Abstract
Importance of the field
Erectile dysfunction (ED) is a major men’s health problem. Although the high success rate of treating ED by phosphodiesterase 5 (PDE5) inhibitors has been reported, there are a significant number of ED patients who do not respond to currently available treatment modalities.
Areas covered in this review
To understand the current status of gene therapy application for ED, gene therapy approaches for ED treatment are reviewed.
What the reader will gain
Gene therapy strategies that can enhance nitric oxide (NO) production or NO-mediated signaling pathways, growth factor-mediated nerve regeneration or K+ channel activity in the smooth muscle could be promising approaches for the treatment of ED. Although the majority of gene therapy studies are still in the preclinical phase, the first clinical trial using non-viral gene transfer of Ca2+-activated, large-conductance K+ channels into the corpus cavernosum of ED patients showed positive results.
Take home message
Gene therapy represents an exciting future treatment option for ED, especially for people with severe ED unresponsive to current first-line therapies such as PDE5 inhibitors although the long-term safety of both viral and non-viral gene therapies should be established.
Keywords: gene therapy, erectile dysfunction, nitric oxide, growth factor, K+ channel
1. Introduction
It has been projected that approximately 150 million men in the world suffer from erectile dysfunction (ED), which is defined as the inability to achieve or maintain an erection of sufficient rigidity for vaginal penetration and completion of the sexual act 1 2. The etiology of ED can be classified into two major categories: organic and psychological3. Major causes for organic ED include aging, particularly in men older than 50 years4–6, cardiovascular diseases such as atherosclerosis and hypertension 7, 8 diabetes 9, and radical prostatectomy10–12. These causes lead to two forms of organic ED, vasculogenic and neurogenic, which often seen together in an ED patient 13.
Although oral phosphodiesterase type 5 (PDE5) inhibitors (sildenafil, tadalafil, and vardenafil) are effective for the treatment of ED, it is not always efficacious in all patients. For example, in the clinical trials using sildenafil, the failure of treatment was often seen in men with diabetes, non-nerve sparing radical prostatectomy, and high disease severity 3, 12. For the patients with organic causes of ED, the overall response rate to sildenafil was 68%, and lower response rates were observed in diabetic patients (for type I, 59%; for type II, 64%) and in patients who had undergone prostatectomy for prostate cancer (43%) 14, 15. The response rate to sildenafil is also known to decreases as the patient age increases 16. Therefore, improved therapies based on a better understanding of the fundamental mechanisms inducing ED are needed especially when the PDE5 inhibitor treatment fails.
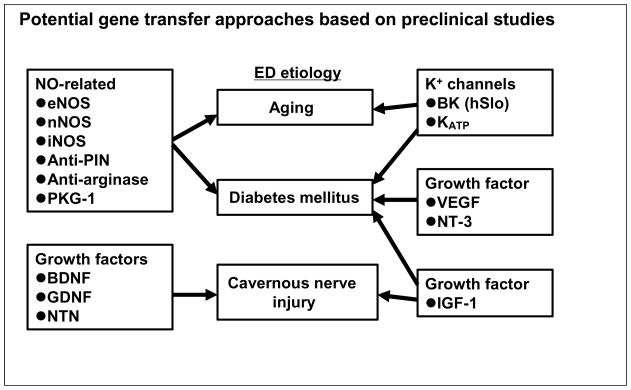
Gene-based therapy has been proposed as one of potential new therapies for PDE5-resistant ED, and preclinical studies have been performed to examine the feasibility of gene therapy for ED induced by various causes such as aging, diabetes and cavernous nerve injury. Since the nitrergic pathway principally contributes to cavernous smooth muscle relaxation, resulting in penile erection, genes involved in NO synthesis such as NO synthase (NOS) have been tested for potential gene therapies of ED. For the neurogenic type of ED induced by diabetes or cavernous nerve injury, genes encoding different types of neurotrophic factors, which can enhance nerve regeneration, have been proposed for ED gene therapy. In addition, K+ channel genes, which functionally enhance relaxation of cavernous smooth muscle, have also been tested as another category of genes for ED gene therapy. The current status of gene therapies for ED has been summarized in a recent thorough review by Harraz et al. 17. Thus, in this article, we will review and update the information on gene therapies for the management of ED, especially focusing on three major categories of gene therapies, respectively, targeting the NO system, growth factors and K+ ion channel mechanisms, including our recent studies using HSV vector-based neurotrophic factor gene therapies in rat models of ED (Fig. 1).
Figure 1. Summary of potential targets of ED gene therapy.
Potential gene transfer approaches targeting the nitric oxide (NO) system, growth factors, K+ channels that are discussed in this review are summarized. Based on the results of preclinical studies, the candidate genes for different ED etiologies such as aging, diabetes mellitus or cavernous nerve injury are connected by arrows.
2. Physiology of penile erection
Penile erection is induced by dilation of penile arteries and relaxation of smooth muscle of the corpus cavernosum that increase penile blood inflow, and venous occlusion that decreases penile blood outflow. Although the venous occlusion is achieved passively by mechanical compression of the emissary veins, smooth muscle relaxation of penile arterial and cavernous tissues is principally induced by nitric oxide (NO), which is a potent vasodilator that is generated by metabolism of arginine via neuronal NO synthase (nNOS) in the nonadrenergic, noncholinergic (NANC) nerves and endothelial NOS (eNOS) in the endothelium of penile arteries and cavernosal sinusoids 18–21. A third isoform (inducible NOS, iNOS), which is Ca2+-insensitive, also plays a role in penile erection 22. NANC nerves that release NO are parasympathetic carried through the cavernous nerves, and activated by parasympathetic nerve stimulation. Sympathetic nerve stimulation in the cavernous nerves release norepinephrine and is responsible for cavernous smooth-muscle contraction and detumescence after orgasm 23. NO produced in nerves or endothelial cells then diffuses to smooth muscle cells and activates soluble guanylate cyclase, resulting in increased cyclic guanosine monophosphate (cGMP) formation, which mediates smooth muscle relaxation 20. Other molecules such as vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide (CGRP), substance P, and pituitary adenylate cyclase-activating polypeptide (PACAP) also play important roles in penile erection 24.
