Abstract
Background
Among individuals with ischemic heart disease, young women with an acute myocardial infarction (AMI) represent an extreme phenotype associated with an excess mortality risk. While women younger than 55 years of age account for less than 5% of hospitalized AMI events, almost 16,000 deaths are reported annually in this group, making heart disease a leading killer of young women. Despite a higher risk of mortality compared with similarly aged men, young women have been the subject of few studies.
Methods
Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) is a large, observational study of the presentation, treatment and outcomes of young women and men with AMI. VIRGO will enroll 2,000 women, 18–55 years of age, with AMI and a comparison cohort of 1,000 men with AMI from more than 100 participating hospitals. The aims of the study are: to determine sex differences in the distribution and prognostic importance of biological, demographic, clinical, and psychosocial risk factors; determine whether there are sex differences in the quality of care received by young AMI patients; and determine how these factors contribute to sex differences in outcomes (including mortality, hospitalization and health status). Blood serum and DNA for consenting participants will be stored for future studies.
Conclusions
VIRGO will seek to identify novel and prognostic factors that contribute to outcomes in this young AMI population. Results from the study will be used to develop clinically useful risk-stratification models for young AMI patients, explain sex differences in outcomes and identify targets for intervention.
Keywords: risk factors, sex, [suggestions] outcomes, young, quality of care
Young women with acute myocardial infarction (AMI) are a relatively large yet understudied population. Almost 16,000 U.S. women 55 years or younger die from coronary heart disease (CHD) each year, ranking it among the leading causes of death in this group.1 Importantly, registries and cohort studies that have published data on young women have identified an excess mortality risk following AMI compared with similarly aged men.2, 3 Moreover, this excess risk appears to be sustained; among young women who initially survive an AMI, their subsequent mortality risk is about 50% higher than men 2 years post-AMI.4 Although recent evidence suggests a narrowing of the mortality gap after AMI between younger women and men, rates still remain higher for younger women.5 Unfortunately, these studies provide limited information about the presentation and natural history of AMI in young women, risk-factor profiles, treatment patterns, and non-mortality outcomes (including subsequent symptoms, function, and quality of life), nor have they been able to identify potential causes for this excess risk. The higher risk may be due to a higher prevalence of various traditional and emerging risk factors in this group compared with men, the greater potency of select risk factors, poorer quality of care, or some combination of these variables. Understanding the underlying causes of the adverse prognosis in young women and men is essential for developing interventions and strategies to improve their care and outcomes.
Conceptualization of the VIRGO Study Design and Selection of Research Domains
The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study is the first national, prospective, observational study of young AMI patients and has a planned enrollment of 2,000 women and 1,000 men. The specific aims of the study are:
To characterize sex differences after hospitalization for AMI in a broad range of outcomes including mortality, all-cause readmission, rehospitalization for cardiovascular causes, and adverse health status.
To evaluate the role of demographic, clinical, metabolic, biochemical, genetic, psychosocial, and lifestyle factors on outcomes for young women and men with AMI, and to examine whether sex-based variation in these factors is associated with variation in outcomes.
To compare the clinical management of young men and women who present to the hospital with AMI, and determine whether differences in the quality of care may be associated with differences in outcomes.
To describe the relationship of female-specific factors including genetic variants, sex hormones, reproductive history, prior use of estrogens, and menstrual cycle history with disease outcomes for women.
To develop comprehensive prognostic scores to stratify risk in this young population and identify predictors of early (within 1 month of discharge) and longer-term (1 year) outcomes.
To create a blood and DNA repository as a resource for future studies.
To partner with the American Heart Association, the American College of Cardiology, and other national organizations to disseminate findings from the VIRGO Study to improve the prevention, care, and outcomes for young patients with AMI.
The VIRGO study will examine a spectrum of patient and clinical factors, from genetic and biological factors, to psychosocial, lifestyle, demographic, and medical care factors that may influence disease trajectories and outcomes in young patients with AMI (Figure 1). The specific research domains were selected based on available evidence from studies of young women as well as literature that has identified sex differences in the presentation, care, and outcomes for older patients with AMI.
Figure 1.
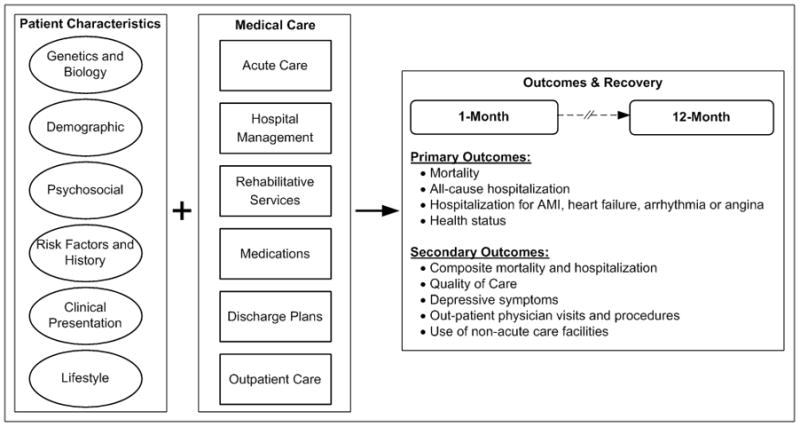
Overview
Genetics and Biology
Twin and family-based studies of CHD have shown that heredity plays an important role in heart disease for young women. These patients represent a unique phenotype likely enriched by a greater prevalence of high-risk genetic profiles than similarly aged men or older women. A study of 21,004 Swedish twins found that premature death from CHD of a female identical twin conferred a 15-fold relative hazard for death from CHD in her co-twin (95 percent confidence interval, 7.1to 31.9), after adjustment for other known CHD risk factorsas compared with a relative hazard of 8.1 for men.6 Several early candidate gene studies indicated that genetic polymorphisms may be associated with AMI and ischemic heart disease risk in postmenopausal women but not in men; the impact on risk in premenopausal women has not been investigated.7, 8 More recently, genome-wide association studies (GWAS) have implicated multiple polymorphisms in the occurrence of premature myocardial infarction, yet their specific impact on risk in young women remains unclear given that young women (especially minority women) were underrepresented in the clinical populations studied.9–13 Although a recent analysis involving the Women’s Genome Health Project revealed that the major risk variant in the 9p21.3 region (rs10757274) does not add substantial incremental value to existing AMI risk prediction models based on traditional cardiac risk factors, it is possible that an allelic risk score based on a multi-gene panel of GWAS-identified genetic risk variants may have a different impact in young women versus young men with AMI.14 In addition, little is known for any clinical population of AMI survivors regarding the prognostic value of newly discovered genetic risk factors following the initial event.
