Abstract
Recently, it has been reported that using multiple signals, murine and human B cells secrete several cytokines with pro-inflammatory and immunoregulatory properties. We present the first comprehensive analysis of 24 cytokines, chemokines, and hematopoietic growth factors production by purified human peripheral blood B cells (CD19+), and naive (CD19+CD27-) and memory (CD19+CD27+) B cells in response to direct and exclusive signaling provided by toll-like receptor (TLR) ligands Pam3CSK (TLR1/TLR2), Imiquimod (TLR7), and GpG-ODN2006 (TLR9). All three TLR ligands stimulated B cells (CD19+) to produce cytokines IL-1α, IL-1β, IL-6, TNF-α, IL-13, and IL-10, and chemokines MIP-1α, MIP-1β, MCP-1, IP-10, and IL-8. However, GM-CSF and G-CSF production was predominantly induced by TLR2 agonist. Most cytokines/chemokines/hematopoietic growth factors were predominantly or exclusively produced by memory B cells, and in general, TLR2 signal was more powerful than signal provided viaTLR7 and TLR9. No significant secretion of eotaxin, IFN-α, IFN-γ, IL-2, IL-3, IL-4, IL-5, IL-7, IL-15, IL-17, IL-12p40, IL-12p70, and TNF-β (lymphotoxin) was observed. These data demonstrate that human B cells can be directly activated viaTLR1/TLR2, TLR7, and TLR9 to induce secretion of cytokines, chemokines, and hematopoietic growth factors and suggest a role of B cells in immune response against microbial pathogenesis and immune homeostasis.
Keywords: Naive B cell, memory B cell, cytokines, chemokines, CD80, CD86, CD25
Introduction
B lymphocytes are known for their role in adaptive immunity as specific antibody-producing cells and as antigen-presenting cells. More recently, a regulatory role of B cells has been established, which appears to be mediated by IL-10 secreted by B cells [1–5]. Furthermore, murine and human B cells have been shown to produce a number of cytokines, which require multiple signals, suggesting their role in innate immunity [6–11]. The toll-like receptors (TLRs) is a family of transmembrane proteins with an extracellular leucin-rich domain and a conserved cytoplasmic domain, which is homologous to the IL-1 receptor [12–14]. Each TLR recognizes specific microbial components termed pathogen-associated molecular patterns including bacterial lipoproteins and peptidoglycan (TLR2), uridine-rich single-stranded ribonucleic acid (ssRNA; TLR7), and microbial deoxyribonucleic acid (DNA; TLR9) [13, 14]. Recently, several TLRs have been reported to be present in B cells [15, 16]. Cognasse et al. [18] have reported secretion of IL-6 by TLR9 expressing memory B cells. Mansson et al. [17] also reported secretion of IL-6 via activation of B cells with IL-2, IL-10, recombinant CD40 ligand, and TLR2, TLR7, and TLR9 ligands.
In this study, we examined the effects of direct signaling by TLR ligands Pam3CSK4 (TLR1/TLR2), Imiquimod (TLR7), and CpG-ODN2006 (TLR9) on B cell-derived 24 cytokines, chemokines, and hematopoietic growth factor production. We observed that all three ligands activated B cells and induced secretion of 11 of 24 cytokines and chemokines, whereas hematopoietic growth factor production was induced predominantly by TLR1/TLR2 ligand. Furthermore, the majority of cytokines, chemokines, and hematopoietic growth factors are predominantly or exclusively produced by CD19+CD27+ memory B cells, and in general TLR2 was a stronger signal than TLR7 and TLR9 to induce their production. To the best of our knowledge, this is the first report of comprehensive analysis of secretion of cytokines, chemokines, and hematopoietic growth factors by direct activation of human naive and memory B cells by TLR ligands. Our data supports a role of B cells in innate immune response against microbial pathogens and immune homeostasis.
Materials and Methods
Subject
Peripheral blood was obtained from young healthy volunteers. Protocol was approved by Human Subject Institution Review Board of the University of California, Irvine.
Reagents
The following monoclonal antibodies and their isotype controls were used — Peridinin chlorophyll protein PerCP-conjugated anti-CD19, PE-conjugated anti-CD27, fluorescein isothiocyanate (FITC)-conjugated anti-CD80, PE-conjugated anti-CD86, PerCP-conjugated anti-HLADR, and APC-conjugated anti-CD25, all purchased from BD Pharmingen (San Jose, CA). B cell enrichment kit was purchased from Stem Cell Technologies (Vancouver, BC, Canada). TLR agonist: Pam3CSK4 (TLR1/TLR2), Imiquimod (TLR7), and CpG-ODN2006 (TLR9) were purchased from InvivoGen (San Diego , California).
