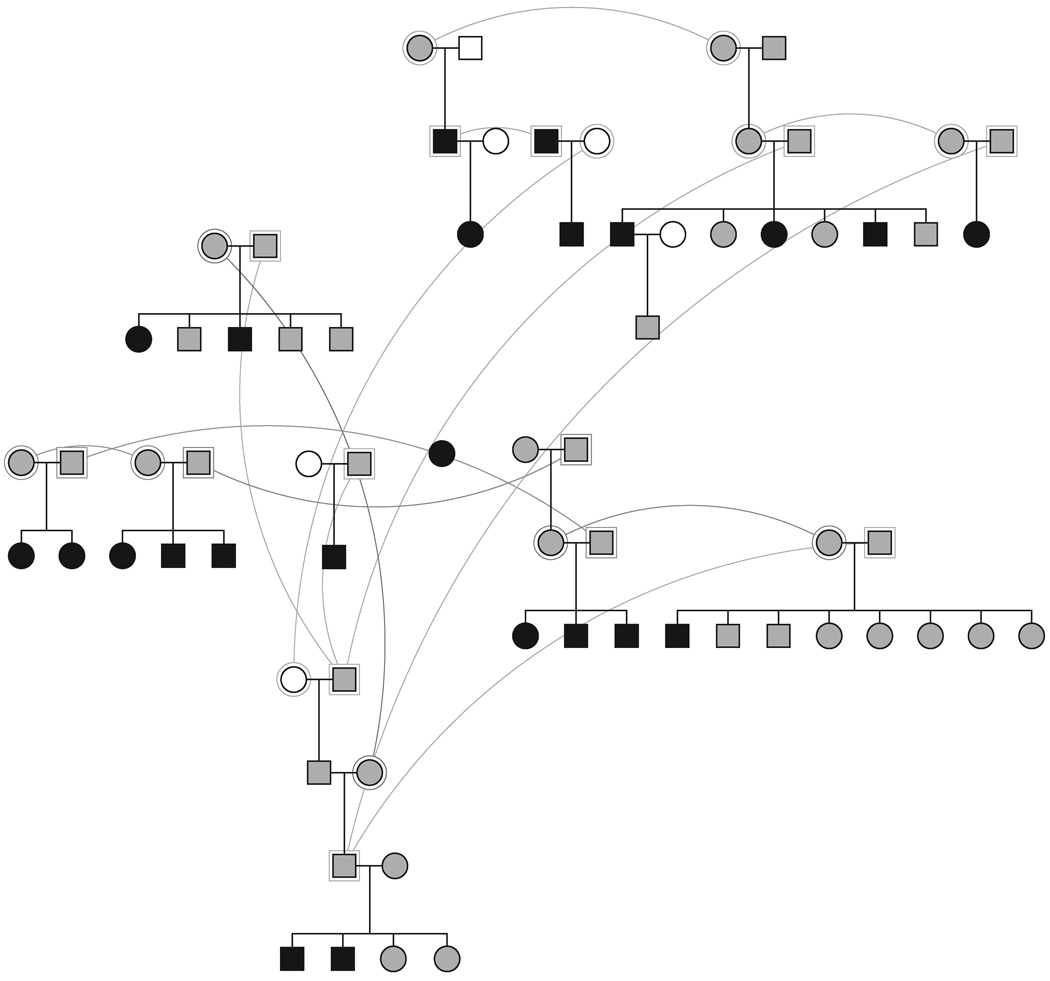
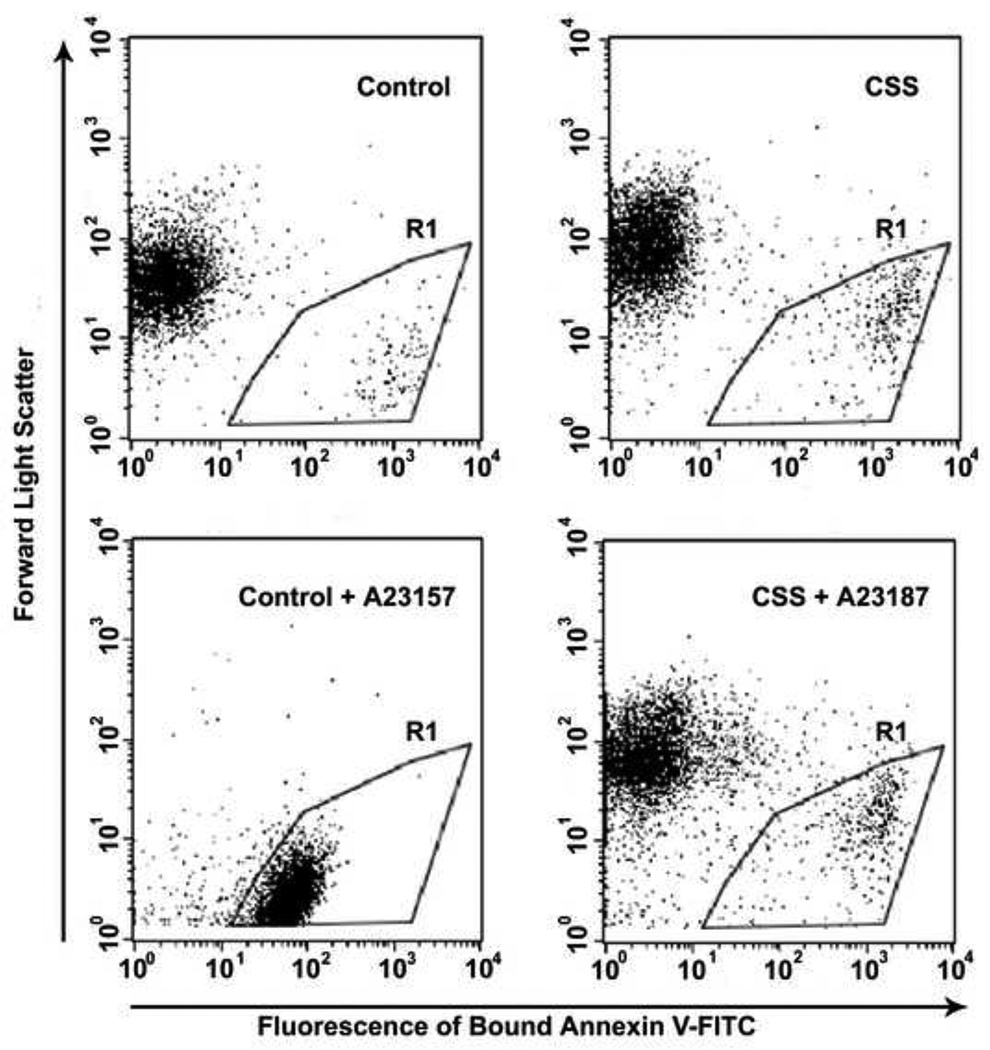
Samples for residual serum PT determinations were analyzed from 112 dogs during the study period. Fifteen dogs had high values (> 90%) indicating a failure of platelet PCA and expression of the CSS trait. Pedigree inspection of the affected dogs demonstrated segregation of CSS through 4 generations (Figure 1). In this pedigree, 6 matings between sires (n=3) and dams (n=5) with normal residual serum PT produced at least 1 CSS affected offspring. Combined, the 6 obligate carrier matings produced 40 total offspring including 12 CSS affected dogs (12/40 = 30% affected). This value is similar to the expected 25% affected offspring produced by mating 2 heterozygous carriers of a single locus, highly penetrant, autosomal recessive trait. Samples for genotyping and linkage analyses were available from 55 of the colony dogs (Figure 2). The linkage pedigree consisted of 28 CSS affected dogs (n=16 male) with group mean residual PT =103% (s.d. = 3.9; ± 95% confidence interval = 1.4) and 27 unaffected dogs (n=13 male) having mean residual PT = 55% (sd = 12.6; ± 95% confidence interval = 4.7). Flow cytometric analyses were also performed on 22 of the CSS affected dogs that could be referred for testing. All had abnormalities pathognomonic for Scott syndrome i.e. impaired PS externalization and diminished microvesiculation in response to calcium ionophore stimulation (Figure 3).