Studies suggest that the pathophysiology of disease in young women may be distinct from that of men and older women. Young women with sudden cardiac death are more likely to have coronary plaque erosion, while plaque rupture is more common in men and in older women.15 The prognostic importance of various inflammatory biomarkers, lipid and metabolic measures, and other biomarkers in explaining the excess risk has not been examined in any large study of young AMI patients. Coronary risk factors such as diabetes, heart failure, and previous stroke have been shown to be more common in younger women with MI than men.3 Sex hormones may play an important role in risk among premenopausal women,16 because younger women may be at greater risk of MI during the menstrual or follicular phase of their ovarian cycle during which their estradiol blood levels are the lowest;17, 18 however, these studies have been based on small populations and have not associated these factors with aspects of recovery or outcomes, nor have they been able to adjust for other potentially confounding factors present in younger women. The VIRGO study will be one of the first to examine the relationship of sex hormones, reproductive history, prior use of estrogens, and menstrual cycle history and phase with aspects of recovery and outcomes in a cohort of young pre-menopausal and peri-menopausal women with AMI.
Sociodemographic and Psychosocial Factors
Results from studies of older populations suggest that sociodemographic and psychosocial factors play an important prognostic role for women with AMI.19–21 Studies in older women suggest that low socioeconomic class, low educational attainment, and double work loads of employment and family are strong risk factors for post-AMI adverse outcomes.19, 22 Furthermore, older women with AMI are more likely to be on sick or disability leave, less likely to return to work, and less likely to adhere to cardiac rehabilitation programs than men; however, comparable studies have not been conducted in younger AMI populations.22, 23 Studies also suggest that depression, marital stress and lack of social support are important and more powerful risk factors for post-AMI adverse outcomes in older women than in older men.19, 24–26 Depressive symptoms are more common among young women hospitalized with AMI,27 but the role of depression, marital stress, and social support on recovery have not been adequately investigated in young patients with AMI. AMI has also been shown to interfere with sexual relationships and functioning, which can exacerbate depression, marital stress, and impede full post-MI recovery.28, 29 More is known about the sexual effects of AMI and sexual dysfunction as a precursor to AMI in men than women.
Clinical Risk Factors and Medical History
The prevalence and prognostic importance of traditional and emerging risk factors may differ by sex for young patients with AMI. Studies conducted in older populations suggest that certain risk factors such as smoking, diabetes, glucose intolerance, elevated cholesterol, and left ventricular hypertrophy are more prevalent and associated with greater mortality risk in women than men with CHD. 23, 30–35 The AMI risk associated with diabetes is particularly important for minority women for whom the prevalence of type II diabetes is 2–4 times that of white women. 32 Obstructive sleep apnea and sleeping disorders are associated with cardiac events and are relatively common among patients with coronary artery disease, but little is known about these associations for women and young AMI patients.36, 37
Clinical Presentation and Medical Care
Studies have shown that women are less likely than men to present with chest pain and more likely to have other symptoms such as nausea and vomiting during an AMI,38–40 though little research has examined symptom presentation among young patients with AMI. Differences in presentation and referral may be the cause of longer time to diagnosis and delays (or omissions) in the administration of life-saving therapies such as aspirin, beta-blockers, fibrinolytic therapy, and revascularization. 41–46 Cardiac procedures are performed less often for women with AMI compared with men,42, 45–49 but whether sex is independently associated with referral for an indicated procedure is largely unexplored in young populations with AMI. The few studies that have examined sex differences in outcomes following percutaneous coronary intervention procedures in younger patients indicate that young women have higher in-hospital mortality and vascular and bleeding complications than similarly aged men;50–54 however, little is known about the long-term outcomes for young patients who undergo these procedures.
Methods
Design Overview
VIRGO will recruit 2,000 women and a comparison sample of 1,000 men from a large, diverse, national network of hospitals over a 3-year enrollment period. Patients will be enrolled after admission for AMI. Data will be collected during the hospitalization, as well as at 1 and 12 months following hospital discharge. To avoid temporal changes in levels of specific biomarkers due to the acute AMI event, blood will be drawn at 1 month for markers of inflammation, lipids, metabolism, and sex hormones.
Site Network
A research network was established in collaboration with more than 100 US and international hospitals. Hospitals were selected based on the following considerations: commitment to women’s heart health; presence of a committed and enthusiastic research team including a Site Coordinator and Principal Investigator; sufficient representation of women and/or minority patients; geographic location of hospitals across the country; prior experience with participating in a research study or registry; ability to identify potential subjects with AMI using daily screening of patients with abnormal troponin or CK-MB levels; and feasibility of conducting the study in the hospital.
The VIRGO study is working collaboratively with investigators from Spain (IMJOVEN; plan to enroll 300 women, 150 men) and Australia (VIRGO-Australia: plan to enroll 90 women, 45 men) to develop an additional international cohort of young AMI patients using the instruments and study design from the parent VIRGO project. Replication of VIRGO in Spain and Australia will facilitate identification of cultural, environmental, health care and health system-related differences in the diagnosis and outcomes for young women and men following AMI.
Study Population and Recruitment
Patients aged 18 to 55 years of age are screened for eligibility based on the most recently adopted AMI criteria.55 To be eligible, participants must have a rise of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit within 24 hours of admission. In addition, there must be evidence of acute myocardial ischemia, including at least 1 of the following: symptoms of ischemia; electrocardiogram (ECG) changes indicative of new ischemia in the ECG (new ST-T changes; new or presumably new left bundle branch block; or the development of pathological Q waves), or other evidence of myocardial necrosis (imaging, pathology; Figure 2). Participants must present at the enrolling institution or have been transferred within the first 24 hours to ensure that the primary clinical decision making is being conducted at the enrolling site. Patients are not eligible for inclusion as a result of the following: elevated cardiac markers as a complication of elective coronary revascularization; previous enrollment in VIRGO; neither English nor Spanish-speaking; unable to provide informed consent; unable to be contacted for follow-up (e.g. no access to phone, not planning on living in the country of enrollment); an AMI due to physical trauma; or currently a prisoner.