Purification of B Cells and B Cell Subsets
B lymphocytes were purified by immunomagnetic human B cell enrichment Kit according to manufacturer’s instructions. In brief, peripheral blood mononuclear cells were suspended at no more than 1 × 108 cells/ml in PBS containing 2% FBS. Negative selection cocktail (100 μl/ml) is added and incubated at room temperature for 15 min. Then the magnetic nanoparticles are added at 50 μl/ml cells and incubated for 10 min. Cells are placed in a 12 × 75 mm polystyrene tube at a volume of 2.5 ml/tube and placed into the magnet for 5 min. The magnet is inverted and the supernatant was poured off. The magnetic labeled unwanted cells remain bound inside the original tube. The purity of negatively selected cells was assessed by flow cytometry (>97%) as detected by the presence of CD20. Furthermore, during the process of purification, B cells were not activated as determined by the expression of CD80, CD86, CD25, and HLA-DR in B cells in whole peripheral blood and in the purified B cells.
Purified B cells were further separated into CD27+ (memory) and CD27- (naive) B cells using PE selection kit (Stem Cell Technologies). Briefly, B cells were labeled with PE-conjugated anti-CD27antibody. The labeled cells were incubated with bispecific tetrameric antibody complexes that recognize PE-labeled cells and dextran. After a 15-min incubation at room temperature, dextran-coated magnetic nanoparticles were added, and magnetically labeled cells (CD27+) were separated from unlabeled cells (CD27-) using a magnet.
Immunophenotyping
Purified B cells were stimulated with Pam3CSK4 (Pam), Imiquimod (ImQ), and CpG for 24 h. Cells are stained with PerCP-conjugated anti-HLA-DR, APC-conjugated anti-CD25, FITC-conjugated anti-CD80, and PE-conjugated anti-CD86 antibodies, and isotype controls. After staining, cells were washed extensively with phosphate buffered saline and analyzed by FACScalibur (Becton-Dickenson, San Jose, CA) equipped with argon ion laser emitting at 488 nm [for FITC, phycoerythrin (PE), and PerCP excitation] and a spatially separate diode laser emitting at 631 nm(for APC excitation). Forward and side scatters were used to gate and exclude cellular debris. Ten thousand cells were acquired and analyzed by FloJo software.
Measurement of Cytokines/Chemokines/Hematopoietic Growth Factors
Cytokine/chemokines secretion was measured in purified total B cells (CD19+) and purified CD19+CD27+ and CD19+CD27- B cell subsets by multiplex biomarker immunoassay. Cells were activated by various concentrations of TLR2, TLR 7, and TLR 9 agonists for 24 h. Supernatant were collected and stored at -20°C until assayed. Cytokines/chemokines were detected by Millipore (St. Charles, MO) MILLIPLEX™ MAP human cytokine/chemokine kit. Following 26 human cytokines, chemokines and hematopoietic growth factors were analyzed: Eotaxin, G-CSF, GM-CSF, IFNα2, INFγ,IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p40), IL-12 (p70),IL-13, IL-15, IL-17, IP-10(CXCL10), MCP-1, MIP-1α, MIP-1βb, TNFα, and TNFβ. MILLIPLEX™ MAP is based on the Luminex® xMAP® technology that uses a technique to internally color-code microspheres with two fluorescent dyes. Through precise concentrations of these dyes, 100 distinctly colored bead sets can be created, each of which is coated with a specific capture antibody. After an analyte from a test sample is captured by the bead, a biotinylated detection antibody is introduced in the reaction mixture, which is then incubated with streptavidin–PE conjugate, the reporter molecule, to complete the reaction on the surface of each microsphere. The microspheres are allowed to pass rapidly through a laser which excites the internal dyes marking the microsphere set. A second laser excites PE, the fluorescent dye on the reporter molecule. Finally, high-speed digital-signal processors identify each individual microsphere and quantify the result of its bioassay based on fluorescent reporter signals. The capability of adding multiple conjugated beads to each sample results in the ability to obtain multiple results from each sample.
Statistical analyses were performed by analysis of variance (ANOVA), and significance was considered at P < 0.05.