Figure 1. Segregation of Scott Syndrome in a German Shepherd Pedigree.
Residual serum prothrombin time (PT) values of 112 related dogs were compiled as a screening test of the canine Scott syndrome (CSS) phenotype. Solid symbols represent dogs that express the CSS phenotype (i.e. residual PT > 90%). Hatched symbols represent obligate carriers of the CSS trait. All dogs with open and hatched symbols have normal residual PT values.
Figure 2. Pedigree for Linkage Analyses of Canine Scott Syndrome.
Solid symbols represent dogs that express the CSS platelet phenotype, grey-filled symbols represent dogs with normal platelet function, and open symbols represent dogs with no samples for phenotype or genotype analyses. Straight lines connect parents and offspring, curved lines connect individual dogs appearing more than once in the pedigree.
Figure 3. Flow Cytometric Characterization of Canine Scott Syndrome.
Dot plots depict forward scatter (FSC, y axis log scale) and intensity of annexin-VFITC labeling (x-axis log scale) of washed platelet suspensions from a healthy dog (control) and CSS affected dog (CSS). Platelet suspensions were reacted in a buffer containing 2 mM Ca2+ with a vehicle control (top row) or calcium ionophore (A23187; bottom row). Stimulation of control dog platelets induces a new cell population (R1) with low FSC and high intensity annexin-V labeling, denoting microparticle release and PS externalization, respectively. In contrast, ionophore stimulated CSS platelets fail to demonstrate either activation response.
3.2 Genotypes and Linkage Analyses
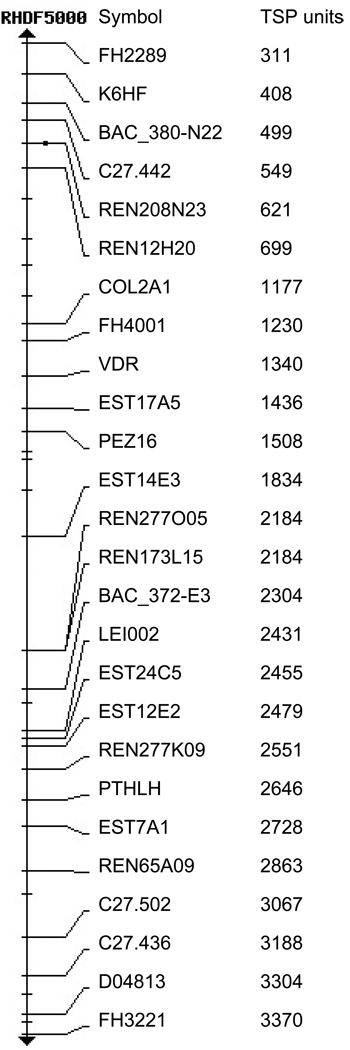
Genotypes were initially obtained for 48 dogs at 280 MSS-2 markers that span the 38 canine autosomes at approximately 10 cM intervals. Preliminary analyses revealed positive 2-point LOD scores (> 1.0) at marker loci on 11 chromosomes, with the highest LOD value of 2.5 on CFA 27. A positive multipoint LOD score, however, was obtained only for marker loci on 2 chromosomes: CFA 27 (maximum multipoint LOD = 1.57) and CFA 30 (maximum multipoint LOD = 1.38). An additional 7 dogs were then genotyped for the MSS-2 markers on these 2 chromosomes and for 1 additional marker on CFA 27 (REN20823) and 1 marker on CFA 30 (C02806). While multipoint LOD scores were no longer positive for CFA 30, the expanded sample and marker set on CFA 27 now yielded a maximal multipoint LOD of 3.8. Genotypes were then obtained for 2 additional markers on CFA 27 (FH4001, FH4079) in the region of the putative trait locus. Ultimately, the pedigree dogs were genotyped for 11 markers on CFA 27; 3 of these markers were found to be monoallelic (FH4001, PEZ16, LEI002). Compilation of 2-point LOD scores and recombination fractions revealed evidence of linkage of CSS to 2 polymorphic markers: REN208N23 (maximal LOD = 5.7; θ = 0.05) and FH4079 (maximal LOD = 5.46; θ = 0.05) (Table 1). These markers are located in a region approximately 8 MB from the centromere on CFA 27, separated from each other by a distance of approximately 1 MB (Figure 4). Multipoint linkage calculated for 10 markers spanning CFA 27 yielded a maximal LOD score of 9.09 at a locus predicted to be approximately 7 MB downstream of FH4079 (Table 2).
