Abstract
Adult bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) exhibit a Sca-1+/Lin–/CD45– phenotype and can differentiate into various cell types, including cardiomyocytes and endothelial cells. We have previously reported that transplantation of a small number (1 × 106) of freshly isolated, non-expanded VSEL-SCs into infarcted mouse hearts resulted in improved left ventricular (LV) function and anatomy. Clinical translation, however, will require large numbers of cells. Because the frequency of VSEL-SCs in the marrow is very low, we examined whether VSEL-SCs can be expanded in culture without loss of therapeutic efficacy. Mice underwent a 30 min. coronary occlusion followed by reperfusion and, 48 hrs later, received an intramyocardial injection of vehicle (group I, n= 11), 1 × 105 enhanced green fluorescent protein (EGFP)-labelled expanded untreated VSEL-SCs (group II, n= 7), or 1 × 105 EGFP-labelled expanded VSEL-SCs pre-incubated in a cardiogenic medium (group III, n= 8). At 35 days after myocardial infarction (MI), mice treated with pre-incubated VSEL-SCs exhibited better global and regional LV systolic function and less LV hypertrophy compared with vehicle-treated controls. In contrast, transplantation of expanded but untreated VSEL-SCs did not produce appreciable reparative benefits. Scattered EGFP+ cells expressing α-sarcomeric actin, platelet endothelial cell adhesion molecule (PECAM)-1, or von Willebrand factor were present in VSEL-SC-treated mice, but their numbers were very small. No tumour formation was observed. We conclude that VSEL-SCs expanded in culture retain the ability to alleviate LV dysfunction and remodelling after a reperfused MI provided that they are exposed to a combination of cardiomyogenic growth factors and cytokines prior to transplantation. Counter intuitively, the mechanism whereby such pre-incubation confers therapeutic efficacy does not involve differentiation into new cardiac cells. These results support the potential therapeutic utility of VSEL-SCs for cardiac repair.
Keywords: myocardial infarction, myocardial repair, VSEL-SCs, stem cell, bone marrow, left ventricular function, remodelling
Introduction
Transplantation of adult bone marrow cells (BMCs) is associated with improvement in left ventricular (LV) structure and function. Following promising results in animal studies, this therapeutic approach has been quickly translated into clinical trials and several different types of BMCs have been used for this purpose [1]. However, a meta-analysis of the results of these small clinical trials indicates that the benefits of BMC therapy are only modest [1]. A vigorous effort has therefore been mounted to identify not only the optimal cell types, but also possible modifications prior to transplantation that may improve the outcome of BMC therapy.
Recent studies from our laboratories have characterized a population of pluripotent CXC chemokine receptor (CXCR)4+ cells that reside in the adult bone marrow, are non-haematopoietic and are able to differentiate into cells of various lineages, including cardiomyocytes and endothelial cells [2–4]. These Sca-1+/Lin–/CD45–‘very small embryonic-like’ stem cells (VSEL-SCs) can acquire a cardiomyocytic phenotype in vitro[3, 5], are mobilized after acute myocardial infarction (MI) [6] and promote cardiac repair following transplantation into the infarct border zone in vivo[7]. In our previous study [7] VSEL-SC transplantation improved LV function and dimensions but was associated with minimal or no differentiation of VSEL-SCs into cardiomyocytes at 35 days after MI; because of the scarcity of VSEL-SCs in the bone marrow and our prior inability to expand them effectively, in that investigation [7] we transplanted only 10,000 freshly isolated, non-expanded VSEL-SCs [7]. Clinical translation of this therapy, however, will require transplantation of much greater numbers of cells, particularly when intracoronary infusion (instead if intramyocardial injection) is used. Consequently, before this new promising cell type can find clinical application in patients with ischemic heart disease, it is important to develop methods to expand VSEL-SCs in culture while maintaining therapeutic efficacy.
Recently, we have been able to expand VSEL-SCs by culturing them on C2C12 myoblast cells. Thus, in the present study we examined whether VSEL-SCs expanded with this method exert salubrious effects on post-MI LV dysfunction and remodelling, analogous to those previously observed with non-expanded VSEL-SCs [7]. Because we have previously observed that incubation in a cardiomyogenic medium (containing transforming growth factor-β1 [TGF-β1], vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF] and insulin-like growth factor-1 [IGF-1]) promotes differentiation of freshly isolated VSEL-SCs towards a cardiac phenotype [3], we compared VSEL-SCs incubated in such a medium for 5 days with non-incubated VSEL-SCs. The results show that expanded VSEL-SCs are still effective in improving LV function and dimensions and attenuating cardiomyocyte hypertrophy when they are pre-incubated in cardiomyogenic medium, but are ineffective in the absence of pre-incubation.