Results
Purified B Cells are Activated by TLR Ligands
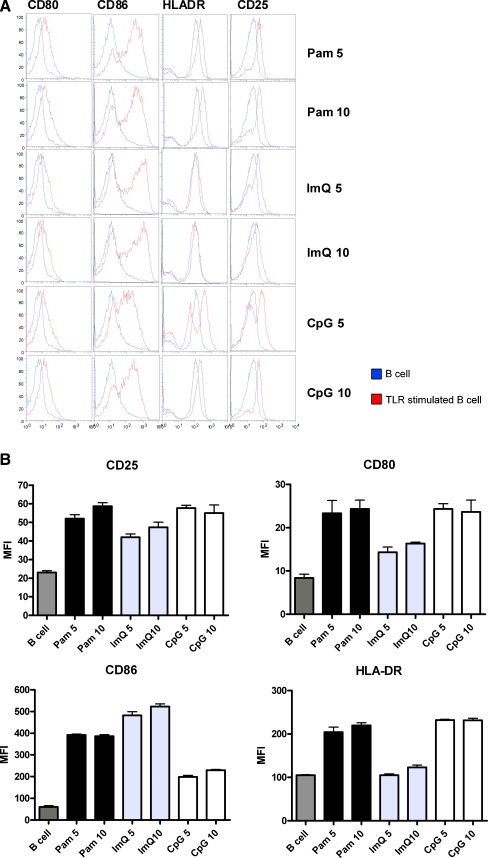
Purified CD19+ B cells were stimulated with two different concentrations (determined by concentration kinetic studies) of Pam, ImQ, and CpG for 24 h. At the end of culture, cells were washed and stained with FITC-anti-CD80, PE-anti-CD86, PerCP-HLA-DR, and APC-anti-CD25 or isotype controls for 30 min on ice. Cells were then washed and examined for the expression of these activation and co-stimulatory antigens with multicolor flow cytometry using FACSCalibur. Ten thousand cells were acquired and analyzed by FlowJo software. Figure 1a displays a representative cytograph, and Fig. 1b shows cumulative data for fluorescence intensity (MFC#, mean±sd) from three separate experiments using three separate normal young subjects. All three TLR ligands significantly (P < 0.05-P < 0.001) upregulated expression of CD80, CD86, and CD25; ImQ did not upregulate HLA-DR expression (P > 0.05). It is also apparent that different TLR ligands upregulated activation antigens to different extent. CD80, HLA-DR, and CD25 were activated to a greater extent (P < 0.05) by Pam and CpG as compared to ImQ, whereas CD86 was upregulated to a greater extent (P < 0.05) by Pam and ImQ than by CpG.
Fig. 1.
TLR-induced activation of B cells. Purified B cells were stimulated by 5 μg/ml and 10 μg/ml of TLR1/TLR2 (Pam), TLR7 (ImQ), and TLR9 (CpG) ligands for 24 h and co-stimulatory and activation molecules were analyzed by multicolor flow cytometry. a. Representative cytoflourograph. b. Cumulative data from three experiments from three healthy individuals
TLR Ligands Induced Secretion of Pro-Inflammatory and Anti-Inflammatory/Immunoregulatory Cytokines
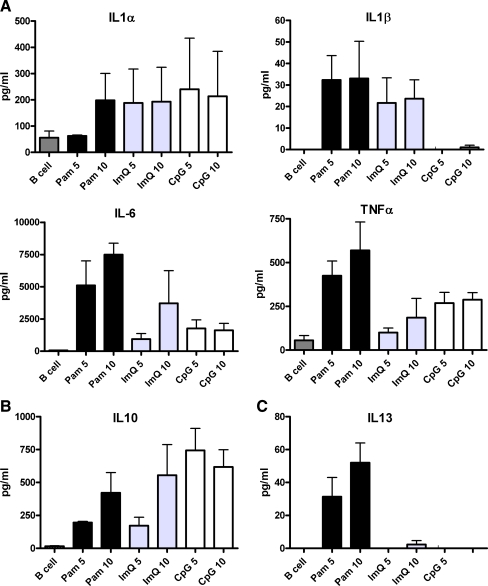
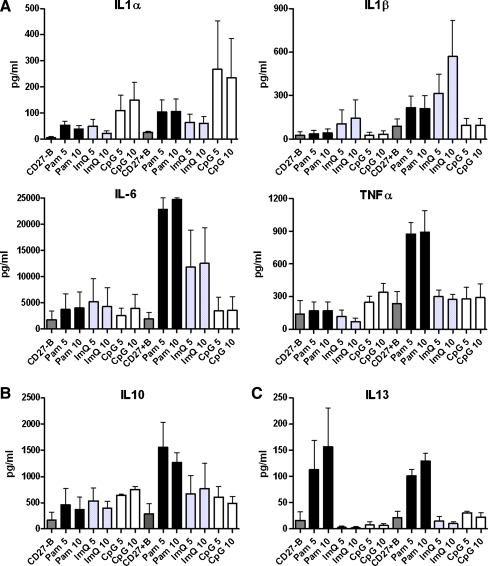
Purified B cells were activated with Pam, ImQ, and CpG as described above for 24 h, supernatants were collected and stored at -20°C until analyzed. Cytokines were analyzed by multiplex biomarker immunoassay and data are expressed (in pg/ml as mean±sd of three separate experiments). Figure 2a shows data of pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α. Although all three TLR ligands induced their secretion, Pam induced significantly greater amount of IL-6 (P < 0.01) as compared to IL-6 produced by ImQ and CpG. CpG failed to induce secretion of IL-1β. All three ligands induced secretion of immunoregulatory IL-10 (Fig. 2b), whereas Pam induced the secretion of anti-inflammatory and immunoregulatory IL-13 (Fig. 2c).