3. Modulation of the nitrergic system
3.1. NOS
It has been well documented that reduced NO production due to a significant decline in NOS expression is often associated with ED induced by aging 25, 26 or diabetes 27, 28. Thus, restoration of endogenous NO synthesis and/or enhancement of the NO-related cascade in the penis have been regarded as potential treatments for ED17.
Adenoviral vector-mediated overexpression of eNOS has been examined in aged or diabetic rats 29. When recombinant adenovirus containing the eNOS gene was injected into the corpus cavernosum of aged rats, eNOS transgene expression as well as cGMP level in cavernous tissues were increased. The increase in intracavernosal pressure in response to cavernous nerve stimulation was also enhanced in eNOS-treated rats. These effects of eNOS gene therapy were detected at 1 day and 5 days after administration in aged rats injected with adenoviruses driven by the Cytomegarovirus (CMV) virus and Rous sarcoma virus (RSV) promoters, respectively 29, 30. In diabetic rats, eNOS gene transfer using adenoviral vectors is associated with an increase in eNOS protein expression and constitutive NOS activity as well as an increase in nitric oxide biosynthesis as shown by increased levels of cavernous nitrate and nitrite formation at 1–2 days after treatment. Furthermore, in eNOS-treated rats, intracavernosal pressure (ICP) after cavernosal nerve stimulation was similar to that observed in normal animals 31. In addition, the combination of adenoviral-mediated eNOS gene therapy and intravenous sildenafil in diabetic rats also resulted in a synergistic erectile response that is greater than either of these therapies 32. The efficacy of eNOS gene therapy was also tested using mesenchymal stem cells (MSC) transduced with adenovirus containing the eNOS gene. When eNOS-modified MSC were injected into the penis of aged rats, the erectile response was improved in association with increased eNOS protein levels, NOS activity, and cGMP levels in corporal tissues at 7 and 21 days after injection 33.
In addition to eNOS, gene delivery of other NOS subtypes such as nNOS and iNOS into the penis also improve erectile function in rats. Magee et al. reported that the adenovirus-mediated gene transfer of an nNOS variant responsible for erection, penile nNOS was effective in stimulating the erection of aged rats at 18 days when given by electroporation, without inducing the expression of cytotoxic genes34. In terms of iNOS gene therapy, plasmid mediated gene transfer of iNOS has been shown to increase ICP during cavernosal nerve stimulation and enhances NOS activity in aged rats at 10 days after treatment 35. Another study using transfection of the iNOS gene via plasmid vectors, adenoviral vectors, or adenovirus-transduced myoblast cells in healthy rats has shown that myoblast mediated transfer produced superior erectile response to cavernous nerve stimulation with enhanced iNOS activity in the cavernous tissue compared with animals treated with adenoviral or plasmid vectors alone 36.
3.2. Other gene therapies targeting the nitrergic mechanisms
3.2.1. Protein Inhibitor of NOS (PIN)
In the rat, the protein inhibitor of NOS (PIN), which binds to nNOS to suppress its activity, is expressed in the pelvic ganglion as well as the cavernosal and dorsal nerve of the penis 37. When plasmid constructs for short hairpin RNA (shRNA) targeting PIN were injected into the penile corpus cavernosum of aged rats, an increase in intracavernosal pressure following electrical field stimulation of the cavernous nerve was raised above the value of young rats 1 month after the injection38. In addition, PIN mRNA and protein expression in penile tissues was decreased by >70% by the shRNA. Thus the antisense PIN gene therapy, which can enhance the nitrergic mechanism, could ameliorate ED in the aged rat38.
3.2.2. Anti-arginase
It has been reported that NO production can be controlled not only by activities of NOS isozymes but also by activities of arginase isozymes and that inhibition of arginase activity can enhance NO-dependent relaxation of smooth muscle tissue in human penile corpus cavernosum39. It is also known that arginase is upregulated in the cavernous tissues of diabetic humans and animals40, 41. Gene transfer of anti-sense arginase via an adenoviral vector reportedly decreases arginase protein and mRNA in the penile tissues of aged mice, in association with improved cGMP production and erectile function in response to cavernous nerve stimulation 41.
3.2.3. cGMP-dependent protein kinase G1 (PKG1)
The release of NO induces elevation of cGMP levels via stimulation of guanylate cyclase activity, which leads to relaxation of smooth muscle tissue in penile corpus cavernosum, resulting in penile erection. One of the effector proteins for cGMP is cGMP-dependent protein kinase-1 (PKG-1), and PKG-1 knockout mice exhibit ED 42. It has also been shown that PKG1 is reduced in the corpus cavernosum smooth muscle of diabetic rabbits and rats 43, 44. Adenoviral-mediated transfer of the PKG-1α gene into the penile corpus cavernosum of diabetic rats restores PKG activity to the levels observed in control rats and improved erectile function during cavernous nerve electrostimulation 44.
4. Growth factors
4.1 Neurotrophin-3 (NT3)
Previous studies suggest autonomic neuropathy as the primary etiology for ED in men with diabetes 45, 46. A significant decrease in NOS containing nerve fibers in the dorsal and intracavernous nerves is found in streptozotocin (STZ)-induced diabetic rats 47. Neurotrophin-3 (NT-3) is a member of the neurotrophin gene family, and Lin et al. reported that male rat major pelvic ganglia (MPG) cultures treated with NT3 induced the most fiber outgrowth48.
The study by Bennett et al. investigated whether herpes simplex virus (HSV) vector-mediated NT-3 delivery is applicable for the treatment of ED induced by diabetes in rats49. After 4 weeks, replication defective HSV vector expressing the β-galactosidase gene (HSV-LacZ) or NT-3 (HSV-NT-3) were injected directly into the cavernous nerve sheath. Four weeks later the animals underwent measurement of intracavernous pressure (ICP) during cavernous nerve electrostimulation. Staining for lacZ and NOS in the major pelvic ganglia was also performed. β-Galactosidase staining revealed lacZ positive neurons in the MPG.. Maximal ICP of the HSV-NT3 treated group was significantly higher than that of the HSV-LacZ treated group. The mean number of NOS positive neurons per section in the NT3 group was significantly higher than that in the lacZ control group. These data suggest that HSV vectors encoding neurotrophic factors such as NT3 might facilitate cavernous nerve regeneration/repair/survival and increased nNOS expression in pelvic ganglion neurons to restore erectile function in diabetic rats49.