Materials and methods
The present study was performed in accordance with the guidelines of the Animal Care and Use Committee of the University of Louisville School of Medicine and with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 85–23, revised 1996).
Experimental protocol
This study was performed in a well-established murine model of MI [7–9]. The experimental protocol is summarized in Figure 1. All mice (groups I–III) underwent a 30 min. coronary occlusion followed by 35 days of reperfusion. At 48 hrs after reperfusion, mice received an intramyocardial injection of vehicle (group I), expanded untreated VSEL-SCs (group II) or expanded VSEL-SCs pre-incubated in cardiogenic medium (TGF-β1, VEGF, bFGF and IGF-1) (group III). Echocardiographic studies were performed 4 days prior to coronary occlusion/reperfusion, at 48 hrs after cell injection (i.e. 96 hrs after MI) and at 35 days after MI (prior to killing).
Fig 1.
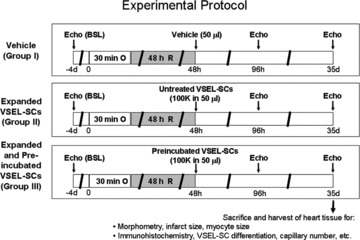
Experimental protocol. Three groups of WT mice were used (groups I–III, n= 7–11 mice/group). Four days after a baseline echocardiogram, mice underwent a 30 min. coronary occlusion followed by reperfusion. Forty-eight hours after MI, mice received intramyocardial injection of vehicle (group I), culture-expanded, untreated VSEL-SCs (group II) or culture-expanded VSEL-SCs incubated in cardiomyogenic medium (group III). Echocardiograms were repeated at 48 hrs after cell transplantation and at 35 days after MI. At 35 days after MI, mice were killed for morphometric and histological studies.
Isolation of Sca-1±/Lin−/CD45− VSEL-SCs
VSEL-SCs were isolated as previously described [3, 7]. Briefly, BMCs were obtained from the femur and tibia of 4–6-week-old male EGFP transgenic mice and red blood cells were lysed with a 0.9% solution of NH4Cl. Freshly isolated BMCs were resuspended in phosphate-buffered solution (PBS) containing 1% foetal bovine serum (FBS). The following primary antibodies were added simultaneously: biotin-conjugated monoclonal rat antimouse Ly-6A/E (Sca-1) (clone E13-161.7), APC-Cy7-conjugated monoclonal rat antimouse CD45 (clone 30-F11) and phycoerythrin (PE) conjugated monoclonal rat antimouse lineage markers [anti-CD45R/ B220 (PE; clone RA3-6B2), anti-Gr-1 (PE; clone RB6-8C5), anti-TCRαβ (PE; clone H57-597), anti-TCRγδ (PE; clone GL3), anti-CD11b (PE; clone M1/70), anti-Ter119 (PE; clone TER-119)]. Secondary staining was performed with PE-Cy5-conjugated streptavidin. All reagents were purchased from BD Pharmingen (San Jose, CA, USA). Staining was performed at 4°C for 20 min., and cells were washed with PBS supplemented with 1% FBS after staining. Flow cytometric cell sorting was performed with a MoFlo machine (Dako, Carpinteria, CA, USA) according to the scheme presented in Figure 2. Bulk-sorted cells were collected into 2 ml DMEM with 10% FBS. The purity was assessed by reanalysing isolated cells immediately following sorting. The viability of sorted cells always exceeded 90%. Sorted cells were pelleted via centrifugation at 1000 ×g for 10 min. and resuspended in DMEM with 10% FBS in a smaller volume proportional to cell number.
Fig 2.
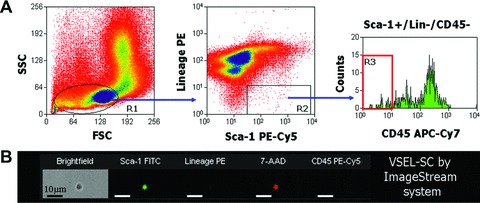
Isolation (flow cytometry) and morphologic characterization (ImageStream analysis) of BM-derived Sca-1+/Lin–/CD45– VSEL-SCs. (A) Representative dot-plots show the scheme for isolating small cells from the lymphoid gate based on expression of Sca-1 (PE-Cy5) and lineage markers (PE), and further separation based on CD45 (APC-Cy7) expression. Region 3 (R3) contains Sca-1+/Lin–/CD45– VSEL-SCs. (B) Visualization of VSEL-SCs by ImageStream analysis demonstrates the very small diameter, positivity for Sca-1 (fluorescein isothiocyanate, green) and negativity for lineage markers (PE) and CD45 (PE-Cy5). The nucleus is identified in red (7-amino-actinomycin D). FSC, forward scatter characteristics; SSC, side scatter characteristics.