Fig. 2.
TLR-induced cytokines (interleukins) production by B cells. Purified B cells were stimulated as in Fig. 1. Supernatants were collected and examined for cytokine production by multiplex biomarker immunoassay. Data are expressed as mean±sd from three separate experiments. All TLRs, although to different levels, induced secretion of proinflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α (a). Immunoregulatory IL-10 (b) IL-13 were also secreted by B cells; however, IL-13 secretion was secreted in low amounts and almost exclusively by TLR1/TLR2 stimulation (c)
TLR Ligands Induced Secretion of Chemokines
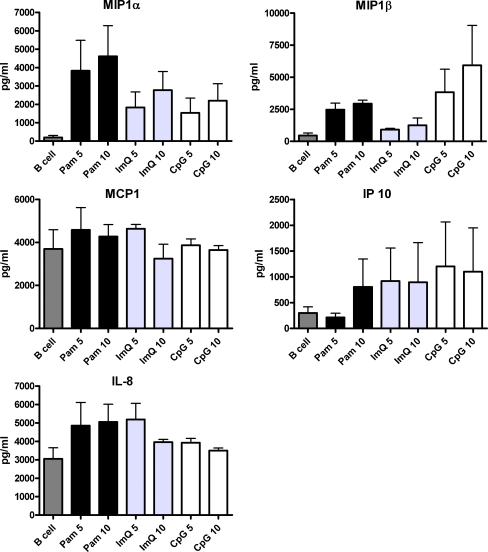
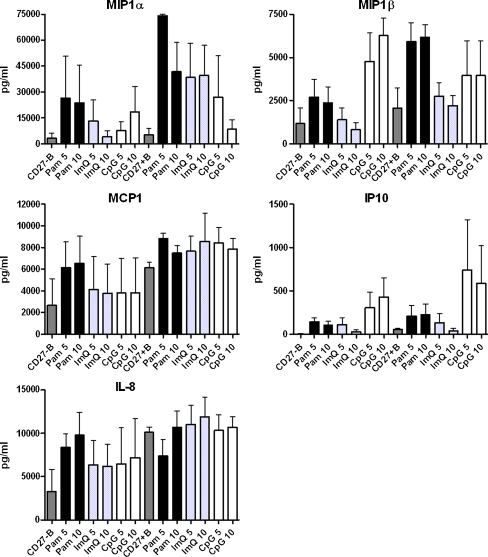
Purified B cells were activated by Pam, ImQ, and CpG as described above, and supernatants were analyzed for chemokines. All three TLR ligands induced secretion of MIP-1α and IP-10; however, IL-8 was not induced by CpG, and MCP1 was not significantly induced by any of the TLR ligands (Fig. 3).
Fig. 3.
TLR-induced production of chemokines. Purified B cells were stimulated as in Fig. 1. Supernatants were collected and examined for chemokines production. All three TLRs induced secretion of MIP-1α and IP-10 (CXCL3); however, secretion of MIP-1β, MCP-1, and IL-8 by various TLR ligands was variable
TLR1/TLR2 Ligand Induces Secretion of GM-CSF and G-CSF
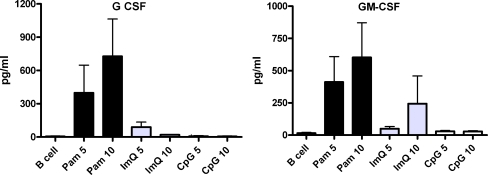
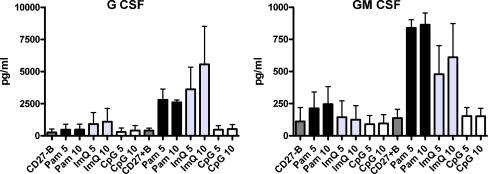
Supernatants from B cells activated with Pam, ImQ, and CpG were analyzed for the secretion of GM-CSF and G-CSF. Both growth factors were produced predominantly by TLR1/TRL2 ligand Pam (Fig. 4).