4.2. Glial cell derived neurotrophic factor (GDNF) and neurturin (NTN)
ED resulting from cavernous nerve injury is a major complication following extirpative surgery of pelvic organs such as the prostate, bladder and rectum. ED is an exceedingly common side effect in men receiving radical prostatectomy50. Surgical trauma associated with removing the prostate where neurovascular bundles run alongside can produce neuropraxia, local inflammation and other immune responses that can ultimately lead to ED. Even with nerve-sparing surgery, it takes many months to recover erectile function11, 12.
Glial cell line-derived neurotrophic factor (GDNF) family ligand such as GDNF and neurturin (NTN) is known to be important for survival and regeneration of cavernous nerves51, 52. GDNF is a member of the transforming growth factor-b (TGF-β) superfamily and is well known for its activities in promoting the survival and extension of sympathetic and parasympathetic axons 53. GDNF is also known to be expressed in the penis of adult rats and retrogradely transported in penile parasympathetic and sensory nerves51, 52. Recent reports also demonstrate that GDNF family receptors are expressed in penis-projecting neurons and they are involved in the survival and regeneration of these neurons51, 54. Neurturin (NTN), another member of GDNF family, has also been known as a target-derived survival and/or neuritogenic factor for postganglionic neurons innervating the penis 51, 54–56. Laurikainen et al. showed that NTN mRNA was expressed in smooth muscle of penile blood vessels and the corpus cavernosum in adult rats, whereas glial cell line-derived neurotrophic factor receptor α 2 (GFRα2) and Ret (common GDNF family receptor) mRNAs were expressed in all cell bodies of the penile postganglionic neurons 55. Furthermore, mice lacking the GFRα2 receptor have significantly less NOS-containing nerve fibers, which are responsible for penile erection, in the dorsal penile and cavernous nerves 55. Other studies also demonstrated the role of the NTN-GFRα2 pathway in survival and regeneration for sacral parasympathetic neurons, especially for those innervating the penis, by examining their signaling pathways in these neurons51, 54, 56. In addition, GFRα2 and nNOS expression both decreased with age, suggesting the possible involvement of a NTN-GFRα2 pathway in the age-related alteration of nNOS expression57. A recent report also showed that treatment with neurturin protein at the site of cavernous nerve crush injury facilitated recovery of erectile function58.
Based on these data, recent studies have shown the feasibility of HSV vector-mediated neurotrophic factor delivery for the treatment of ED induced by cavernous nerve injury in rats 59, 60. GDNF and NTN were used as penile neurotrophic factors in these studies, and erectile function by measurement of ICP along with arterial pressure and nerve regeneration by a retrograde tracing study using Fluorogold (FG) following the nerve injury and the HSV vector administration was investigated.
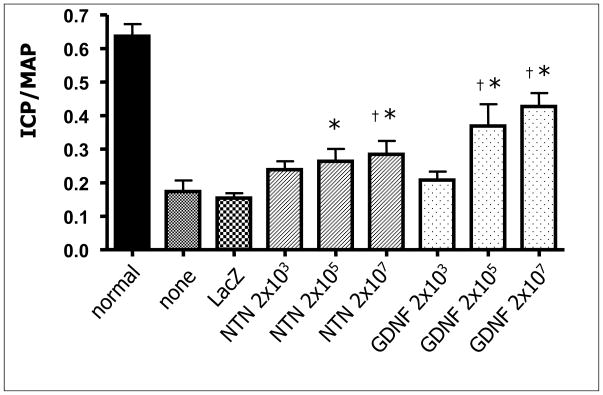
In male rats with bilateral cavernous nerve injury induced by using a clamp and dry ice without transection, the effecte of HSV-GDNF and HSV-NTN (1.0 × 105, 107 and 109 pfu) administered around the damaged nerve after the injury were evaluated. HSV vector expressing green fluorescent protein (GFP) and LacZ (HSV-LacZ) were used as a control vector. ICP during pelvic nerve stimulation was measured at 2 and 4 weeks after the nerve injury. FG was injected into the penile crus 7 days before histological experiments. Approximately 60% of MPG cells were GFP-positive after the viral administration, indicating that the inserted genes were well transported to MPG. ICP of the groups which received the high titer HSV-GDNF and HSV-NTN exhibited significant recovery of ICP compared with the HSV-LacZ group or HSV-untreated group at 4 weeks after the nerve injury (Fig. 2). However, since the post-treatment ICP value did not reach the control level, further studies may be needed to evaluate whether the increased ICP level after GDNF or NTN gene transfer would be sufficient for intercourse. The high titer HSV-GDNF and HSV-NTN groups had more FG-positive cells in MPG than the HSV-LacZ group, indicating that delivery of these vectors is capable of rescuing the nerve damage 59, 60. These data suggest that administration of HSV vector-mediated neurotrophic factors such as GDNF or NTN around injured cavernous nerves could hasten the process of nerve regeneration and promote the recovery of erectile function after cavernous nerve injury.
Figure 2. Intracavernous pressure (ICP) responses at 4 weeks after the bilateral cavernous nerve injury.
Rats treated with higher titers (2×105 and 107 pfu) of HSV-GDNF or NTN exhibited significant recovery of ICP along with mean arterial pressure (ICP/MAP) compared with the HSV-LacZ control vector group or HSV-untreated nerve-injured group at 4 weeks after the nerve injury. Asterisk indicates P<0.05 versus “none” (virus-untreated group) by student’s t-test and ANOVA. Cross mark indicates P<0.05 versus “LacZ” (HSV-LacZ group) by student’s t-test and ANOVA.
4.3. Brain derived neurotrophic factor (BDNF)
Brain derived neurotrophic factor (BDNF) is known to enhance nerve growth. In rats with cavernous nerve injury induced by freezing, adenoviral vector-mediated BDNF gene transfer into the corpus cavernosum exhibited significantly higher ICP during cavernous nerve stimulation and had greater nNOS staining in major pelvic ganglion neurons compared with the control gene (LacZ)-treated group 61. It is also shown that adenoviral BDNF gene transfer is effective to restore erectile function in response to cavernous nerve stimulation and reduce neural and vascular damages of cavernous tissues in a rat model of hypercholesterolemia induced by high fat diet 62.