Figure 2.
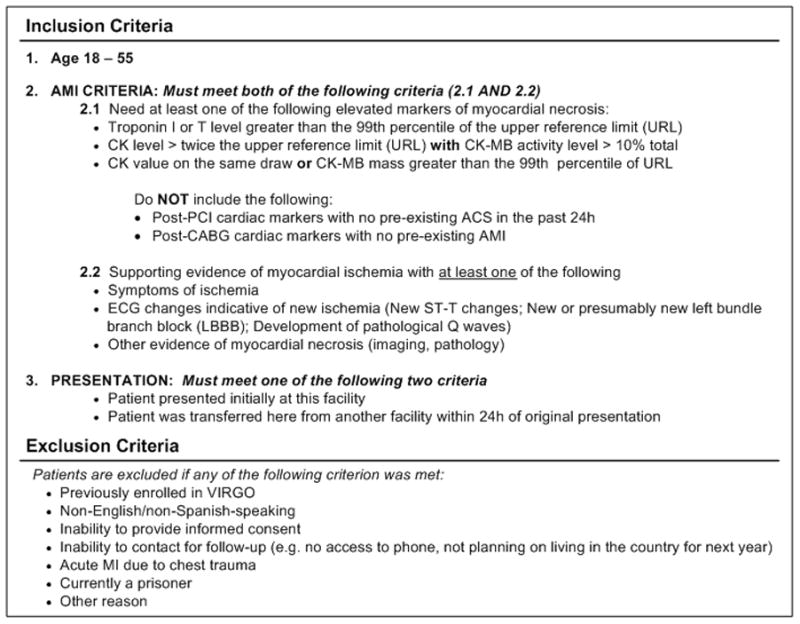
VIRGO Study Inclusion and Exclusion Criteria
Data Collection Overview
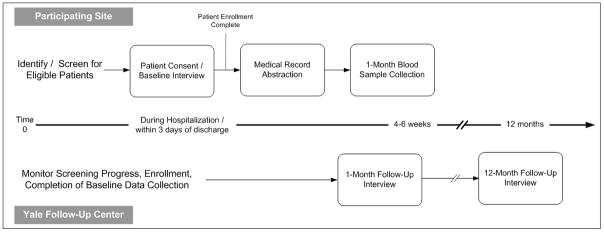
Data will be collected from patient interviews (at baseline, 1, and 12 months), physical assessments at baseline, review of the medical record, and the analysis of blood specimens (Figure 3). Enrolled participants will sign a consent form in accordance with the Health Insurance Portability and Accountability Act (HIPAA) and Institutional Review Board regulations. The coordinator at each site will perform the routine screening, enroll eligible participants, administer the baseline interview, collect height, weight, waist, and neck circumference measurements during the index hospitalization, and complete the medical record review. Screening data on all eligible patients will be collected for comparison of enrolled and non-enrolled patients. Blood will be obtained 4-6 weeks after hospital discharge for biomarker analyses and storage for future genetic studies. The 1- and 12-month interviews are administered by the Yale Follow-Up Center. Screening, enrollment, medical record review and interview data are entered onto web-based forms provided through VIRGO’s online electronic data management system.
Figure 3.
Overview of Study Design
Medical Record Abstraction
Information on clinical variables, including cardiac and non-cardiac history, vital signs and laboratory results, in-hospital complications, cardiac function testing, data from all cardiac procedures, discharge medications, and discharge disposition will be abstracted from the medical chart by the local site coordinator. All site coordinators receive standardized training and certification, and undergo routine assessment from the Yale Coordinating Center. A comprehensive data dictionary is provided to ensure standardization of abstracted data. Several test and procedure reports including the qualifying ECG, CT imaging report, cardiac catheterization, echocardiogram, stress test, as well as the admission and discharge summaries are forwarded to the Yale Coordinating Center where an expert team of reviewers will abstract detailed clinical information.
Participant Interviews
Participants are interviewed in-person during the index hospitalization by the local site coordinator. The 1-month and 12-month interviews are conducted by field staff from the Yale Follow-up Center. For Spanish speaking participants, the baseline, 1-month and 12-month interviews are completed using linguistically valid translations. All site interviewers receive standardized training and certification, and undergo routine assessment from the Yale Coordinating Center.
The specific domains of the data collection instruments are presented in Table 1.26, 56–67 Many of the selected instruments have been included in prior longitudinal studies of patients with AMI.68 Demographic information includes the patients’ address and contact information for follow-up purposes, race/ethnicity, country of origin, marital/relationship status, education level, living situation, employment, income and monthly finances. Participants are asked about their usual source of health care, satisfaction with the care they receive, difficulty obtaining medical care, insurance coverage, the impact of medical costs on their finances, and beliefs about decision making in the health care setting (Deber-Kraetschmer).61
Table 1.