Fig. 4.
TLR-induced production of hematopoietic growth factors by B cells. Purified B cells were stimulated as in Fig. 1. Supernatants were collected and examined for the production of G-CSF and GM-CSF by multiplex biomarker immunoassay. Both G-CSF and GM-CSF were produced predominantly in response to TLR1/TLR2 agonist
TLR Ligands did Induce Secretion of Multiple Cytokines
None of the TLR ligands induced significant secretions of eotaxin, IFN-α, IFN-γ, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-7, IL-15, IL-17, IL-12p40, IL-12p70, and TNF-β (data not shown). This suggests that either priming of B cells by other signal may be required for TLRs to induce secretion of these cytokines, or B cells do not produce these cytokines regardless of the nature and multiplicity of signals.
Next, we investigated whether naive and memory B cells respond differently to TLRs signaling. Therefore, we purified total (CD19+) B cells into naive (CD27-) and memory (CD27+) B cells and stimulated with TLR ligands as described above, and supernatants were analyzed for cytokines, chemokines, and hematopoietic growth factors.
Cytokine Production by Naive and Memory B Cells
Significantly (P < 0.5) greater amounts of IL-1α, IL-1β, IL-6, and TNFα were produced by memory B cells upon stimulation with Pam as compared to naive B cells (Fig. 5a). ImQ induced significantly greater amounts of IL-1β, and IL-6 in memory B cells as compared to naive B cells. CpG induced significantly greater amounts (P < 0.05) of IL-1α by the memory B cells as compared to naive B cells. Immunoregulatory IL-10 was induced by all three ligands in both naive and memory B cells but significantly more (P < 0.05) by memory B cells as compared to naive B ells in response to Pam (Fig. 5b). IL-13 was produced by both naive and memory B cells predominantly in response to Pam (Fig. 5c).
Fig. 5.
TLR-induced cytokines (interleukins) produced by naive and memory B cells. Purified B cells were further separated into naive (CD27-) and memory (CD27+) B cells and stimulated with TLR ligands as described in Fig. 1. Data are expressed as mean±sd from three separate experiments. All four pro-inflammatory cytokines (IL-1α, IL-1β, TNF-α, and IL-6) were produced predominantly by memory B cells, in response to all three TLRs ligands (a). Immunoregulatory IL-10 was produced by both naive and memory B cells by all three TLRs; however, significantly (P < 0.01) more by memory B cells in response to TLR1/TLR2 stimulation (b). In contrast, similar levels of immunoregulatory IL-13 were produced by naive and memory B cells and almost exclusively by TLR1/TLR2 stimulation (c)
Chemokines Produced by Naive and Memory B Cells
Both naive and memory B cells produced IP-10, IL-8, MCP-1, MIP-1α, and MIP1β in response to Pam, ImQ, and CpG (Fig. 6). However, Pam induced significantly greater (P < 0.05) amounts of MIP1α and MIP1β by memory B cells as compared to naive B cells.
Fig. 6.
TLR-induced production of chemokines by naive and memory B cells. Purified B cells were further separated into naive (CD27-) and memory (CD27+) B cells and stimulated with TLR ligands as described in Fig. 1. All TLRs induced chemokines production by both naive and memory B cells
Hematopoietic Growth Factor Production by Naive and Memory B Cells
Both G-CSF and GM-CSF were predominantly produced by memory B cells upon signaling with Pam and ImQ. CpG failed to induce significant amounts of G-CSF and GM-CSF (Fig. 7).
Fig. 7.