In vitro expansion and pre-differentiation of VSEL-SCs in cardiomyogenic medium
Following isolation by FACS, EGFP-labelled VSEL-SCs were plated over a feeder layer of unlabelled C2C12 cells in DMEM with low concentration of FBS. The expansion of VSEL-SCs was continued for 9 days with change of medium every 3–4 days. Following expansion, cells were trypsinized and EGFP-labelled expanded VSEL-SCs were isolated from C2C12 cells by flow cytometry. In some studies, VSEL-SCs were subsequently plated in medium containing TGF-β1 (10 ng/ml), VEGF (10 ng/ml), bFGF (10 ng/ml) and IGF-1 (10 ng/ml) for 5 days [3]. Following this treatment, VSEL-SCs were harvested, thoroughly washed in DMEM to remove the cardiogenic ingredients, and aliquoted (100,000 cells in 50 μl volume for each mouse) for intramyocardial injection in group III. The same number of expanded VSEL-SCs, cultured for 5 days in identical medium but without cardiogenic growth factors (i.e. without pre-incubation) was injected in group II. Because we were unable to obtain a large number of freshly isolated VSEL-SCs (the primary reason for expansion), and in order to keep the number of injected cells similar among groups, we did not include a treatment group with freshly isolated VSEL-SCs.
Myocardial infarction and cell transplantation
Three groups of wild-type (WT) mice (C57/BL6 strain, body wt. 20–25 g, age 10–12 weeks; Jackson Laboratories, Bar Harbor, ME, USA) were used. The experimental preparation has been described in detail [7–9]. Briefly, mice were anesthetized with pentobarbital sodium (50 mg/kg i.p.), intubated and ventilated using a small rodent ventilator. Body temperature, heart rate and arterial pH were carefully maintained within the physiological range throughout the experiments [8]. Using a sterile technique, the chest was opened through a midline sternotomy. An 8–0 nylon suture was passed with a tapered needle under the left anterior descending coronary artery 2 mm from the tip of the left auricle, and a non-traumatic balloon occluder was applied on the artery. Coronary occlusion was induced by inflating the balloon occluder. Successful performance of coronary occlusion and reperfusion was verified by visual inspection (i.e. by noting the development of a pale colour in the distal myocardium upon inflation of the balloon and the return of a bright red colour due to hyperaemia after deflation) and by observing ST segment elevation and widening of the QRS complex on the ECG during ischemia and their resolution after reperfusion [8]. The chest was then closed in layers, and a small catheter was left in the thorax for 10–20 min. to evacuate air and fluids. The mice were removed from the ventilator, kept warm with heat lamps, given fluids (1.0–1.5 ml of 5% dextrose in water intraperitoneally) and allowed 100% oxygen via nasal cone. Forty-eight hours later, mice were re-anesthetized and ventilated and the chest reopened via aseptic technique. Vehicle (50 μl, group I), expanded untreated VSEL-SCs (100,000 cells in 50 μl, group II), or expanded pre-incubated VSEL-SCs (100,000 cells in 50 μl, group III) were injected intramyocardially using a 30 gauge needle. A total of five injections were made to deliver a total of 100,000 cells per heart in the peri-infarct region in a circular pattern, at the border between infarcted and surviving myocardium. Because in our previous study [5] we found that the expression of chemoattractants in the infarcted myocardium was maximal at 48 hrs after reperfusion, this time-point was chosen for VSEL-SC transplantation to ensure maximal retention of injected cells. The chest was closed in layers and the mice allowed to recover as described above.
Echocardiographic studies
Echocardiograms were obtained using an HDI 5000 SonoCT echocardiography machine (Philips Medical Systems, Bothell, WA, USA) equipped with a 15–7 MHz linear broadband and a 12–5 MHz phased array transducers [9]. The mice were anesthetized with pentobarbital (25 mg/kg i.p.). The anterior chest was shaved and the mice were placed in the left lateral decubitus position. Using a rectal temperature probe, body temperature was carefully maintained close to 37.0°C with a heating pad throughout the study. Modified parasternal long-axis and parasternal short-axis views were used to obtain two-dimensional, M-mode and spectral Doppler images [9]. Systolic and diastolic anatomic parameters were obtained from M-mode tracings at the mid-papillary level. LV volume was estimated by the Teichholz formula. LV mass was estimated by the area-length method. Images were analysed off-line using the Prosolv data analysis software (version 2.5, Problem Solving Concepts, Inc., Indianapolis, IN, USA) by an investigator who was blind to the treatment allocation.