VIRGO Study Domains
| Domain | Scale | Baseline | 1-month | 12-month |
|---|---|---|---|---|
| Patient Interviews | ||||
| Demographics | x | x | x | |
| Income/Socioeconomic Status | x | x | x | |
| Health Care Variables | x | x | x | |
| Presenting Symptoms/Perceived Risk | x | |||
| Medical History | ||||
| General Health | SF-1256 | x | x | x |
| CVD Functional Status | SAQ57 | x | x | x |
| Health-Related Quality of Life | EQ-5D58 | x | x | x |
| Menstrual/Reproductive History | x | x | x | |
| CVD Risk Factors | ||||
| Family History of CVD | x | x | ||
| Diabetes | x | x | ||
| Hypertension | x | x | ||
| Hypercholesterolemia | x | x | ||
| Smoking History | x | x | x | |
| Smoking Environment | x | x | ||
| Physical Activity | x | x | x | |
| Alcohol Consumption | CAGE59 | x | x | x |
| Substance Abuse | x | x | ||
| BMI/Weight/Hip Circumference | x | x | x | |
| Sleep Apnea | Berlin60 | x | ||
| Decision Making | Deber- | |||
| Kraetschmer61 | x | x | x | |
| Stress | PSS62, Life | |||
| Events63 | x | x | x | |
| Depression | PHQ-964 | x | x | x |
| Social Support | ESSI65 | x | x | |
| Marital Strain | Stockholm26 | x | x | |
| Discrimination | Everyday66 | x | ||
| Sexual Activity | Lindau67 | x | x | x |
| Medications | x | x | x | |
| Medication Adherence | x | x | ||
| Rehabilitation | x | x | ||
| Outpatient visits | x | x | ||
| Outcomes/Rehospitalization | x | x | ||
| Medical Record Abstraction | ||||
| ECG | x | |||
| Vitals and Laboratory Values | x | |||
| Reperfusion and Acute Therapies | x | |||
| In-Hospital Procedures | x | |||
| In-Hospital Events | x | |||
| Discharge Medications | x |
SF-12 = The Short-Form 12-Item Health Survey
SAQ = Seattle Angina Questionnaire CAGE = The
CAGE Questionnaire
Berlin = Berlin Questionnaire
Deber = Deber-Kraetschmer Problem-Solving Decision-Making Scale
PSS = Perceived Stress Scale
Life Events = Stressful Life Events
PHQ-9 = The PHQ depression scale
ESSI = ENRICHD Social Support Inventory
Stockholm = The Stockholm Marital Stress Scale
Everyday = DAS-DQ Frequency of Everyday Mistreatment
Clinical information includes the patients’ prodromal and presenting symptoms, medical history, acute symptoms, help-seeking experience, perceived risk, general health (SF-12),56 cardiovascular functional status (Seattle Angina Questionnaire, SAQ),57 sexual activity and function,67 and health-related quality of life (EQ-5D).58 For women, information on their menstrual and reproductive history is obtained. Detailed information will be collected for both traditional and novel cardiovascular risk factors including family history of cardiovascular disease, diabetes, hypertension, hypercholesterolemia, smoking status, smoking environment, physical activity, alcohol consumption,59 substance abuse, weight change and sleep disorders.60 Site coordinators will collect physical measurements including height and weight, and hip, waist and neck circumference during the hospital interview.
Psychosocial variables include stress and major life events within the past year (Perceived Stress Scale, Stressful Life Events),62,63 social support (ENRICHD Social Support Scale),65 depression (PHQ-9),64 prior depressive episodes and treatment, quality of sexual life, communication with a physician about sexuality or sexual problems,67 marital strain (The Stockholm Marital Strain Scale),26 and discrimination (The Detroit Area Study Discrimination Questionnaire (DAS-DQ), Frequency of Everyday Mistreatment).66
Information on clinical management and treatment will be assessed during the index hospitalization as well as during follow-up interviews. Details related to pharmacological treatments (drugs, doses and timing), invasive interventions (coronary angiography, percutaneous or surgical coronary revascularization), and noninvasive studies received by patients during hospitalization and at discharge will be recorded. Patients will be asked about their current medications, medication adherence, follow-up physician visits (all-cause and cardiac-related), and participation in cardiac rehabilitation programs during the 1- and 12-month interviews.
Study outcomes will be assessed during the follow-up interviews. The primary outcomes are: mortality; hospitalization (all-cause and hospitalization for AMI, heart failure, arrhythmia or angina); and health status as measured by the Seattle Angina Questionnaire57 and the Medical Outcomes Study Short-Form 12 (SF-12).56 Secondary outcomes will include the composite of mortality and hospitalization, components of the health status assessments, quality of care, depressive symptoms, outpatient physician visits, admissions at non-acute care facilities (including home health care, skilled nursing facility, or rehabilitation hospital), and receipt of out-patient diagnostic tests and procedures.
Blood Collection and Analysis
Blood will be obtained 4 to 6 weeks after hospital discharge for biomarker analyses and storage for future genetic studies using 1 of the following strategies selected by the VIRGO participant: 1) return to the enrollment hospital for a blood draw arranged by the local site coordinator; 2) receive a blood collection kit by mail that can be brought to the participant’s local physician for a blood draw; or 3) for select locations, receive a visit from a home-health agency to perform an in-home blood draw. Enrolled participants will provide a separate informed consent for blood specimen and DNA storage. A study specific patient identification number will be used on all study materials, and will not contain any patient identifying information.
Blood analyses are planned to include lipid profiles (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, Apolipoprotein AI, Lipoprotein(a), and Apolipoprotein B), glucose, insulin, high sensitivity C-reactive protein, total homocysteine levels, hemoglobin A1c, estradiol, bioavailable estradiol, estrone, progesterone, follicle-stimulating hormone, sex hormone binding globulin, and luteinizing hormone.
Data Management
We have developed data management procedures using web-based technology to ensure accurate and efficient data collection and analysis, confidentiality, and real-time, on-demand study monitoring reports. Screening and baseline interview data are entered by local site coordinators using an electronic data management system. Follow-up interviews are entered real-time into this system by Yale Follow-up Center staff. The collection, shipping, and receipt of blood samples are tracked using this online technology. The system has been constructed specifically with the goal of managing multiple, simultaneous, and integrated information from multiple data entry sources. It is validated according to the Code of Federal Regulations and industry standards, and meets other federal standards including those of HIPAA.
Analyses
To address the primary objectives of the study, we will compare health outcomes, risk factors, treatment measures, genetic factors and biomarkers between sex groups. We will report summary results for all collected baseline, treatment, genetic/metabolic and outcome data by sex, both overall and by racial/ethnic and age subgroups. For each aim, we will use standard parametric and non-parametric techniques for observational data, including t-tests, chi-squared tests, linear regression models, Wilcoxon rank sum tests, and ANOVA. Because patient characteristics, treatment and outcomes may be correlated within study sites, analyses will account for the effect of clustering of patients within sites. To examine and adjust for differences between comparison groups, we will use linear, logistic, Cox proportional hazard and Poisson models that either explicitly model inter-site correlation or adjust estimated variances accordingly; for models other than survival models we will primarily employ hierarchical generalized linear models to account for correlation of measures within recruitment site. We will develop risk models to examine the differences between men and women, and to stratify young women according to risk of adverse outcomes. For each model, we will identify a set of candidate variables based on clinical relevance and the relationship between the variables and the outcome using appropriate statistical techniques according to whether the dependent variable is time to event, other continuous, or binary.