TLR-induced production of hematopoietic growth factors by naive and memory B cells. Purified B cells were further separated into naive (CD27-) and memory (CD27+) B cells and stimulated with TLR ligands as described in Fig. 1. TLR1/TLR2 and TLR7 induced G-CSF and GM-CSF secretion almost exclusively by memory B cells. TLR9 stimulations did not induce secretion of either growth factor
Discussion
Although a role of B cells in antibody response and antigen presentation is well known, its role in innate immune response and immune regulation has recently been appreciated [6–12]. Recently, B cells have been reported to express a number of TLRs [15–20]. In mice and most human studies, cytokine production by B cells utilized multiple signals including BCR, CD40L, and TLR; however, cytokine production by B cells in response to primary and sole signaling by TLR has not been studied in detail. In this study, we have demonstrated that human B cells and B cell subsets (naive and memory) may be directly activated by TLR1/TLR2, TLR7, and TLR9 ligands to induced expression of co-stimulatory antigens and secretion of 11 of 24 cytokines, chemokines, and hematopoietic growth factors studied. A number of investigators have reported that human B cells require priming by another ligand to be sensitive to TLR signaling [6–12]; however, this may be a matter of end-point assay measured. In the majority of these reports, B cell proliferation and differentiation into memory and plasma cells were assays, whereas in our study, an early event of cytokine/chemokine secretion was assayed. Therefore, it is likely that B cells may be directly activated via TLR1/TLR2, TLR7, and TLR9 to secrete cytokines/chemokines/hematopoietic growth factors and to upregulate co-stimulatory molecules and antigen-presenting function. However, they may require more than one signal to induce cell division and differentiation into memory and plasma cells.
We observed that TLR1/TLR2 and TLR9 agonists significantly upregulated the expression of CD80, CD86, HLA-DR, and CD25. TLR7 agonist upregulated CD80, CD86, and CD25 but failed to upregulate HLA-DR. It remains to be determined whether the lack of upregulation of HLA-DR by Pam results in the failure to augment antigen-presenting function of B cells. Krieg et al. [21] reported that CpG stimulation augments the expression of CD80, CD86, and HLA-DR in murine B cells, and Jiang et al. [22] have reported that CpG stimulates naive B cells to undergo proliferation and enhanced antigen-presenting function; however, CpG did induce differentiation into memory B cells or immunoglobulin (Ig)-secreting plasma cells.
It has been reported that murine B cells are strongly responsive to direct stimulation with TLR2 ligand [22]; however, human B cells require priming for TLR2 ligand by cross-linking of the BCR with anti-Ig or protein A [8, 23]. In the present study, we demonstrate that human B cells and B cell subsets can be directly activated without priming to secrete cytokines, chemokines, and hematopoietic growth factors. The reason for this discrepancy may be due to differences in assays for B cells activation used. Bekeredjian-Ding et al. [24] used B cell proliferation assay, a much later event as compared to cytokine and chemokines secretion.
Human B cells also express TLR7 and TLR9 [18, 19]. In human B cells, TLR7 expression is IFN type I dependent, which sensitized human B cells for signaling via TLR7 [24]. Therefore, for B cells to respond toTLR7 ligand, B cells require priming by IFN type I produced by dendritic cells [25]. However, our data demonstrate that human B cells and naive and memory B cell subsets can be stimulated directly by TLR7 agonist without prior sensitization to secrete cytokines and chemokines. Since our data show that IFNs are not produced by human B cells, our study also excludes any possibility of IFN type I produced by B cells to sensitize B cells to respond to TLR7 ligand in an autocrine or paracrine manner. We also show that direct activation of B cells and B cell subsets by CpG (TLR9) induces secretion of chemokines and cytokines. Hornung et al. [19] has also reported direct responsiveness of human B cells to CpG (TLR9) as determined by its effect on the expression of TLR7 and TLR9. Cognasse et al. [18] reported that TLR9+ purified B cells secrete IL-6 upon stimulation with IL-2, IL-10, soluble CD40L, and TLR9 ligand CpG. Duddy et al. [10], using BCR and CD40 signaling system, showed that memory B cells produce TNF-α and lymphotoxin (TNF-β). We also observed that all three TLR agonists induce TNF-α but not TNF-β, suggesting that the nature of the signal may dictate the profile of certain cytokine production by B cells.