4.4. Vascular Endothelial derived Growth Factor (VEGF)
Vascular endothelial growth factor (VEGF) is a multifunctional protein that stimulates angiogenesis and increases vascular permeability. Previous studies showed that VEGF treatment could restore erectile function in animal models of ED such as hypercholesterolemic rabbits and diabetic rats62–65. In diabetic rats, transfection with DNA encoding VEGF into the corpus cavernosum showed increased smooth muscle content as well as improved ICP during cavernous nerve electrostimulation compared to controls 66. In another study using diabetic rats, adenovirus vector mediated gene transfer VEGF and another angiogenic factor, angiopoietin-1, into the corpus cavernosum has been demonstrated to completely restore erectile function during electrical stimulation of the cavernous nerve in association with increased angiogenesis, eNOS phosphorylation, and cGMP expression in cavernous tissues 2 and 8 weeks after treatment while intracavernous injection of either angiopoietin or VEGF adenoviral vector alone elicited partial improvement 67. Intracorporal treatment with adenoviral VEGF has also been shown to prevent castration-associated ED in rats68.
4.5. Insulin-like growth factor-1 (IGF-1)
It has been demonstrated that IGF-I can enhance regeneration of NOS-containing nerves after cavernous nerve injury in rats 69, 70. In addition, expression of IGF-1 has been shown to be reduced in cavernous tissues of diabetic rats71. Adenoviral vector-mediated gene transfer of IGF-1 into the corpus cavernosum in diabetic rats reportedly improves erectile function during cavernous nerve stimulation in association with increases expression of IGF-1 mRNA and protein in cavernous tissues 72.
5. Modulation of K+ channels
K+ ion channels are important to stabilize the membrane potential and reduce the excitability of nerves and muscle cells including penile smooth muscle cells 73, 74. Thus the feasibility of gene transfer of K+ channels for the treatment of ED has been investigated in animals as well as humans.
5.1. Large-conductance Ca2+-activated potassium channel (BK or Maxi-K)
Ca2+ activated K+ channels are one of major groups of six/seven transmembrane potassium-selective channels. These channels are divided into several groups according to the genetic, electrical and structural properties of each channel75. Large-conductance, Ca2+ activated K+ (BK or Maxi-K) channels, which are activated by membrane potential depolarization, increases in cytosolic Ca2+ concentration and/or NO/cGMP mediated mechanisms, contribute to hyperpolarization of smooth muscle cell membranes, resulting in a decrease in calcium influx and penile corporeal muscle relaxation75–77. Several previous studies by the Christ and Melman’s group investigated the efficacy of intracavernous gene transfer of naked hSlo cDNA that encodes the human BK channel α-subunit for the treatment of ED. The hSlo cDNA was inserted into a mammalian plasmid, where expression is driven by the cytomegalovirus promoter. The plasmid cannot replicate itself, but is designed to efficiently replicate the inserted DNA sequence in the presence of the appropriate enzymes and substrates in the host nucleus. The plasmid containing the desired DNA sequence (i.e., hSlo DNA) enters the nucleus of the host cell, and transcribe the desired mRNA, leading to production of a functional BK channel protein 78. In aged or diabetic rats, intracavernous injection of the plasmid containing hSlo DNA induced significant elevation in ICP in response to cavernous nerve stimulation in association with the overexpression of hSlo in cavernous tissues for up to 4 months after the treatment79–81. More recently, the same group has reported that intracavernous injection of a smooth-muscle-specific gene transfer vector (pSMAA-hSlo) encoding the pore-forming α-subunit of the human large-conductance, Ca2+-sensitive K+ (BK) channel also induces increased ICP response to cavernous nerve stimulation in aged rats82 or an improvement of sexual behavior and papaverine-induced erectile responses in male cynomolgus monkeys with ED secondary to diet-induced atherosclerosis83.
To date, a phase 1 safety clinical trial using the plasmid containing hSlo DNA has been completed, and this is the first and only clinical trial of gene therapy for humans with ED 84, 85. The trial was an open-label, sequential, single-dose, intracavernous instillation of seven doses of the hSlo plasmid in 20 men with moderate-to-severe erectile dysfunction as defined by the international index of erectile function (IIEF) score. The recent review by Melman et al. 86 summarized the encouraging results of the trial as follows: (1) of greatest importance in a phase 1 trial, that is, the primary endpoint, safety, showed no serious short or long-term (2 years) transfer-related adverse events, (2) there was no plasmid evident in the semen of the participants, (3) there were no clinically relevant changes in any of the physical or chemical parameters measured including ECG. and (4) in two of the men who responded with improved erections after transfer, the response lasted for 6 months as in the preclinical trials in aged or diabetic male rats.
5.2. ATP-sensitive K+ channels (KATP)
ATP-sensitive K+ channel (KATP) is also involved in the control of smooth muscle tone. A previous study has shown using isolated penile corporal tissue strips from diabetic patients have shown a significant decrease in the relaxation responses to KATP channel modulators such as pinacidil and levcromakalim 87. Thus a recent study examined the efficacy of intracavernous injection with naked cDNA of the KATP channel gene (Kir6.1 + SUR2B or Kir6.2 + SUR2B) for erectile function in aged rats. The KATP channel gene transfer enhanced ICP responses to cavernous nerve stimulation compared to age-matched control rats, which were associated with an increase in cavernosal levels of K KATP channel mRNA (Kir 6.1 and 6.2)88.
6. Viral vs. non-viral gene therapy
Both non-viral and viral delivery vehicles have been employed to deliver genes to the penis and other tissues. Each delivery system has its own advantages and disadvantages for delivery to different target cells and tissues. Overall, a major advantage of the non-viral delivery systems has been the low immunogenicity of this approach compared to viral vectors which all show some level host response to the vector itself which may only amplify any host response to the therapeutic gene. In addition, the non-viral systems generally exhibit the short-term nature of vector-delivered transgene expression due to the limited maintenance of the delivery vehicle in the transduced cells. On the other hand, viral vectors are more efficient at delivering their genetic payload to the target cell compared to non-viral systems because over millennia viruses have acquired efficient methods to deliver their own genetic material to cells in order to replicate their genomes and further propagate themselves. Another advantage is that most viral vectors can be readily produced and purified for in vivo gene transfer while the non-viral methods are limited by production of sufficient quantities of DNA for transduction 89. However there are potential risks with viral vectors that have limited the use of viral vectors in humans, such as endogenous viral recombination, cancer development and immunological reactions90.