Sample Size Calculations
Because survival differences by sex will be smaller and more difficult to detect than other primary outcomes, the sample size was determined by the number of patients needed to detect an absolute difference in 1-year mortality rates between women and men of 2%, assuming a 3% 1-year mortality rate for men. We estimated statistical power to detect the corresponding ratio of hazard rates, after accounting for enrollment rate and annual loss-to-follow-up. We assumed a uniform patient entry rate over the first 3 years, with a total 4-year study period and an annual drop-out of 20%. Accounting for the effect of clustering on survival times is problematic; there is no natural or accepted way of partitioning the variance of the hazardsλ into within- and between-cluster components.69 To adjust our anticipated sample size for our sampling design we considered the intra-hospital correlation, denotedρ, suggested by Xie and Waksman.70 Using a conservatively inflated value of ρ = 0.05, we adjusted the effective sample size by the design effect of VIF = 1+(m -1)*ρ, where m is the average number of subjects per hospital. Thus, with 2,000 women and 1,000 men we will have a power of 82% to detect a 1-year mortality hazard ratio of 1.68 of women relative to men. We anticipate higher prevalence rates for the other outcomes and will have adequate power to detect relative differences between groups.
Strategies for Dissemination of Research
We have developed collaborations with the American Heart Association Go Red for Women Movement, the American College of Cardiology, and the National Heart Lung and Blood Institute to facilitate the rapid dissemination of information from the study. Reports and publications will be targeted for enrolled participants and their families, participating VIRGO study centers, clinicians, the scientific community, and policymakers. A VIRGO website was developed (www.VIRGOStudy.org) to provide current information about our progress fielding the study, as well as to highlight current research topics about heart disease in young women and men.
Summary
Despite perceptions that young women are protected from heart disease, it is one of the leading causes of death in women 55 years and younger, accounting for more than 16,000 deaths annually in the United States. Young women with AMI represent an extreme phenotype of ischemic heart disease that is associated with an excess mortality risk. Even though more than a decade has passed since the publication of initial studies reporting an excess mortality risk for young women with heart disease, there is currently little information about the etiology of premature heart disease or factors that may contribute to this excess risk. The VIRGO study combines the complementary disciplines of biology, the social sciences, health services research, and clinical medicine to examine the spectrum of factors, ranging from genetics to bedside, that influence disease onset and recovery for young AMI patients. The VIRGO study will be the first and most comprehensive study of young women and men with heart attacks to identify the key determinants of recovery and discover knowledge that will assist us in improving their care.
Supplementary Material
Acknowledgments
We appreciate the contributions of Maria Johnson, Norrina Allen, and Emi Watanabe during the preparation of the grant application and initial phases of the VIRGO study. We appreciate the support of the Yale Center for Clinical Investigation (YCCI) for their assistance with the collection of blood specimens for New Haven participants. YCCI is funded by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
Funding Source: This research project is supported by R01 HL081153-01A1K from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. Dr. Stacy T. Lindau was supported in part by K23AG032870-01A from the National Institute on Aging, National Institute of Health, Department of Health and Human Services.
IMJOVEN is supported in Spain by PI 081614 from the Fondo de Investigaciones Sanitarias del Instituto Carlos III, Ministry of Science and Technology, and additional funds from the Centro Nacional de Investigaciones Cardiovasculares (CNIC).
VIRGO Investigators (enrolling participants as of July 15, 2010)
United States: Cynthia D Adams RN MSN APRN-BC PhD, The Indiana Heart Hospital; Lee A Arcement MD MPH FACC, Leonard J Chabert Medical Center; James Arrowood MD, Virginia Commonwealth University; Richard G Bach MD, Washington University School of Medicine; Kenneth Berkovitz MD, Summa Health System (Akron City Hospital); Vera Bittner MD MSPH, University of Alabama at Birmingham; David Bohle MD, Forsyth Medical Center; Beth Boyer-Kollas MS PhD CPCP, Orlando Health; John Brush MD, Sentara Hospitals; Linda P Calhoun MD, New Hanover Regional Medical Center; George Charos MD FACC, St. Joseph’s Medical Center; Howard A Cooper MD, Washington Hospital Center; Francis H Corcoran MD FACC, St John’s Regional Medical Center; Neil Dashkoff MD, Erie County Medical Center; Thomas