A role of B cells in the regulation of T cell responses has been reported, especially in murine models [1–5]. This appears to be mediated predominantly by IL-10 [3, 4, 26]. We have also observed that significant amounts of IL-10 is produced by purified B cells stimulated with all three TLR agonists Pam, ImQ, and CpG. This is in agreement with reported IL-10 production in response to TLR9 ligand [6]; however, there are no reports of IL-10 production in response to TLR1/TLR2 and TLR7. Duddy et al. [10] reported that IL-10 is produced exclusively by naive B cells when stimulated by BCR and CD40-mediated signaling. Recently, Blair et al. [27] reported production of IL-10 by stimulation of CD19hi+CD24+CD38+hi, a subset of naive B cells by CD40 ligation. However, no IL-10 was produced by three different subsets of B cells upon stimulation with anti-IgM, anti-IgM plus CHO-CD154 cells. This would suggest that B cell subsets produce different amounts of cytokines depending upon type of signal. Here, we show that memory B cells produce significantly greater amounts of IL-10 by memory B cells especially upon stimulation with TLR1/TLR2 agonist. Production of IL-10 by B cells via TLR-mediated mechanism may have evolved as a mechanism to protect against development of microbial pathogen-induced chronic inflammatory states, or else microbes use this mechanism for their persistence in the host by inducing suppression of immune response. Since IL-10 can induce the differentiation of B cell to antibody-producing plasma-like cells, production of endogenous IL-10 may be also a means to induce the production of antimicrobial antibodies by antigen-specific B cells. Giordani et al. [28] have reported that INF-α amplifies human naive B cells, TLR-9-mediated activation, and Ig production. However, Hanten et al. [29] reported IgG and IgM production by human B cells following direct activation by TLR ligand without the presence of IFN-α. Zhang et al. [30] reported that type I interferons protect neonates from acute inflammation through IL-10-producing B cells; however, type I interferon do not potentiate IL-10 production by adult B cells. It is interesting to note that we have observed that type I interferon responsive cytokine IP-10 [31] is induced by all TLR agonists; however, B cells did not secrete IFN-α in response to any of three TLR agonists. Taken together, it is likely that IP-10 and IL-10 are produced in direct response to TLR stimulation rather than induced indirectly by type I interferon, and naive B cells can be activated directly by TLRs to differentiate and induce Ig secretion.
IL-13 is an immunoregulatory cytokine secreted predominantly by activated T-helper type 2 cells [32].This cytokine is involved in several stages of B-cell maturation and differentiation. It upregulates CD23 and MHC class II expression and promotes IgE isotype switching of B cells. In contrast, IL-13 downregulates macrophage activity, thereby inhibiting the production of pro-inflammatory cytokines and chemokines, and appears to be critical to the pathogenesis of allergen-induced asthma through IgE-independent mechanisms. We observed that B cells, and both naive and memory B cells, secrete IL-13, which is induced predominantly and almost exclusively by TLR-2 ligand.
In this study, we also show that direct activation of total B cells, naive B cells, and memory B cells with TLR1/TLR2, TLR7, and TLR9 agonists resulted in the secretion of chemokines IL-8, IP-10, MCP1, MIP1α, and MIP1β. Furthermore, TLR1/TLR2 signal induced significantly greater amounts of MIP1α and MIP1β by memory B cells as compared to naive B cells. IP-10 (CXCL10) was initially identified as a 10 kD inflammatory chemokine that is induced by IFN-γ and secreted by various cell types, including monocytes, neutrophils, endothelial cells, keratinocyte, fibroblasts, mesenchymal cells, dendritic cells, and astrocytes [33]. IP-10 suppresses neovascularization and functions as an “angiostatic” chemokine [34]. CXCL10 binds to CXCR3 and regulates immune responses by activation and recruitment of leukocytes such as T cells, eosinophils, monocytes, and NK cells [31]. Recent reports have shown that the serum and/or the tissue expressions of CXCL10 are increased in various autoimmune diseases [31]. This would suggest that B cells, in addition to producing autoantibodies, may play a role in autoimmune disease through production of chemokines and cytokines. Our data also suggest that B cells may play an important role (via secretion of IP-10) in leukocyte homing to inflamed tissues and in the perpetuation of inflammation and thus may importantly contribute to tissue damage.
MIP-1α (CCL-3L1) is a most potent ligand for CCR5, the major co-receptor for HIV, and is a dominant HIV-suppressive cytokines [35]. We show that TLRs directly activate B cells and B cell subsets to produce large amounts of MIP-1α. It remains to be determined whether B cells via secretion of MIP-1α regulate HIV infection and HIV replication.
G-CSF and GM-CSF are two hematopoietic growth factors that play an important role on hematopoiesis [36]. We show that direct activation of B cells and memory B cells greater than naive B cells via TLR2, and to a minor extent via TLR7, results in the secretion of both GM-CSF and G-CSF. Therefore, it is likely that B cells might regulate hematopoiesis, especially in response to TLR2 stimulation (bacterial lipoprotein and lipoteochoic acid).