Morphometric analysis
At the end of the study, the thorax was opened, the abdominal aorta cannulated, and the heart arrested in diastole with KCl and CdCl2, excised and perfused retrogradely through the aorta with 10% neutral-buffered formalin. The right atrium was cut to allow drainage. The perfusion pressure was adjusted to match the mean arterial pressure. The LV chamber was filled with fixative from a pressure reservoir set at a height equivalent to the in vivo measured LV end-diastolic pressure [9–11]. The LV was sectioned serially into four rings perpendicular to its longitudinal axis, processed and embedded in paraffin. The infarct area fraction was calculated by computerized planimetry (Image-Pro Plus, Media-Cybernetics, Carlsbad, CA, USA) of digital images of three Masson’s trichrome-stained serial LV sections taken at 0.5–1.0 mm intervals along the longitudinal axis [9, 10]. The mid-section was used to measure LV diameter. The thickness of the infarct wall, septal wall and posterior wall was calculated in serial sections and averaged [9, 10]. An average sarcomere length of 2.1 μm was utilized in all cases to correct the raw measurements of LV anatomical parameters [11].
For the assessment of cardiomyocyte cross-sectional area, digital images were acquired from trichrome-stained myocardial sections. Cardiomyocyte cross-sectional area was measured in transversely sectioned myocytes with a circular profile and a central nucleus [7, 12]. On average, a total of 100 myocytes were measured in each heart. All morphometric analyses were performed by investigators who were blind to the treatment allocation.
Immunohistochemistry
Immunohistochemistry was performed in formalin-fixed 4-μm-thick histological sections. Cardiomyogenic differentiation was recognized by the presence of α-sarcomeric actin (Sigma-Aldrich, St. Louis, MO, USA) and troponin T (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); endothelial differentiation by PECAM-1 (Santa Cruz Biotechnology, Inc.) and von Willebrand factor (Sigma); and smooth muscle cell differentiation by α-smooth muscle actin (Sigma) [9, 13]. Colocalization of cell-specific markers with EGFP was used to identify cells that originated from BMCs [9, 14]. Nuclei were identified with 4′-6-diamidino-2-phenylindole (DAPI).
For the assessment of capillary density [12, 15], sections were stained with an anti-CD31 (Santa Cruz Biotechnology, Inc.) primary antibody followed by the addition of a tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary antibody [7, 13]. CD31+ capillary profiles were counted at 100× magnification in contiguous fields in the infarct zone, border zone and non-ischemic zone. On average, a total of 40–50 fields were counted in each heart. Myocardial sections were immunostained with directly fluorochrome-conjugated hairpin-1 probe and anti-Ki67 antibody to quantitatively determine the number of apoptotic and cycling cardiomyocytes, respectively [7, 9].
Statistical analysis
Data are reported as means ± S.E.M. Morphometric, histological and echocardiographic data were analysed with a one-way ANOVA followed by Student’s t-tests with the Bonferroni correction as appropriate [16]. For data with unequal variance, Kruskal-iWallis one-way ANOVA on ranks and pairwise multiple comparison (Dunn’s method) were performed. All statistical analyses were performed with the SPSS software (version 8, SPSS, Inc., Chicago, IL, USA).
Results
Exclusions
A total of 197 mice (55 WT and 142 EGFP transgenic) were used. Forty-nine WT mice were assigned to the MI studies (groups I–III), 142 EGFP transgenic mice were used as BM donors for cell isolation, and 6 mice were used for the determination of myocyte area. Five mice died in the early post-infarction period and 10 mice died within 72 hrs after intramyocardial injection. Eight mice were excluded from the study due to technical reasons, leaving a total of 11, 7 and 8 mice in groups I–III, respectively.
Myocardial infarct size
The average infarct area fraction did not differ significantly among the three groups (15.0 ± 1.4%, 14.2 ± 3.9% and 14.8 ± 2.8% in groups I, II and III, respectively, Fig. 3). The infarct area fraction measures the average area of scarred tissue, expressed as a percent of the LV area in three LV sections 0.5–1.0 mm apart [7, 9, 10].
Fig 3.