In addition, HSV vectors have several significant advantages over other viral vectors for the treatment of peripheral nervous system disorders. Replication-defective recombinant vectors, which lack multiple essential gene functions and are non-toxic in vivo91–94 have been generated to increase the overall safety for clinical therapeutic applications. These vectors can be prepared to high titer and purity without contamination from wild-type 95–97. Thus, delivery of the replication defective HSV vector to neurons innervating the site of vector delivery at the time of surgery could reduce the complications associated with systemic delivery of trophic factors, as the vector would express the neurotrophin solely within the targeted neurons 59, 60.
7. Expert opinion
The high success rate of treating ED has been reported because of the recent advances of new oral therapies including PDE5 inhibitors. However, there are still a significant number of ED patients who do not respond to currently available treatment modalities, especially severe ED cases with diabetes, aging or cavernous nerve injury, for whom new treatment alternatives are of great demand. Thus the possibility of gene therapy application for ED has been explored in the last decade (Fig. 1). However, there are significant safety concerns that delay the clinical translation of gene therapy modalities to human patients. The recent clinical study using non-viral gene therapy of the Ca2+ activated BK (Max-K) channel for ED patients shows great promise for future development of gene-based therapy of ED. In addition, the phase I safety studies using non-replicating HSV vectors encoding human enkephalin, an opiate gene, are underway in patients with cancer pain to elucidate the safety of locally applied viral gene therapy98, 99. Thus it is hoped that the long-term safety of both viral and non-viral gene therapy can be established in near future before large scale human studies are considered. Overall, gene therapy for ED represents an exciting future treatment option, especially for people with severe ED unresponsive to current first-line therapies such as PDE5 inhibitors.
References
- 1.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999 Jul;84(1):50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 2.McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000 Oct;12( Suppl 4):S6–S11. doi: 10.1038/sj.ijir.3900567. [DOI] [PubMed] [Google Scholar]
- 3.Deng W, Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ. Gene and stem cell therapy for erectile dysfunction. Int J Impot Res. 2005 Dec;17( Suppl 1):S57–63. doi: 10.1038/sj.ijir.3901430. [DOI] [PubMed] [Google Scholar]
- 4.Seftel AD. Erectile dysfunction in the elderly: epidemiology, etiology and approaches to treatment. J Urol. 2003 Jun;169(6):1999–2007. doi: 10.1097/01.ju.0000067820.86347.95. [DOI] [PubMed] [Google Scholar]
- 5.Montorsi F, Briganti A, Salonia A, Deho F, Zanni G, Cestari A, et al. The ageing male and erectile dysfunction. BJU Int. 2003 Sep;92(5):516–20. doi: 10.1046/j.1464-410x.2003.04378.x. [DOI] [PubMed] [Google Scholar]
- 6.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000 Feb;163(2):460–3. [PubMed] [Google Scholar]
- 7.Kloner RA. Erectile dysfunction in the cardiac patient. Curr Urol Rep. 2003 Dec;4(6):466–71. doi: 10.1007/s11934-003-0028-9. [DOI] [PubMed] [Google Scholar]
- 8.Kloner RA, Speakman M. Erectile dysfunction and atherosclerosis. Curr Atheroscler Rep. 2002 Sep;4(5):397–401. doi: 10.1007/s11883-002-0078-3. [DOI] [PubMed] [Google Scholar]
- 9.Richardson D, Vinik A. Etiology and treatment of erectile failure in diabetes mellitus. Curr Diab Rep. 2002 Dec;2(6):501–9. doi: 10.1007/s11892-002-0120-4. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AL. Strategies to promote recovery of cavernous nerve function after radical prostatectomy. World J Urol. 2003 May;20(6):337–42. doi: 10.1007/s00345-002-0303-2. [DOI] [PubMed] [Google Scholar]
- 11.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000 Dec;164(6):1929–34. [PubMed] [Google Scholar]
- 12.Shimizu T, Hisasue S, Sato Y, Kato R, Kobayashi K, Tsukamoto T. Erectile dysfunction following nerve-sparing radical retropubic prostatectomy and its treatment with sildenafil. Int J Urol. 2005 Jun;12(6):552–7. doi: 10.1111/j.1442-2042.2005.01088.x. [DOI] [PubMed] [Google Scholar]
- 13.Siroky MB, Azadzoi KM. Vasculogenic erectile dysfunction: newer therapeutic strategies. J Urol. 2003 Aug;170(2 Pt 2):S24–9. doi: 10.1097/01.ju.0000075361.35942.17. discussion S9–30. [DOI] [PubMed] [Google Scholar]
- 14.Boulton AJ, Selam JL, Sweeney M, Ziegler D. Sildenafil citrate for the treatment of erectile dysfunction in men with Type II diabetes mellitus. Diabetologia. 2001 Oct;44(10):1296–301. doi: 10.1007/s001250100656. [DOI] [PubMed] [Google Scholar]
- 15.Briganti A, Salonia A, Deho F, Zanni G, Barbieri L, Rigatti P, et al. Clinical update on phosphodiesterase type-5 inhibitors for erectile dysfunction. World J Urol. 2005 Dec;23(6):374–84. doi: 10.1007/s00345-005-0022-6. [DOI] [PubMed] [Google Scholar]
- 16.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999 Feb 3;281(5):421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 17.Harraz A, Shindel AW, Lue TF. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol. 2010 Mar;7(3):143–52. doi: 10.1038/nrurol.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992 Jan 9;326(2):90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 19.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 20.Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005 May;106(2):233–66. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Feifer A, Carrier S. Pharmacotherapy for erectile dysfunction. Expert Opin Investig Drugs. 2008 May;17(5):679–90. doi: 10.1517/13543784.17.5.679. [DOI] [PubMed] [Google Scholar]