In summary, signaling via TLR1/TLR2, TLR7, and TLR9 directly activates B cells to secrete proinflammatory and immunoregulatory cytokines and chemokines. In general, memory B cells appear to secrete greater amounts of cytokines and chemokines, and TLR1/TLR2 appear to mediate stronger signals as compared to the signals provided by TLR7 and TLR9. Therefore, B cells, in addition to their well-established role in adaptive immunity, may also regulate immune responses to infectious pathogens via production of cytokines and chemokines and in immune homeostasis through production of immunoregulatory cytokines.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10875-010-9471-9
References
- 1.Fillatreau S, Gray D, Anderson SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8:391–396. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 2.Hoehlig K, Lampropoulou V, Roch T, Neves P, Calderon-Gomez E, Anderton SM, Steinhoff U, Fillatreau S. Immune regulation by B cells and antibodies a view towards the clinic. Adv Immunol. 2008;98:1–38. doi: 10.1016/S0065-2776(08)00401-X. [DOI] [PubMed] [Google Scholar]
- 3.Lund FE. Cytokine-producing B lymphocytes — key regulator of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 5.Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 6.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, Nadler LM. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cells signals CD40 ligand, interferon γ, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–11. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray D, Gray M, Barr T. Innate responses of B cells. Eur J Immunol. 2007;37:3304–3310. doi: 10.1002/eji.200737728. [DOI] [PubMed] [Google Scholar]
- 9.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 10.Duddy M, Niino M, Adatia F, Herbert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profile of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 11.Gargo A, Servis D, Cepika AM, Toellner KM, Grafton G, Taylor DR, Branica S, Gordon J. Type I cytokine profile of human naive and memory B lymphocytes: a potential for memory cells to impact polarization. Immunology. 2006;118:66–77. doi: 10.1111/j.1365-2567.2006.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, Turna E, Giese T, Ellwart JW, Endres S, Hartmann G. IL12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:854–963. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2002;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. TLR signaling. Sem Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 16.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B cells. Cell Immunol. 2005;236:140–145. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Mansson A, Adner M, Hockerfelt C, Cardell L-O. A distinct toll-like receptor repertoire in human tonsil B cells, directly activated by Pam3CSK4, R-837 and Cpg-200 stimuation. Immunology. 2006;118:539–548. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cognasse F, Hamzeh-Cogness H, Lafarge S, Chavarin P, Pozzeto B, Richard Y, Gerraud O. Identification of two subppulations of purified human Blood B cells, CD27–CD23+ and CD27highCD80+, that strongly express cell surface toll-like receptor 9 and secrete high levels of interleukin-6. Immunology. 2008;125:430–437. doi: 10.1111/j.1365-2567.2008.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 20.Pistota V. Production of cytokines by human B cells in health and diseases. Immunol Today. 1997;18:343–350. doi: 10.1016/S0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM, Yi AK, Matson S, Waldscmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motif in bacterial DNA trigger direct B cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 23.Borsutzky S, Kreschmer K, Becker PD, Muhlradt PF, Kirsching CJ, Weiss S, Guzman CA. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol. 2005;174:6308–6313. doi: 10.4049/jimmunol.174.10.6308. [DOI] [PubMed] [Google Scholar]
- 24.Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, Zahringer U, Hartmann G. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol. 2007;178:2803–2812. doi: 10.4049/jimmunol.178.5.2803. [DOI] [PubMed] [Google Scholar]
- 25.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 26.Booth JS, Griebel PJ, Babiuk LA, Mutwiri GK. A novel regulatory B-cell population in sheep Peyer’s patches spontaneously secretes IL-10 and downregulates TLR9-induced IFNalpha responses. Mucosal Immunol. 2009;2(3):265–75. doi: 10.1038/mi.2009.6. [DOI] [PubMed] [Google Scholar]
- 27.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19+CD24hiCD38hi exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Giordani L, Sanchez M, Libri I, Quaranta MG, Mattioli B, Viora M. IFN-α amplifies human naive B cell TLR-9-mediated activation and Ig production. J Leukoc Biol. 2009;86:261–271. doi: 10.1189/jlb.0908560. [DOI] [PubMed] [Google Scholar]
- 29.Hanten JA, Vasilakos JP, Riter CI, Neys L, Lipson KE, Alkan SS, Birmachu W. Comparision of human B cell activation by TLR7 and TLR9. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Deriaud E, Jiao X, Braun D, LeClerc C, Lo-Man R. Type I interferons protect neonates from acute inflammation through interleukin-10 producing B cells. J Exp Med. 2007;204:1107–1118. doi: 10.1084/jem.20062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupardus PJ, Birnbaum ME, Garcia KC. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure. 2010;18:332–342. doi: 10.1016/j.str.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EY, Lee Z-H, Song YW. CXCL10 and autimmune diseases. Autoimmun Rev. 2009;8:379–383. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan S-Y, Roczniak S, Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 35.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein 1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/S1359-6101(02)00045-X. [DOI] [PubMed] [Google Scholar]
- 36.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:554–559. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]