Table 1.
Results of 2-Point Linkage to the CSS Trait for 8 Polymorphic Markers on CFA 27
| Marker | Distance a | Recombination Fraction (θ) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TSP | MB | 0.0 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | |
| FH2289 | 311 | 5.03 | −7.1 | −1.5 | 0.939 | 1.646 | 1.637 | 1.091 | 0.442 |
| REN208N23 | 621 | 7.66 | 4.326 | 5.692 | 5.758 | 5.260 | 3.897 | 2.349 | 0.848 |
| FH4079 | 791 | 8.68 | −0.4 | 4.787 | 5.464 | 5.190 | 3.955 | 2.376 | 0.778 |
| FH3221 | 3370 | −10.6 | −3.8 | −0.44 | 0.727 | 1.191 | 0.851 | 0.313 | |
| FH3924 | 4290 | 39.44 | −3.6 | −2.4 | −0.6 | 0.148 | 0.617 | 0.530 | 0.219 |
| PEZ6 | 5050 | 43.47 | −18.5 | −8.5 | −3.4 | −1.2 | 0.221 | 0.483 | 0.294 |
| REN181L14 | 5506 | 45.72 | −21.3 | −9.1 | −5.1 | −3.1 | −1.2 | −0.4 | −0.18 |
| REN72K15 | 6288 | 47.68 | −7.2 | −6.2 | −4.0 | −2.4 | −0.8 | −0.22 | −0.02 |
Marker distances from centromere displayed in radiation hybrid map units (TSP) and Megabase (MB) from NCBI Dog genome build 2.1, LOD values < 3 are displayed in grey font
Figure 4. Schematic of Markers on Dog Map CFA 27.
The genetic map distances in TSP units are displayed for markers in the region of the CSS trait locus on canine chromosome 27 (RH Map, NCBI Dog Map Build 2.1). The CSS trait maximal 2-point LOD score is at marker REN208N23 and maximal multipoint LOD score is placed near marker PEZ16
Table 2.