Donohue MD, Hospital of St. Raphael; Fiona L Dulbecco MD, California Medical Center; Thanh H Duong-Wagner MD FACC Baptist Health Care-Pensacola; Cara East MD, Baylor Hamilton Heart and Vascular Hospital; Joan Eldridge MD, Parker Adventist Hospital; James A Fox MD, Munson Medical Center; Jules Gardin MD, Hackensack University Medical Center; Michael Gault MD, Saddleback Memorial Medical Center; Mindy Gentry MD, Medical College of Georgia; John Gill MD, Memorial Health System; Eve Gillespie MD PhD, Glacier View Cardiology; Robert W Godley MD FACC, Parkview Research Center/Fort Wayne Cardiology; Deepika Gopal MD, Baylor Research Institute; Mayra Guerrero MD, Henry Ford Hospital; Kelly Hayes MD PhD, Hamot Medical Center; Cynthia Holt RN MN CCRN, Overlook Hospital; Phillip A Horwitz MD, University of Iowa Hospitals and Clinics; Elizabeth Jackson MD MPH, University of Michigan Health Systems; Alice K Jacobs MD, Boston University Medical Center; Paul A Jones MD, Mercy Hospital and Medical Center; Sunny S. Jhamnani MB BS, Beth Israel Deaconess Medical Center; Peggy Kalowes RN PhD CNS, Long Beach Memorial Medical Center; Milind M Karve MD, Sparrow Hospital; Friederike Keating MD, Fletcher Allen Health Care; Eileen Kelly-Hensing MD, NorthShore University HealthSystem; Anita Kelsey MD, St Francis Hospital and Medical Center; Amit Khera MD FACC, University of Texas Southwestern Medical Center at Dallas (UTSW); Harlan Krumholz MD SM, Judith Lichtman PhD MPH, Yale-New Haven Hospital; Charles R Lambert MD PhD MBA, Pepin Heart Hospital; Matthew Lindberg MD, Samaritan Heart and Vascular Institute; Mark Lurie MD, Torrance Memorial Medical Center; Susan Mani MD, Danbury Hospital; Walt Marquardt MD, Mercy General Hospital; Kevin P Marzo MD, Winthrop University Hospital; Fred Masoudi MD, Denver Health Medical Center; John McLachlan MD, Cardiovascular Research Foundation of Louisiana; Richard McNamara MD, Spectrum Health; Laxmi S Mehta MD FACC, The Ohio State University Medical Center; Rupa Mehta MD, University of Chicago Medical Center; C Noel Bairey Merz MD, Cedars-Sinai Heart Institute; Nancy Mesiha MD, St. John Hospital & Medical Center; Paula Miller MD, University of North Carolina at Chapel Hill; Margo Minissian AACN-BC MSN CNS AACC FAHA, Cedars-Sinai Heart Institute; Judith E. Mitchell MD FACC FAHA, SUNY Downstate Medical Center; Vivian Mo MD, University of Southern California; Karen Moncher MD, Meriter Hospital; Adefisayo Oduwole MD, Morehouse School of Medicine; Soubhagyalak Parvathaneni MD, St John’s Mercy Medical Research Institute; Amar Pohwani MD, Memorial Medical Center (Sutter Gould Medical Foundation); Indu Poornima MD, Allegheny General Hospital; Vijay Rajendran MD FCCP FACC, Genesis Medical Center; John Reefer MD, Butler Memorial Hospital; Harmony Reynolds MD, Cardiovascular Clinical Research Center, New York University School of Medicine; Arthur L Riba MD FACC, Oakwood Hospital and Medical Center; Steven Rowe MD, Cox South Hospital; Daniel A Rubin MD FACC, Jefferson Regional Medical Center; Jane E Schauer MD PhD FACC, Presbyterian Heart Group; Anil Shah MD FACC, St. Elizabeth’s Hospital; Barry Sharaf MD, Rhode Island Hospital; Charles A Shoultz III MD FACC, Providence Healthcare Network; Ashley Simmons MD, University of Kansas Hospital; Kimberly A Skelding MD, Geisinger Clinic - Cardiology; Diane Sobkowicz MD, Sutter Medical Center Sacramento; Gerald Sotsky MD, The Valley Hospital; John A Spertus MD MPH, Mid America Heart Institute St Luke’s Hospital; Daniel Sporn MD, D. Guthrie Foundation for Educ & Research; Marcus St. John MD, Baptist Cardiac & Vascular Institute, Baptist Hospital of Miami; Richard A Staudacher MD FACC, Waukesha Memorial Hospital; Kathleen Stergiopoulos MD PhD FASE FACC, Stony Brook University Medical Center; John E Stone MD, Providence Hospital; Deborah Sybrant BSN PA-C MPAS, The International Heart Institute of Montana; Peter N Tadros MD FACC FSCAI FASE, University of Kansas Hospital; Cynthia Taub MD, Montefiore/Albert Einstein Medical Center; Norma Thiessen MD, Minneapolis Heart Institute; Jennifer Tremmel MD, Stanford University Medical Center; Srikanth Upadya MD FACC, Citrus Memorial Health Systems; Elisabeth von der Lohe MD, Krannert Institute of Cardiology Clarian Cardiovascular Center Methodist Hospital; William Vosik MD, Good Samaritan Hospital; Mary Norine Walsh MD, St. Vincent Heart Center of Indiana; Nancy Weeks RN BA, Los Robles Hospital and Medical Center; Paulette Wehner MD, St Mary’s Medical Center; Robert Weiss MD, Central Maine Heart and Vascular Institute; Francine K Welty MD PhD, Beth Israel Deaconess Medical Center; Ryan Whitney MD, BryanLGH Heart Institute; Mark Wiley MD, University of Kansas Hospital;Jerome E Williams Jr. MD FACC, Presbyterian Hospital - Mid Carolina Cardiology; J Scott Wolery MD FACC, St. Rita’s Medical Center; Lambert A Wu MD FACP FACC, Cotton-O’Neil Clinical Research Center; Mark Zainea MD, Mount Clemens Regional Medical Center; Stuart Zarich MD, Bridgeport Hospital; Suhail Zavaro MD, Sharp Grossmont Hospital.