- 22.Hung A, Vernet D, Xie Y, Rajavashisth T, Rodriguez JA, Rajfer J, et al. Expression of inducible nitric oxide synthase in smooth muscle cells from rat penile corpora cavernosa. J Androl. 1995 Nov-Dec;16(6):469–81. [PubMed] [Google Scholar]
- 23.Saenz de Tejada I, Blanco R, Goldstein I, Azadzoi K, de las Morenas A, Krane RJ, et al. Cholinergic neurotransmission in human corpus cavernosum. I. Responses of isolated tissue. Am J Physiol. 1988 Mar;254(3 Pt 2):H459–67. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004 Nov 15;83(2):189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Garban H, Vernet D, Freedman A, Rajfer J, Gonzalez-Cadavid N. Effect of aging on nitric oxide-mediated penile erection in rats. Am J Physiol. 1995 Jan;268(1 Pt 2):H467–75. doi: 10.1152/ajpheart.1995.268.1.H467. [DOI] [PubMed] [Google Scholar]
- 26.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20(7):527–50. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- 27.Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–30. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- 28.Lue TF. Erectile dysfunction. N Engl J Med. 2000 Jun 15;342(24):1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 29.Champion HC, Bivalacqua TJ, Hyman AL, Ignarro LJ, Hellstrom WJ, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):11648–52. doi: 10.1073/pnas.96.20.11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bivalacqua TJ, Champion HC, Mehta YS, Abdel-Mageed AB, Sikka SC, Ignarro LJ, et al. Adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to the penis improves age-related erectile dysfunction in the rat. Int J Impot Res. 2000 Sep;12( Suppl 3):S8–17. doi: 10.1038/sj.ijir.3900556. [DOI] [PubMed] [Google Scholar]
- 31.Bivalacqua TJ, Usta MF, Champion HC, Adams D, Namara DB, Abdel-Mageed AB, et al. Gene transfer of endothelial nitric oxide synthase partially restores nitric oxide synthesis and erectile function in streptozotocin diabetic rats. J Urol. 2003 May;169(5):1911–7. doi: 10.1097/01.ju.0000051881.14239.4a. [DOI] [PubMed] [Google Scholar]
- 32.Bivalacqua TJ, Usta MF, Champion HC, Leungwattanakij S, Dabisch PA, McNamara DB, et al. Effect of combination endothelial nitric oxide synthase gene therapy and sildenafil on erectile function in diabetic rats. Int J Impot Res. 2004 Feb;16(1):21–9. doi: 10.1038/sj.ijir.3901054. [DOI] [PubMed] [Google Scholar]
- 33.Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007 Mar;292(3):H1278–90. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- 34.Magee TR, Ferrini M, Garban HJ, Vernet D, Mitani K, Rajfer J, et al. Gene therapy of erectile dysfunction in the rat with penile neuronal nitric oxide synthase. Biol Reprod. 2002 Jul;67(1):20–8. doi: 10.1093/biolreprod/67.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Garban H, Marquez D, Magee T, Moody J, Rajavashisth T, Rodriguez JA, et al. Cloning of rat and human inducible penile nitric oxide synthase. Application for gene therapy of erectile dysfunction. Biol Reprod. 1997 Apr;56(4):954–63. doi: 10.1095/biolreprod56.4.954. [DOI] [PubMed] [Google Scholar]
- 36.Chancellor MB, Tirney S, Mattes CE, Tzeng E, Birder LA, Kanai AJ, et al. Nitric oxide synthase gene transfer for erectile dysfunction in a rat model. BJU Int. 2003 May;91(7):691–6. doi: 10.1046/j.1464-410x.2003.04219.x. [DOI] [PubMed] [Google Scholar]
- 37.Magee TR, Ferrini MG, Davila HH, Zeller CB, Vernet D, Sun J, et al. Protein inhibitor of nitric oxide synthase (NOS) and the N-methyl-D-aspartate receptor are expressed in the rat and mouse penile nerves and colocalize with penile neuronal NOS. Biol Reprod. 2003 Feb;68(2):478–88. doi: 10.1095/biolreprod.102.007310. [DOI] [PubMed] [Google Scholar]
- 38.Magee TR, Kovanecz I, Davila HH, Ferrini MG, Cantini L, Vernet D, et al. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (Protein Inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J Sex Med. 2007 May;4(3):633–43. doi: 10.1111/j.1743-6109.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim NN, Cox JD, Baggio RF, Emig FA, Mistry SK, Harper SL, et al. Probing erectile function: S-(2-boronoethyl)-L-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry. 2001 Mar 6;40(9):2678–88. doi: 10.1021/bi002317h. [DOI] [PubMed] [Google Scholar]
- 40.Bivalacqua TJ, Hellstrom WJ. Potential application of gene therapy for the treatment of erectile dysfunction. J Androl. 2001 Mar-Apr;22(2):183–90. [PubMed] [Google Scholar]
- 41.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007 Mar;292(3):H1340–51. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 42.Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000 Feb 29;97(5):2349–54. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang S, Hypolite JA, Velez M, Changolkar A, Wein AJ, Chacko S, et al. Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am J Physiol Regul Integr Comp Physiol. 2004 Oct;287(4):R950–60. doi: 10.1152/ajpregu.00639.2003. [DOI] [PubMed] [Google Scholar]
- 44.Bivalacqua TJ, Kendirci M, Champion HC, Hellstrom WJ, Andersson KE, Hedlund P. Dysregulation of cGMP-dependent protein kinase 1 (PKG-1) impairs erectile function in diabetic rats: influence of in vivo gene therapy of PKG1alpha. BJU Int. 2007 Jun;99(6):1488–94. doi: 10.1111/j.1464-410X.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- 45.Melman A, Gingell JC. The epidemiology and pathophysiology of erectile dysfunction. J Urol. 1999 Jan;161(1):5–11. [PubMed] [Google Scholar]
- 46.Bemelmans BL, Meuleman EJ, Doesburg WH, Notermans SL, Debruyne FM. Erectile dysfunction in diabetic men: the neurological factor revisited. J Urol. 1994 Apr;151(4):884–9. doi: 10.1016/s0022-5347(17)35113-3. [DOI] [PubMed] [Google Scholar]