CSS Trait Multipoint LOD Scores at 10 Microsatellite Markers Spanning CFA 27
| Order from centromere on chromosome |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Marker Name | FH2289 | REN208N23 | FH4079 | PEZ16 | LEI002 | FH3221 | FH3924 | PEZ6 | REN181L4 | REN72K15 |
| Distancea from adjacent marker (MB) | 0 | 3.1 | 1.7 | 7.17 | 9.23 | 9.39 | 9.2 | 7.6 | 4.56 | 7.82 |
| Multipoint LOD Score | −20.46 | 2.13 | 0.39 | 9.09 | 6.92 | −28.02 | −12.34 | −33.99 | −25.67 | −11.0 |
Marker distances displayed in Megabase (MB) from NCBI Dog genome build 2.1, LOD values < 3 are displayed in grey font
3.3 Human Syntenic Region
Multipoint linkage analyses suggest that the CSS trait locus resides within an approximately 14 MB interval, spanning 7.5 MB to 21.5 MB on CFA 27. This region corresponds to a macrosyntenic block near the centromere on human chromosome 12, with orthologous genes located from 12p.11 to 12 q.12.(Goodstadt et al. 2006) The region is gene rich and contains approximately 130 defined or predicted canine genes. Although none of the defined genes in this region have a known role in membrane lipid transport or platelet activation, the protein products of at least 12 of these genes have been identified in the platelet proteome.(Dittrich et al. 2008)
4. Discussion
Initial candidate gene studies of Scott syndrome focused on a phospholipid scramblase (PLSCR1) with a putative role in platelet PCA.(Janel et al. 1999) This protein was first isolated from red blood cells and characterized as having the ability to promote rapid transmembrane lipid movement in response to elevated intracellular calcium.(Zhou et al. 1997) Subsequent studies have revealed that PLSCR1 acts as a cell-signaling molecule that mediates inflammatory cell response to cytokines via upregulation of inositol triphosphate receptor expression.(Bateman et al. 2009; Sahu et al. 2007) Palmitoylation of PLSCR1 apparently influences its cellular localization and activity; when palmitoylated PLSCR1 is associated with lipid rafts in the plasma membrane and when not palmitoylated the protein is imported into the nucleus where it demonstrates transcription factor activity. Mouse PLSCR1 knock-outs provide additional insight into the protein’s biologic activities. PLSCR1 null mice demonstrate normal hemostasis, with no impairment of platelet membrane PS externalization.(Zhou et al. 2002) Rather, the lack of PLSCR1 affects granulopoiesis and neutrophil differentiation in response to cytokine stimulation. Studies of PLSCR1 mRNA and protein from human Scott patients have failed to identify abnormalities indicating that PLSCR1 is the Scott syndrome disease-gene. Lymphoblast cell-lines derived from the index Scott patient expressed normal levels of PLSCR1 mRNA and protein, with no evidence of protein dysfunction. (Zhou et al. 1998) Northern blot and sequence analyses of lymphocyte PLSCR1 mRNA from a second, unrelated Scott patient revealed no differences from healthy controls. (Janel et al. 1999)
Candidate gene studies have also examined Scott leukocyte-expression of members of the ATP-binding cassette (ABC) family of membrane transporters. While no differences were found in sequence or expression levels of ABCB1 or ABCB4 in 1 patient,(Zhou et al. 1998) heterozygosity for a missense mutation in ABCA1 was reported in an unrelated Scott patient.(Albrecht et al. 2005) The variant sequence was associated with reduced lymphocyte expression of both mutant and wild-type ABCA1 mRNA, and impaired intracellular trafficking of the mutant protein in a cell expression system. A role for ABCA1 in platelet PS externalization, however, has not been defined. The best-characterized biologic activity of ABCA1 is its ability to promote cholesterol efflux by mediating lipidation of a carrier protein, ApoA-1 at the plasma membrane and within endocytic vesicles.(Zarubica et al. 2007) Inactivating ABCA1 mutations in human patients and murine knock-out models cause the disease phenotype Tangier disease (OMIM: 205400) manifest clinically by a lack of plasma high density lipoprotein cholesterol and cholesterol accumulation in a variety of tissues. Functional analyses of human and murine Tangier platelets reveal abnormal collagen-induced aggregation response and a spectrum of other abnormalities that can be attributed to defective vesicular trafficking.(Nofer et al. 2004) In further contrast to Scott platelets, Tangier platelets from both species demonstrate normal PS externalization in response to agonist stimuli. (Nofer et al. 2004; Schmitz et al. 2006)