Spain - IMJOVEN: In Spain, IMJOVEN is developed with the partnership of the Centro Nacional de Investigaciones Cardiovasculares (CNIC); the Working Group on Ischemic Heart Disease of the Spanish Society of Cardiology; and the Red de Investigación Cardiovascular (RECAVA) and HERACLES ISCIII research networks. IMJOVEN Executive Committee: Valentín Fuster, MD PhD; Héctor Bueno, MD PhD; Ginés Sanz, MD PhD; Ana Dopazo, PhD; Eliseo Guallar, MD PhD; Magda Heras, MD PhD; Alfredo Bardaji, MD PhD; Jaume Marrugat, MD PhD. Victoria Lorente MD, Albert Ariza MD, Hospital Universitario de Bellvitge, l’Hospitalet de Llobregat; Manuel F Jiménez Navarro MD PhD, Eduardo de Teresa MD PhD, Fernando Cabrera MD PhD; Hospital Clínico “Virgen de la Victoria”, Málaga; Manuel Piqué MD, Fernando Worner MD PhD, Hospital Arnau de Vilanova, Lleida; Joaquina Belchí MD Hospital General de Valencia; Rosana Hernández-Antolin MD PhD, Tamara Gorgadze MD, Hospital Clínico San Carlos, Madrid; Héctor Bueno MD PhD, Hospital General Universitario Gregorio Marañón, Madrid; Rosa-Maria Lidon MD PhD, Hospital Vall d’Hebron, Barcelona; Martín J García-González MD, Hospital Universitario de Canarias, Tenerife; José M García-Acuña MD PhD, Hospital Clínico de Santiago de Compostela; Montserrat Vila MD, Hospital de la Santa Creu i San Pau, Barcelona; Mercè Roqué MD PhD, Magda Heras MD PhD, Hospital Clínic de Barcelona; Norberto Alonso-Orcajo MD PhD, Complejo Asistencial de León; Antonio Curós MD PhD, Carolina Bosch MD, Hospital Universitari Germans Trias, Badalona; Eduardo Pinar MD PhD, Juan Ramón Gimeno MD, PhD, Hospital Virgen Arrixaca, Murcia; Sara M Ballesteros MD, Hospital Virgen del Rocío, Sevilla; Antonio Amaro MD, Juan Manuel Lamas Touza MD PhD, Maria Bastos Fernández MD PhD, Hospital Montecelo, Pontevedra; Vicente Barriales MD, Hospital Universitario Central de Asturias, Oviedo; Alfredo Bardaji MD PhD, Hospital Universitario de Tarragona Joan XXIII; Antonio L Arrebola MD, Rafael Melgares MD PhD, Hospital Universitario Virgen de las Nieves, Granada; Alberto San Román MD PhD, Carolina Hernández MD PhD, Hospital Clínico Universitario de Valladolid (ICICOR); Dona Meroño MD, Hospital del Mar, Barcelona; Antonio Tello MD, José Moreu MD, Hospital Virgen de la Salud, Toledo; Gloria Oller MD, Hospital General de Catalunya, Barcelona.
Australia: John Beltrame, BSc, BMBS, FRACP, PhD, FESC, FACC, FCSANZ, The Queen Elizabeth Hospital, Lyell McEwin Hospital, Adelaide.
See Online Supplement for List of VIRGO Coordinators.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.AHA. Women and Cardiovascular Diseases - Statistics. 2004. [Google Scholar]
- 2.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134:173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med. 2009;169:1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 7.Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, van Leeuwen JP, de Jong FH, Zillikens MC, Hofman A, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291:2969–2977. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 8.Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 9.Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der Harst P, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ Wellcome Trust Case Control C, Cardiogenics C. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nature Genetics. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 10.Myocardial Infarction Genetics Consortium; Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O’Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O’Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall AS, Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, Konig IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O’Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Altshuler D Wellcome Trust Case Control C. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nature Genetics. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, Linsel-Nitschke P, Kathiresan S, Wright B, Tregouet DA, Cambien F, Bruse P, Aherrahrou Z, Wagner AK, Stark K, Schwartz SM, Salomaa V, Elosua R, Melander O, Voight BF, O’Donnell CJ, Peltonen L, Siscovick DS, Altshuler D, Merlini PA, Peyvandi F, Bernardinelli L, Ardissino D, Schillert A, Blankenberg S, Zeller T, Wild P, Schwarz DF, Tiret L, Perret C, Schreiber S, El Mokhtari NE, Schafer A, Marz W, Renner W, Bugert P, Kluter H, Schrezenmeir J, Rubin D, Ball SG, Balmforth AJ, Wichmann HE, Meitinger T, Fischer M, Meisinger C, Baumert J, Peters A, Ouwehand WH, Deloukas P, Thompson JR, Ziegler A, Samani NJ, Schunkert H Italian Atherosclerosis Thrombosis and Vascular Biology Working G, Myocardial Infarction Genetics C, Wellcome Trust Case Control C, Cardiogenics C. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nature Genetics. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction.[see comment] Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 14.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3.[see comment] Ann Intern Med. 2009;150:65–72. doi: 10.7326/0003-4819-150-2-200901200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer MCA, Rittersma SZH, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, Virmani R. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J Am Coll Cardiol. 2009;54:0000–0000. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 17.Hamelin BA, Methot J, Arsenault M, Pilote S, Poirier P, Plante S, Bogaty P. Influence of the menstrual cycle on the timing of acute coronary events in premenopausal women. Am J Med. 2003;114:599–602. doi: 10.1016/s0002-9343(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 18.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Variation in the risk of onset of acute myocardial infarction during the menstrual cycle. Am J Cardiol. 2002;90:49–51. doi: 10.1016/s0002-9149(02)02386-x. [DOI] [PubMed] [Google Scholar]
- 19.Kristofferzon ML, Lofmark R, Carlsson M. Myocardial infarction: gender differences in coping and social support. J Adv Nurs. 2003;44:360–374. doi: 10.1046/j.0309-2402.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 20.Lacey EA, Walters SJ. Continuing inequality: gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health. 2003;57:622–627. doi: 10.1136/jech.57.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogels EA, Lagro-Janssen AL, van Weel C. Sex differences in cardiovascular disease: are women with low socioeconomic status at high risk? Br J Gen Pract. 1999;49:963–966. [PMC free article] [PubMed] [Google Scholar]
- 22.Brezinka V, Kittel F. Psychosocial factors of coronary heart disease in women: a review. Soc Sci Med. 1996;42:1351–1365. doi: 10.1016/0277-9536(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 23.Johansson S, Bergstrand R, Ulvenstam G, Vedin A, Wilhelmsson C, Wedel H, Wilhelmsen L, Aberg A. Sex differences in preinfarction characteristics and longterm survival among patients with myocardial infarction. Am J Epidemiol. 1984;119:610–623. doi: 10.1093/oxfordjournals.aje.a113778. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Butler J, Miller J, Vaccarino V, Williams CS, Mendes de Leon CF, Seeman TE, Kasl SV, Berkman LF. Prognostic importance of emotional support for elderly patients hospitalized with heart failure. Circulation. 1998;97:958–964. doi: 10.1161/01.cir.97.10.958. [DOI] [PubMed] [Google Scholar]