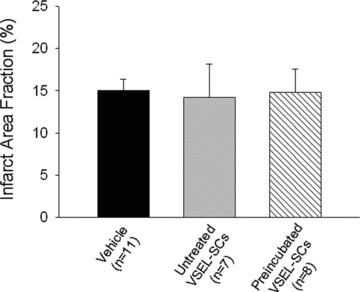
Myocardial infarct size. Myocardial infarct area fraction [(infarct area/LV area) × 100] assessed from Masson’s trichrome-stained hearts in groups I, II and III, which were treated with vehicle, expanded untreated VSEL-SCs and expanded pre-incubated VSEL-SCs, respectively. Data are means ± S.E.M. n= 7–11 mice/group.
Transplantation of pre-incubated VSEL-SCs attenuates LV systolic dysfunction
Before coronary occlusion (baseline), all parameters of LV function, measured by echocardiography, were similar in groups I, II and III (Fig. 4). At 48 hrs after cell transplantation (96 hrs after reperfusion), the degree of LV systolic functional impairment was also similar among the groups (Fig. 4), indicating that both the injury sustained during ischemia/reperfusion and that associated with intramyocardial injection were comparable. In mice that received vehicle (group I) or expanded but untreated VSEL-SCs (group II), there was further functional deterioration between 96 hrs and 35 days after reperfusion (Fig. 4). In contrast, LV systolic function was better preserved in mice treated with expanded and pre-incubated VSEL-SCs (group III), resulting in significantly greater LV ejection fraction (Fig. 4D) in group III compared with groups I and II at 35 days after MI. In group III there was also enhanced regional myocardial function in the infarct region, as evidenced by a 98% (P < 0.05) and 47% greater systolic wall thickening fraction compared with groups I and II, respectively (Fig. 4E).
Fig 4.
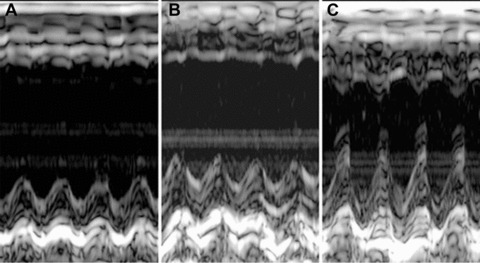
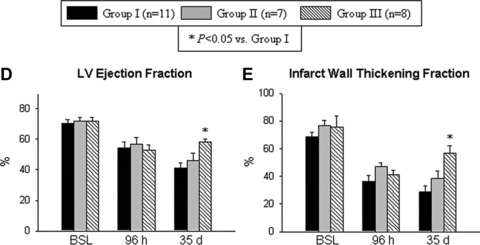
Impact of cell therapy on LV function. (A)–(C) Representative M-mode images at 35 days after coronary occlusion/reperfusion from mice that were given vehicle (group I) (A), expanded and untreated VSEL-SCs (group II) (B) and expanded pre-incubated VSEL-SCs (group III) (C). Compared with the hearts in groups I and II, the heart in group III exhibited a smaller LV cavity, a thicker infarct wall and improved motion of the infarct wall. (D–E) Quantitative echocardiographic analysis revealed improvement in LV functional parameters following transplantation of pre-incubated VSEL-SCs at 35 days after MI. Data are means ± S.E.M. n= 7–11 mice/group. *P<0.05 versus group I at 35 days.
Transplantation of pre-incubated VSEL-SCs attenuates LV expansion
The extent of LV remodelling at 35 days after infarction was assessed both morphometrically and echocardiographically (Fig. 5). By morphometry, the differences among groups did not reach statistical significance (Fig. S1). By echocardiography, LV end-diastolic volume at 35 days in group III (63 ± 11 μl) was 26% smaller compared with group I (86 ± 8 μl), albeit without statistical significance (Fig. 5D). These data suggest a possible trend towards improvement in post-MI LV expansion in mice that were given pre-incubated VSEL-SCs as compared with vehicle-treated mice.
Fig 5.
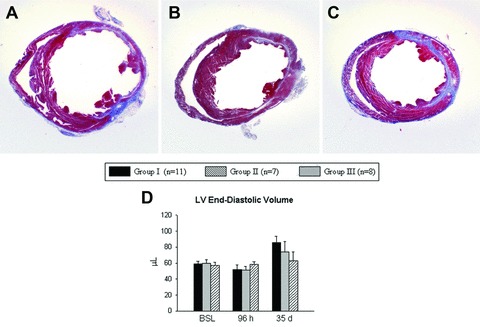
Impact of cell therapy on LV anatomy. (A)–(C) Representative Masson’s trichrome-stained myocardial sections from mice that were given vehicle (group I) (A), expanded and untreated VSEL-SCs (group II) (B) and expanded pre-incubated VSEL-SCs (group III) (C). (D) Echocardiographic analysis of LV end-diastolic volume at 35 days after MI. Data are means ± S.E.M. n= 7–11 mice/group.