The pedigree studies described in this report indicate that CSS is a single-gene defect with autosomal recessive expression pattern associated with a novel trait locus on CFA 27. This region does not contain the canine homologues of PLSCR1 or ABCA1, located on CFA 23 and CFA 11, respectively. In addition, our previous phenotypic studies failed to demonstrate impaired granulopoiesis, lipid metabolism, or platelet granule release suggestive of functional defects of either of these genes.(Brooks et al. 2002; Brooks et al. 2007; Catalfamo et al. 2006) The CSS phenotype is defined as a bleeding disorder caused by a lack of stimulated platelet membrane PS externalization and resultant failure of platelet PCA. Platelets from CSS dogs, however, do not have an absolute inability to externalize PS. Treatment of CSS platelets with tetracaine, a local anesthetic agent, induces PS externalization equivalent to that of control dog platelets.(Brooks et al. 2002) A similar platelet response to tetracaine was initially demonstrated in studies of a human Scott patient.(Toti et al. 1996) Proposed mechanisms for tetracaine-induced PS exposure include direct perturbation of the membrane lipid bilayer and depolarization of the mitochondrial membrane with subsequent activation of apoptotic pathways.(Augereau et al. 2004) Recent studies indicate that mitochondrial membrane depolarization is also an integral event leading to platelet membrane PS externalization in response to the physiologic agonists, thrombin and collagen.(Dale et al. 2006; Lopez et al. 2008) While CSS platelets treated with these agonists demonstrate loss of mitochondrial potential, PS externalization fails to ensue. (Brooks et al. 2007) These findings suggest that the events downstream of mitochondrial depolarization vary among stimuli, or perhaps differences in timing or extent of mitochondrial depolarization dictate a variable downstream response.
Studies of murine platelets lacking key mitochondria-associated proteins demonstrate the importance and complexity of mitochondrial signaling for platelet procoagulant response. Targeted deletion of murine Ppif generates mice lacking cyclophilin D (CypD), a critical component of the mitochondrial permeability transition pore (mPTP).(Baines et al. 2005) Platelets from CypD null mice fail to externalize PS in response to thrombin and convulxin stimulation, and generate little thrombin activity in prothrombinase assays.(Jobe et al. 2006) In contrast, platelet function studies of mice lacking Bax and Bak, key components of the mitochondrial apoptosis channel (mAC), reveal normal PS exposure in response to physiologic dual agonist (thrombin plus CRP) stimulation. (Schoenwaelder et al. 2009) A defect in PS externalization manifests, however, upon treatment of Bax/Bak null platelets with ABT 737, a BH3 mimetic compound that initiates apoptosis with mitochondrial release of cytochrome C and caspase activation. Together, these murine model studies suggest the presence of two pathways leading to platelet PS externalization, perhaps mediated by mPTP versus mAC signaling.(Smith et al. 2008) The CSS trait locus identified in our study includes canine homologs of several mitochondria-associated proteins and apoptosis regulators, such as BID (BH3 interacting death domain agonist) and LAG1 (longevity assurance homolog 5) that may be considered candidate disease genes. The CSS platelet phenotype entails a uniquely impaired response to calcium ionophore, distinct from either murine knock-out model. Consequently, putative candidates should not be restricted to mitochondrial genes and must include unknown downstream effectors. Ultimately, discovery and characterization of the CSS disease gene will provide important insights into basic mechanisms of activated and apoptotic cell membrane PS externalization.
Acknowledgements
This work was supported in part by Cornell University’s Vertebrate Genomics Center and The Seeing Eye Foundation. Some genotype data for markers on CFA 27 were obtained through a submission to the NHLBI Mammalian Genotyping Service (HV4814). The authors thank Jennifer Cruikshank and Peter Schweitzer for their assistance in performing Genemapper analyses.