- 47.Dahiya R, Chui R, Perinchery G, Nakajima K, Oh BR, Lue TF. Differential gene expression of growth factors in young and old rat penile tissues is associated with erectile dysfunction. Int J Impot Res. 1999 Aug;11(4):201–6. doi: 10.1038/sj.ijir.3900405. [DOI] [PubMed] [Google Scholar]
- 48.Lin G, Chen KC, Hsieh PS, Yeh CH, Lue TF, Lin CS. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 2003 Oct;92(6):631–5. doi: 10.1046/j.1464-410x.2003.04439.x. [DOI] [PubMed] [Google Scholar]
- 49.Bennett NE, Kim JH, Wolfe DP, Sasaki K, Yoshimura N, Goins WF, et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J Urol. 2005 May;173(5):1820–4. doi: 10.1097/01.ju.0000158056.66236.1f. [DOI] [PubMed] [Google Scholar]
- 50.Talcott JA, Rieker P, Propert KJ, Clark JA, Wishnow KI, Loughlin KR, et al. Patient-reported impotence and incontinence after nerve-sparing radical prostatectomy. J Natl Cancer Inst. 1997 Aug 6;89(15):1117–23. doi: 10.1093/jnci/89.15.1117. [DOI] [PubMed] [Google Scholar]
- 51.Palma CA, Keast JR. Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci. 2006;7:41. doi: 10.1186/1471-2202-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurikainen A, Hiltunen JO, Vanhatalo S, Klinge E, Saarma M. Glial cell line-derived neurotrophic factor is expressed in penis of adult rat and retrogradely transported in penile parasympathetic and sensory nerves. Cell Tissue Res. 2000 Dec;302(3):321–9. doi: 10.1007/s004410000273. [DOI] [PubMed] [Google Scholar]
- 53.Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002 Oct;12(5):523–31. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 54.Wanigasekara Y, Keast JR. Neurturin has multiple neurotrophic effects on adult rat sacral parasympathetic ganglion neurons. Eur J Neurosci. 2005 Aug;22(3):595–604. doi: 10.1111/j.1460-9568.2005.04260.x. [DOI] [PubMed] [Google Scholar]
- 55.Laurikainen A, Hiltunen JO, Thomas-Crusells J, Vanhatalo S, Arumae U, Airaksinen MS, et al. Neurturin is a neurotrophic factor for penile parasympathetic neurons in adult rat. J Neurobiol. 2000 May;43(2):198–205. doi: 10.1002/(sici)1097-4695(200005)43:2<198::aid-neu9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 56.Wanigasekara Y, Airaksinen MS, Heuckeroth RO, Milbrandt J, Keast JR. Neurturin signalling via GFRalpha2 is essential for innervation of glandular but not muscle targets of sacral parasympathetic ganglion neurons. Mol Cell Neurosci. 2004 Feb;25(2):288–300. doi: 10.1016/j.mcn.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Hisasue S, Kato R, Suetomi T, Kato K, Suzuki K, Kobayashi K, et al. Age-related alteration of neurturin receptor GFRa2 and nNOS in pelvic ganglia. Neurobiol Aging. 2006 Oct;27(10):1524–30. doi: 10.1016/j.neurobiolaging.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Bella AJ, Fandel TM, Tantiwongse K, Brant WO, Klein RD, Garcia CA, et al. Neurturin enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Brachial Plex Peripher Nerve Inj. 2007;2:5. doi: 10.1186/1749-7221-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kato R, Wolfe D, Coyle CH, Huang S, Wechuck JB, Goins WF, et al. Herpes simplex virus vector-mediated delivery of glial cell line-derived neurotrophic factor rescues erectile dysfunction following cavernous nerve injury. Gene Ther. 2007 Sep;14(18):1344–52. doi: 10.1038/sj.gt.3302990. [DOI] [PubMed] [Google Scholar]
- 60.Kato R, Wolfe D, Coyle CH, Wechuck JB, Tyagi P, Tsukamoto T, et al. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 2009 Jan;16(1):26–33. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, Lue TF. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001 Jun;165(6 Pt 1):2103–9. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 62.Gholami SS, Rogers R, Chang J, Ho HC, Grazziottin T, Lin CS, et al. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J Urol. 2003 Apr;169(4):1577–81. doi: 10.1097/01.ju.0000055120.73261.76. [DOI] [PubMed] [Google Scholar]
- 63.Shirai M, Yamanaka M, Shiina H, Igawa M, Kawakami T, Ishii N, et al. Vascular endothelial growth factor restores erectile function through modulation of the insulin-like growth factor system and sex hormone receptors in diabetic rat. Biochem Biophys Res Commun. 2006 Mar 17;341(3):755–62. doi: 10.1016/j.bbrc.2005.12.226. [DOI] [PubMed] [Google Scholar]
- 64.Yamanaka M, Shirai M, Shiina H, Tanaka Y, Enokida H, Tsujimura A, et al. Vascular endothelial growth factor restores erectile function through inhibition of apoptosis in diabetic rat penile crura. J Urol. 2005 Jan;173(1):318–23. doi: 10.1097/01.ju.0000141586.46822.44. [DOI] [PubMed] [Google Scholar]
- 65.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998 May 22;273(21):13313–6. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 66.Dall’Era JE, Meacham RB, Mills JN, Koul S, Carlsen SN, Myers JB, et al. Vascular endothelial growth factor (VEGF) gene therapy using a nonviral gene delivery system improves erectile function in a diabetic rat model. Int J Impot Res. 2008 May-Jun;20(3):307–14. doi: 10.1038/ijir.2008.1. [DOI] [PubMed] [Google Scholar]
- 67.Ryu JK, Cho CH, Shin HY, Song SU, Oh SM, Lee M, et al. Combined angiopoietin-1 and vascular endothelial growth factor gene transfer restores cavernous angiogenesis and erectile function in a rat model of hypercholesterolemia. Mol Ther. 2006 Apr;13(4):705–15. doi: 10.1016/j.ymthe.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 68.Rogers RS, Graziottin TM, Lin CS, Kan YW, Lue TF. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res. 2003 Feb;15(1):26–37. doi: 10.1038/sj.ijir.3900943. [DOI] [PubMed] [Google Scholar]
- 69.Jung GW, Kwak JY, Yoon S, Yoon JH, Lue TF. IGF-I and TGF-beta2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int J Impot Res. 1999 Oct;11(5):247–59. doi: 10.1038/sj.ijir.3900402. [DOI] [PubMed] [Google Scholar]
- 70.Bochinski D, Hsieh PS, Nunes L, Lin GT, Lin CS, Spencer EM, et al. Effect of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 complex in cavernous nerve cryoablation. Int J Impot Res. 2004 Oct;16(5):418–23. doi: 10.1038/sj.ijir.3901190. [DOI] [PubMed] [Google Scholar]