Transplantation of pre-incubated VSEL-SCs attenuates LV hypertrophy
Because post-infarct LV remodelling is associated with myocyte hypertrophy and increased LV mass, we investigated the effects of VSEL-SC therapy on these parameters. To this end, we compared the three infarcted groups (groups I–III) with a separate control group (n= 6) of non-infarcted mice that were of similar age (10–12 weeks) and did not undergo surgery. Although the differences did not reach statistical significance, in infarcted mice treated with vehicle (group I) or expanded but not pre-incubated VSEL-SCs (group II), the cross-sectional myocyte area at 35 days after MI (219 ± 21 μm2 and 188 ± 31 μm2 in groups I and II, respectively) was 48% and 27% higher compared with non-infarcted control mice (148 ± 16 μm2). However, in mice given expanded and pre-incubated VSEL-SCs (group III), the myocyte area (147 ± 17 μm2) was virtually identical to that in non-infarcted mice and 30% smaller than that in group I (Fig. 6). These results were corroborated by the echocardiographic estimates of LV mass, which were significantly increased from baseline to 35 days in groups I and II but not in group III; in the latter group, the LV mass was 24% smaller than in group I (P<0.05) (Fig. 6E). Together, these data indicate that transplantation of expanded and pre-incubated VSEL-SCs is associated with attenuation of myocyte hypertrophy.
Fig 6.
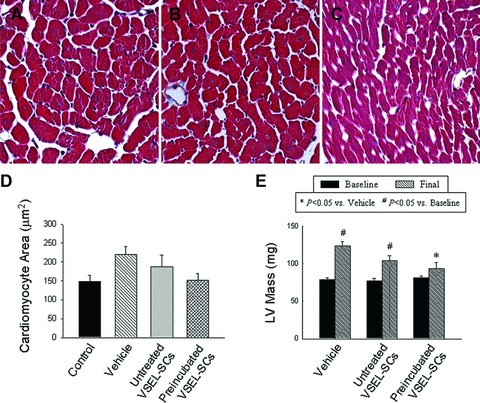
Impact of cell therapy on cardiomyocyte hypertrophy and LV mass. (A)–(C) Representative images of cardiomyocytes in the viable myocardium from Masson’s trichrome-stained sections of hearts of mice that were given vehicle (group I) (A), expanded and untreated VSEL-SCs (group II) (B) and expanded pre-incubated VSEL-SCs (group III) (C). (D) Quantitative assessment of myocyte cross-sectional area in groups I–III compared with non-infarcted control hearts. (E) Compared with group I, echocardiographically estimated LV mass was significantly less in group III. Data are means ± S.E.M. n= 7–11 mice/group. (E) *P<0.05 versus vehicle (final); #P < 0.05 versus respective baseline values.
Myocyte regeneration
Cardiomyogenic specification of transplanted VSEL-SCs was inferred by concomitant positivity for α-sarcomeric actin and EGFP [9, 17]. Although scattered EGFP+/α-sarcomeric actin+ cells were identified in the peri-infarct zone in both groups II and III (Fig. 7), the number of such cells was very small in both groups and the morphology did not resemble that of adult myocytes, indicating that the functional and structural benefits of VSEL-SCs could not be accounted for by their differentiation into new cardiac myocytes.
Fig 7.
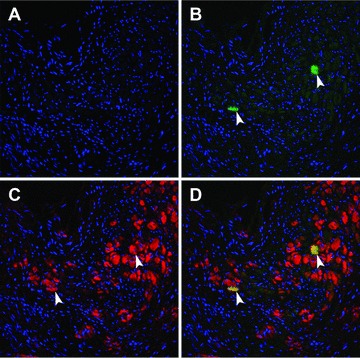
Colocalization of EGFP and cardiac proteins after transplantation of pre-incubated VSEL-SCs. VSEL-SC-derived cells are identified by EGFP immunoreactivity [(B)–(D), green]. Cells with possible cardiogenic commitment are identified by α-sarcomeric actin immunoreactivity [(C), (D), red]. (D) shows the merged image. Two cells are shown that are positive for both EGFP and α-sarcomeric actin (arrowheads, D, yellow).
Impact of cell therapy on capillary density, myocyte apoptosis and myocyte cycling
Either in the infarct border zone or in the non-ischemic zone, there was no significant difference among the three groups with respect to capillary density (Fig. S2), Ki67 immunoreactivity (for identification of cycling cells) (Fig. S3) and immunoreactivity for the hairpin-1 probe (for detection of apoptosis) (data not shown).