- 25.Mendes de Leon CF, Krumholz HM, Seeman TS, Vaccarino V, Williams CS, Kasl SV, Berkman LF. Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982–1991. Established Populations for the Epidemiologic Studies of the Elderly. Arch Intern Med. 1998;158:2341–2348. doi: 10.1001/archinte.158.21.2341. [DOI] [PubMed] [Google Scholar]
- 26.Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- 27.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 28.Addis IB, Ireland CC, Vittinghoff E, Lin F, Stuenkel CA, Hulley S. Sexual activity and function in postmenopausal women with heart disease. Obstetrics & Gynecology. 2005;106:121–127. doi: 10.1097/01.AOG.0000165276.85777.fb. [DOI] [PubMed] [Google Scholar]
- 29.Archer SL, Gragasin FS, Webster L, Bochinski D, Michelakis ED. Aetiology and management of male erectile dysfunction and female sexual dysfunction in patients with cardiovascular disease. Drugs & Aging. 2005;22:823–844. doi: 10.2165/00002512-200522100-00003. [DOI] [PubMed] [Google Scholar]
- 30.Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 31.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 32.Prevalence of physical activity, including lifestyle activities among adults--United States, 2000–2001. MMWR Morb Mortal Wkly Rep. 2003;52:764–769. [PubMed] [Google Scholar]
- 33.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 34.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 36.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 37.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 38.Arslanian-Engoren C, Patel A, Fang J, Armstrong D, Kline-Rogers E, Duvernoy CS, Eagle KA. Symptoms of men and women presenting with acute coronary syndromes. Am J Cardiol. 2006;98:1177–1181. doi: 10.1016/j.amjcard.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 39.Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, Long T. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med. 2007;167:2405–2413. doi: 10.1001/archinte.167.22.2405. [DOI] [PubMed] [Google Scholar]
- 40.Lovlien M, Schei B, Hole T. Women with myocardial infarction are less likely than men to experience chest symptoms. Scand Cardiovasc J. 2006;40:342–347. doi: 10.1080/14017430600913199. [DOI] [PubMed] [Google Scholar]
- 41.Richards HM, Reid ME, Watt GC. Why do men and women respond differently to chest pain? A qualitative study. J Am Med Womens Assoc. 2002;57:79–81. [PubMed] [Google Scholar]
- 42.Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT, Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–566. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 43.Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, Ornato JP, Barron HV, Kiefe CI. Prevalence, Clinical Characteristics, and Mortality Among Patients With Myocardial Infarction Presenting Without Chest Pain. JAMA. 2000;283:3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 44.Clarke KW, Gray D, Keating NA, Hampton JR. Do women with acute myocardial infarction receive the same treatment as men? BMJ. 1994;309:563–566. doi: 10.1136/bmj.309.6954.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, Sollano J, Katz S, Moye L, Basta LL, Lewis SJ, Gottlieb SS, Bernstein V, McEwan P, Jacobson K, Brown EJ, Kukin ML, Kantrowitz NE, Pfeffer MA for the Survival and Ventricular Enlargement Investigators. Sex differences in the management of coronary artery disease. N Engl J Med. 1991;325:226–230. doi: 10.1056/NEJM199107253250402. [DOI] [PubMed] [Google Scholar]
- 46.Kudenchuk PJ, Maynard C, Martin JS, Wirkus M, Weaver WD. Comparison of presentation, treatment, and outcome of acute myocardial infarction in men versus women (the Myocardial Infarction Triage and Intervention Registry) Am J Cardiol. 1996;78:9–14. doi: 10.1016/s0002-9149(96)00218-4. [DOI] [PubMed] [Google Scholar]
- 47.Giles WH, Anda RF, Casper ML, Escobedo LG, Taylor HA. Race and sex differences in rates of invasive cardiac procedures in US hospitals. Data from the National Hospital Discharge Survey. Arch Intern Med. 1995;155:318–324. [PubMed] [Google Scholar]
- 48.Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med. 1998;158:981–988. doi: 10.1001/archinte.158.9.981. [DOI] [PubMed] [Google Scholar]
- 49.Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, Labresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L. Get With the Guidelines Steering Committee and I. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 50.Abramson JL, Veledar E, Weintraub WS, Vaccarino V. Association between gender and in-hospital mortality after percutaneous coronary intervention according to age. Am J Cardiol. 2003;91:968–971. A964. doi: 10.1016/s0002-9149(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs S, Kornowski R, Teplitsky I, Brosh D, Lev E, Vaknin-Assa H, Ben-Dor I, Iakobishvili Z, Rechavia E, Battler A, Assali A. Major bleeding complicating contemporary primary percutaneous coronary interventions-incidence, predictors, and prognostic implications. Cardiovascular Revascularization Medicine. 2009;10:88–93. doi: 10.1016/j.carrev.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Argulian E, Patel AD, Abramson JL, Kulkarni A, Champney K, Palmer S, Weintraub W, Wenger NK, Vaccarino V. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 53.Narins CR, Ling FS, Fischi M, Peterson DR, Bausch J, Zareba W. In-hospital mortality among women undergoing contemporary elective percutaneous coronary intervention: a reexamination of the gender gap. Clin Cardiol. 2006;29:254–258. doi: 10.1002/clc.4960290606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivas VS, Garg S, Negassa A, Bang JY, Monrad ES. Persistent sex difference in hospital outcome following percutaneous coronary intervention: results from the New York State reporting system. J Invasive Cardiol. 2007;19:265–268. [PubMed] [Google Scholar]
- 55.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 58.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 59.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 60.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 61.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–1420. [PubMed] [Google Scholar]
- 62.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 63.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 64.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enhancing recovery in coronary heart disease patients (ENRICHD): Study design and methods. The ENRICHD investigators. Am Heart J. 2000;139:1–9. doi: 10.1016/s0002-8703(00)90301-6. [DOI] [PubMed] [Google Scholar]
- 66.Williams JR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socioeconomic status, stress and discrimination. Journal of Health Psycology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 67.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H Cardiovascular Outcomes Research C. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 69.Segal MR, Neuhaus JM, James IR. Dependence estimation for marginal models of multivariate survival data. Lifetime Data Anal. 1997;3:251–268. doi: 10.1023/a:1009601031424. [DOI] [PubMed] [Google Scholar]
- 70.Xie T, Waksman J. Design and sample size estimation in clinical trials with clustered survival times as the primary endpoint. Stat Med. 2003;22:2835–2846. doi: 10.1002/sim.1536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.