Footnotes
Cornell University Life Sciences Core Laboratories Center, Cornell University, Ithaca, NY
References
- Albrecht C, et al. A novel missense mutation in ABCA1 results in altered protein trafficking and reduced phosphatidylserine translocation in a patient with Scott syndrome. Blood. 2005;106:542–549. doi: 10.1182/blood-2004-05-2056. [DOI] [PubMed] [Google Scholar]
- Augereau O, et al. Apoptotic-like mitochondrial events associated to phosphatidylserine exposure in blood platelets induced by local anaesthetics. Thromb Haemost. 2004;92:104–113. doi: 10.1160/TH03-10-0631. [DOI] [PubMed] [Google Scholar]
- Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bateman A, et al. Phospholipid scramblases and Tubby-like proteins belong to a new superfamily of membrane tethered transcription factors. Bioinformatics. 2009;25:159–162. doi: 10.1093/bioinformatics/btn595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers EM, Comfurius P, Zwaal RF. Platelet procoagulant activity: physiological significance and mechanisms of exposure. Blood Rev. 1991;5:146–154. doi: 10.1016/0268-960x(91)90031-7. [DOI] [PubMed] [Google Scholar]
- Boudreaux MK. Characteristics, diagnosis, and treatment of inherited platelet disorders in mammals. J Am Vet Med Assoc. 2008;233:1251–1259. doi: 10.2460/javma.233.8.1251. 1190. [DOI] [PubMed] [Google Scholar]
- Brooks M. A review of canine inherited bleeding disorders: biochemical and molecular strategies for disease characterization and carrier detection. J Hered. 1999;90:112–118. doi: 10.1093/jhered/90.1.112. [DOI] [PubMed] [Google Scholar]
- Brooks MB, Catalfamo JL, Brown HA, Ivanova P, Lovaglio J. A hereditary bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Blood. 2002;99:2434–2441. doi: 10.1182/blood.v99.7.2434. [DOI] [PubMed] [Google Scholar]
- Brooks MB, Catalfamo JL, Friese P, Dale GL. Scott syndrome dogs have impaired coated-platelet formation and calcein-release but normal mitochondrial depolarization. J Thromb Haemost. 2007;5:1972–1974. doi: 10.1111/j.1538-7836.2007.02683.x. [DOI] [PubMed] [Google Scholar]
- Castaman G, Yu-Feng L, Battistin E, Rodeghiero F. Characterization of a novel bleeding disorder with isolated prolonged bleeding time and deficiency of platelet microvesicle generation. Br J Haematol. 1997;96:458–463. doi: 10.1046/j.1365-2141.1997.d01-2072.x. [DOI] [PubMed] [Google Scholar]
- Castelhano MG, et al. Development and use of DNA archives at veterinary teaching hospitals to investigate the genetic basis of disease in dogs. J Am Vet Med Assoc. 2009;234:75–80. doi: 10.2460/javma.234.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalfamo JL, Brooks MB, Ammersbach M, deMaster A. Platelet ABCA1 expression is normal in Scott syndrome dogs. Blood. 2006;108:57b. [Google Scholar]
- Clark LA, et al. Chromosome-specific microsatellite multiplex sets for linkage studies in the domestic dog. Genomics. 2004;84:550–554. doi: 10.1016/j.ygeno.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Dachary-Prigent J, Pasquet JM, Freyssinet JM, Nurden AT. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: a study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34:11625–11634. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- Dale GL, Friese P. Bax activators potentiate coated-platelet formation. J Thromb Haemost. 2006;4:2664–2669. doi: 10.1111/j.1538-7836.2006.02211.x. [DOI] [PubMed] [Google Scholar]
- Dittrich M, et al. Platelet protein interactions: map, signaling components, and phosphorylation groundstate. Arterioscler Thromb Vasc Biol. 2008;28:1326–1331. doi: 10.1161/ATVBAHA.107.161000. [DOI] [PubMed] [Google Scholar]