Discussion
Although basic as well as clinical studies indicate that transplantation of adult BMCs induces cardiac repair and improves LV function after MI [1], the improvement in LV ejection fraction and the reduction in infarct scar size observed in clinical trials have been relatively modest [1], prompting the search for specific cell types that may confer more consistent benefits. We have previously shown that VSEL-SCs, a recently discovered population of pluripotent stem cells found in adult bone narrow [3, 4, 6], can be effectively used to alleviate post-MI cardiac dysfunction and remodelling in mice [7]. However, if this new form of cell therapy is to be used in humans, methods need to be developed that will enable expansion of VSEL-SCs prior to transplantation, because their numbers in the bone marrow are very low [3]. In this study we explored the feasibility of expanding VSEL-SCs on a C2C12 cell feeder layer prior to injection into infarcted hearts.
Our salient findings are the following: (i) in a murine model of reperfused MI, intramyocardial injection of 105 VSEL-SCs expanded and then pre-incubated in cardiomyogenic medium resulted in improvement in LV function and attenuation of LV hypertrophy; (ii) in contrast, injection of the same number of expanded but not pre-incubated VSEL-SCs failed to impart any beneficial effect and (iii) at 35 days after MI, very few cells derived from the transplanted VSEL-SCs were found that expressed markers of cardiomyocytic or endothelial differentiation, implying that the salubrious effects of pre-incubated VSEL-SCs are mediated by paracrine mechanisms. Together, these results demonstrate that VSEL-SCs expanded in vitro can be used to alleviate cardiac dysfunction and remodelling after MI, but for this strategy to be effective, the cells need to be exposed to a combination of cardiogenic growth factors and cytokines (TGF-β1, VEGF, bFGF and IGF-1) prior to transplantation.
Because the number of cells transplanted may be a major determinant of their efficacy [18, 19], and because many cells are lost following myocardial transplantation as a result of washout and death [20, 21], injecting large numbers of cells is important, at least in principle. VSEL-SCs are very rare in the adult bone marrow (∼0.01% of all nucleated BMCs); consequently, in our previous study [7] we were unable to inject more than 10,000 freshly isolated VSEL-SCs in each mouse, a number that may have been too low to produce an optimal therapeutic effect. This problem is likely to be more severe when repair is attempted in large hearts and when cells are given by intracoronary infusion (the most clinically applicable route) rather than intramyocardial injection. To overcome this limitation, which will likely impede clinical translation, in the present study we expanded VSEL-SCs on a layer of C2C12 myoblast cells in culture and isolated the expanded EGFP-labelled VSEL-SCs by flow cytometry. Interestingly, although in this study we injected a 10-fold greater number of VSEL-SCs than in our previous study of freshly isolated VSEL-SCs [7], transplantation of expanded VSEL-SCs without prior exposure to cardiogenic factors (group II) failed to produce statistically significant improvements in LV function and remodelling. In contrast, pre-incubation of the same number of expanded VSEL-SCs in cardiomyogenic medium (group III) resulted in significant improvements in LV function and dimensions. Because transplantation of only 10,000 freshly isolated VSEL-SCs is sufficient to effect functional and structural improvements in this murine model [7], the present findings suggest that the process of expansion itself somehow causes the cells to lose their ability to alleviate post-MI dysfunction and remodelling (although expansion in culture enables the use of greater numbers of VSEL-SCs), and that incubation with cardiomyogenic medium can obviate this problem. Importantly, following expansion and isolation by flow cytometry, expanded VSEL-SCs were repeatedly washed to remove the various components of the expansion medium; therefore, the observed beneficial effects cannot be ascribed to actions exerted by the medium. Thus, both the mechanism whereby expansion causes loss of reparative ability and the mechanism whereby exposure to TGF-β1, VEGF, bFGF and IGF-1 restores this property remain unknown and represent important areas for future investigations. Furthermore, whether pre-incubation would improve the benefits observed with freshly isolated VSEL-SCs, and whether transplantation of larger numbers of expanded untreated (or expanded pre-incubated) VSEL-SCs would prove more effective compared with small number of non-expanded, freshly isolated VSEL-SCs also remain to be examined in future studies.