- 71.El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999 Jun;11(3):123–32. doi: 10.1038/sj.ijir.3900392. [DOI] [PubMed] [Google Scholar]
- 72.Pu XY, Hu LQ, Wang HP, Luo YX, Wang XH. Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian J Androl. 2007 Jan;9(1):83–91. doi: 10.1111/j.1745-7262.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 73.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006 Dec;7(12):921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 74.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995 Jan;75(1):191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 75.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005 Dec;57(4):463–72. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 76.Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol. 2002 Jun;167(6):2628–35. [PubMed] [Google Scholar]
- 77.Fan SF, Brink PR, Melman A, Christ GJ. An analysis of the Maxi-K+ (KCa) channel in cultured human corporal smooth muscle cells. J Urol. 1995 Mar;153(3 Pt 1):818–25. [PubMed] [Google Scholar]
- 78.Lau DH, Kommu SS, Siddiqui EJ, Thompson CS, Morgan RJ, Mikhailidis DP, et al. Gene therapy and erectile dysfunction: the current status. Asian J Androl. 2007 Jan;9(1):8–15. doi: 10.1111/j.1745-7262.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 79.Christ GJ, Rehman J, Day N, Salkoff L, Valcic M, Melman A, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998 Aug;275(2 Pt 2):H600–8. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- 80.Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004 Oct;287(4):H1544–53. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 81.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003 Jul;170(1):285–90. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 82.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008 Mar;15(5):364–70. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 83.Christ GJ, Andersson KE, Williams K, Zhao W, D’Agostino R, Jr, Kaplan J, et al. Smooth-Muscle-Specific Gene Transfer with the Human Maxi-K Channel Improves Erectile Function and Enhances Sexual Behavior in Atherosclerotic Cynomolgus Monkeys. Eur Urol. 2009 Dec 25;52:1055–66. doi: 10.1016/j.eururo.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006 Dec;17(12):1165–76. doi: 10.1089/hum.2006.17.1165. [DOI] [PubMed] [Google Scholar]
- 85.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: results of a phase I trial. Isr Med Assoc J. 2007 Mar;9(3):143–6. [PubMed] [Google Scholar]
- 86.Melman A, Rojas L, Christ G. Gene transfer for erectile dysfunction: will this novel therapy be accepted by urologists? Curr Opin Urol. 2009 Nov;19(6):595–600. doi: 10.1097/MOU.0b013e3283314985. [DOI] [PubMed] [Google Scholar]
- 87.Venkateswarlu K, Giraldi A, Zhao W, Wang HZ, Melman A, Spektor M, et al. Potassium channels and human corporeal smooth muscle cell tone: diabetes and relaxation of human corpus cavernosum smooth muscle by adenosine triphosphate sensitive potassium channel openers. J Urol. 2002 Jul;168(1):355–61. [PubMed] [Google Scholar]
- 88.So I, Chae MR, Lee SW. Gene transfer of the K(ATP) channel restores age-related erectile dysfunction in rats. BJU Int. 2007 Nov;100(5):1154–60. doi: 10.1111/j.1464-410X.2007.07050.x. [DOI] [PubMed] [Google Scholar]
- 89.Goins WF, Goss JR, Chancellor MB, de Groat WC, Glorioso JC, Yoshimura N. Herpes simplex virus vector-mediated gene delivery for the treatment of lower urinary tract pain. Gene Ther. 2009 Apr;16(4):558–69. doi: 10.1038/gt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gur S, Kadowitz PJ, Hellstrom WJ. A review of current progress in gene and stem cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2008 Oct;8(10):1521–38. doi: 10.1517/14712598.8.10.1521. [DOI] [PubMed] [Google Scholar]
- 91.Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997 Jun;71(6):4614–25. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Samaniego LA, Webb AL, DeLuca NA. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995 Sep;69(9):5705–15. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marconi P, Krisky D, Oligino T, Poliani PL, Ramakrishnan R, Goins WF, et al. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11319–20. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krisky DM, Marconi PC, Oligino T, Rouse RJ, Fink DJ, Glorioso JC. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 1997 Oct;4(10):1120–5. doi: 10.1038/sj.gt.3300497. [DOI] [PubMed] [Google Scholar]
- 95.Jiang C, Wechuck JB, Goins WF, Krisky DM, Wolfe D, Ataai MM, et al. Immobilized cobalt affinity chromatography provides a novel, efficient method for herpes simplex virus type 1 gene vector purification. J Virol. 2004 Sep;78(17):8994–9006. doi: 10.1128/JVI.78.17.8994-9006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozuer A, Wechuck JB, Goins WF, Wolfe D, Glorioso JC, Ataai MM. Effect of genetic background and culture conditions on the production of herpesvirus-based gene therapy vectors. Biotechnol Bioeng. 2002 Mar 20;77(6):685–92. doi: 10.1002/bit.10162. [DOI] [PubMed] [Google Scholar]
- 97.Ozuer A, Wechuck JB, Russell B, Wolfe D, Goins WF, Glorioso JC, et al. Evaluation of infection parameters in the production of replication-defective HSV-1 viral vectors. Biotechnol Prog. 2002 May-Jun;18(3):476–82. doi: 10.1021/bp010176k. [DOI] [PubMed] [Google Scholar]
- 98.Wolfe D, Mata M, Fink DJ. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther. 2009 Apr;16(4):455–60. doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfe D, Wechuck J, Krisky D, Mata M, Fink DJ. A clinical trial of gene therapy for chronic pain. Pain Med. 2009 Oct;10(7):1325–30. doi: 10.1111/j.1526-4637.2009.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]