- Goodstadt L, Ponting CP. Phylogenetic reconstruction of orthology, paralogy, and conserved synteny for dog and human. PLoS Comput Biol. 2006;2:e133. doi: 10.1371/journal.pcbi.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon R, et al. A 1-Mb resolution radiation hybrid map of the canine genome. Proc Natl Acad Sci U S A. 2003;100:5296–5301. doi: 10.1073/pnas.0831002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk JW, Siljander PR, Bevers EM, Farndale RW, Lindhout T. Receptors and signalling mechanisms in the procoagulant response of platelets. Platelets. 2000;11:301–306. doi: 10.1080/09537100050144704. [DOI] [PubMed] [Google Scholar]
- Janel N, et al. Assessment of the expression of candidate human plasma membrane phospholipid scramblase in Scott syndrome cells. Thromb Haemost. 1999;81:322–323. [PubMed] [Google Scholar]
- Jobe SM, et al. Critical role for cyclophilin D and mitochondrial permability transition pore (MPTP) in platelet activation. Blood. 2006;108 doi: 10.1182/blood-2007-05-092684. 435a(Abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Thrombin induces activation and translocation of Bid, Bax and Bak to the mitochondria in humanplatelets. J Thromb Haemost. 2008;6:1780–1788. doi: 10.1111/j.1538-7836.2008.03111.x. [DOI] [PubMed] [Google Scholar]
- Nofer JR, et al. Impaired platelet activation in familial high density lipoprotein deficiency (Tangier disease) J Biol Chem. 2004;279:34032–34037. doi: 10.1074/jbc.M405174200. [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Kruglyak L. Unleashing the canine genome. Genome Res. 2000;10:1271–1274. doi: 10.1101/gr.155900. [DOI] [PubMed] [Google Scholar]
- Sahu SK, Gummadi SN, Manoj N, Aradhyam GK. Phospholipid scramblases: an overview. Arch Biochem Biophys. 2007;462:103–114. doi: 10.1016/j.abb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Salles II, et al. Inherited traits affecting platelet function. Blood Rev. 2008;22:155–172. doi: 10.1016/j.blre.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Schambeck CM. Molecular defects in the ABCA1 pathway affect platelet function. Pathophysiol Haemost Thromb. 2006;35:166–174. doi: 10.1159/000093563. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder SM, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86:266–275. [PubMed] [Google Scholar]
- Smith DJ, Ng H, Kluck RM, Nagley P. The mitochondrial gateway to cell death. IUBMB Life. 2008;60:383–389. doi: 10.1002/iub.44. [DOI] [PubMed] [Google Scholar]
- Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–1415. [PubMed] [Google Scholar]
- van der Planken MG, et al. Platelet prothrombinase activity, a final pathway platelet procoagulant activity, is overexpressed in type 1 diabetes: no relationship with mean platelet volume or background retinopathy. Clin Appl Thromb Hemost. 2000;6:65–68. doi: 10.1177/107602960000600202. [DOI] [PubMed] [Google Scholar]
- Weiss HJ. Impaired platelet procoagulant mechanisms in patients with bleeding disorders. Semin Thromb Hemost. 2009;35:233–241. doi: 10.1055/s-0029-1220331. [DOI] [PubMed] [Google Scholar]
- Zarubica A, Trompier D, Chimini G. ABCA1, from pathology to membrane function. Pflugers Arch. 2007;453:569–579. doi: 10.1007/s00424-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Sims PJ, Wiedmer T. Expression of proteins controlling transbilayer movement of plasma membrane phospholipids in the B lymphocytes from a patient with Scott syndrome. Blood. 1998;92:1707–1712. [PubMed] [Google Scholar]
- Zhou Q, et al. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99:4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- Zwaal RF, Comfurius P, Bevers EM. Platelet procoagulant activity and microvesicle formation. Its putative role in hemostasis and thrombosis. Biochim Biophys Acta. 1992;1180:1–8. doi: 10.1016/0925-4439(92)90019-j. [DOI] [PubMed] [Google Scholar]
- Zwaal RF, Comfurius P, Bevers EM. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim Biophys Acta. 2004;1636:119–128. doi: 10.1016/j.bbalip.2003.07.003. [DOI] [PubMed] [Google Scholar]