The mechanism that underlies the improvement in LV function observed with pre-incubated VSEL-SCs is unclear. We have previously reported that VSEL-SCs isolated from the adult bone marrow express markers indicative of commitment to several unrelated lineages, including neuronal, skeletal muscle, endothelial and cardiac lineages [2, 3, 5]. As mentioned above, in our previous study [7] transplantation of freshly isolated VSEL-SCs improved LV function and dimensions, but only a small number of VSEL-SC-derived cells expressing cardiac markers were observed after 35 days. We reasoned that enhancing the cardiomyogenic specification of the transplanted cells would improve efficacy and that exposure of VSEL-SCs to cardiomyogenic factors would achieve this goal (i.e. it would increase the number of VSEL-SC-derived cardiomyocytes and thereby further improve LV function and geometry). Several components of our cardiomyogenic medium, including TGF-β1[22], bFGF [23] and IGF-1 [24], have been implicated in cardiogenesis in embryonic developmental studies. These factors have also been used successfully to induce cardiac differentiation of various embryonic and adult cells in vitro[25, 26], suggesting that priming of cells with these factors is likely to augment cardiomyocytic differentiation following transplantation in vivo. Indeed, pre-treatment of bone marrow derived CD117+ cells with TGF-β1 followed by intramyocardial injection has been reported to enhance cardiomyocyte regeneration and improve LV function after acute MI [27]. Similarly, pre-treatment of mesenchymal stem cells with FGF, IGF-1 and BMP-2 has been shown to induce the expression of cardiac-specific transcription factors in vitro and improve cardiomyocytic differentiation and infarct repair in vivo[28]. However, in the present study we detected only a small number of VSEL-SC-derived cells expressing cardiac-specific proteins, regardless of whether the VSEL-SCs had been incubated with (group III) or without (group II) cardiogenic medium. Therefore, generation of new cardiomyocytes was not an important mechanism whereby pre-incubation with TGF-β1, VEGF, bFGF and IGF-1 improved the outcome.
A similar pattern has been observed in several other studies [7, 29, 30]. Indeed, whether differentiation of transplanted cells into cells of cardiac and vascular lineages serves as the primary mechanism of infarct repair with cell therapy remains controversial [31–33]. For this reason, paracrine actions of beneficial growth factors/cytokines secreted by transplanted cells have been proposed to be a major mechanism of cell therapy-induced cardiac repair [30]. It is plausible that exposure to cardiomyogenic factors altered the secretome of expanded VSEL-SCs in a favourable manner. Such priming may also enhance the ability of VSEL-SCs to inhibit myocyte apoptosis and/or activate endogenous cardiac stem cells [34, 35], resulting in preservation of cardiac mass and/or new myocyte formation. Although at 35 days after MI the number of cardiomyocytes positive for hairpin-1 and Ki67 did not differ significantly among the three groups, it remains possible that transplantation of expanded pre-treated VSEL-SCs was associated with reduction of apoptosis and/or increased cardiomyocyte proliferation at earlier time-points. It is also possible that the primary beneficial effect of enhanced secretion of growth factors by pre-incubated VSEL-SCs was inhibition of LV hypertrophy, which in turn positively impacted LV function. This hypothesis is supported by the attenuation of cardiomyocyte hypertrophy in hearts treated with pre-incubated VSEL-SCs (Fig. 6). Further studies will be necessary to fully characterize the genetic properties of expanded VSEL-SCs [36] and to identify the molecular mechanisms via which exposure to cardiomyogenic medium influenced the reparative properties of expanded VSEL-SCs.
In conclusion, VSEL-SCs expanded in culture retain their ability to alleviate LV dysfunction and remodelling after a reperfused MI, provided that prior to transplantation they are exposed to cardiomyogenic growth factors and cytokines (TGF-β1, VEGF, bFGF and IGF-1). The mechanism whereby such pre-incubation confers therapeutic efficacy remains unclear but, counter intuitively, does not involve differentiation of VSEL-SCs into new cardiac cells. The present results support the potential utility of VSEL-SCs as cellular substrates for cardiac repair after MI and should facilitate clinical translation of VSEL-SC transplantation. Future investigations will be necessary to identify the specific molecular mechanisms whereby pre-incubation with TGF-β1, VEGF, bFGF and IGF-1 enhances the cardiac reparative benefits of VSEL-SC therapy.
Acknowledgments
This study was supported in part by NIH grants R01 HL-72410, HL-55757, HL-70897, HL-76794, HL-78825, HL-89939, CA-106281, DK-074720 and R21 HL-89737, and the Stella & Henry Hoenig endowment, and the ‘Homing’ grant from the Foundation for Polish Science (HOM/2008/15).
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Morphometric assessment of LV anatomy. Thefigure illustrates LV chamber diameter measured postmortemin hearts arrested in diastole. The differences in chamber diameteramong the three groups did not reach statistical significance. Dataare means ± SEM. n = 7--11 mice/group.
Fig. S2. Quantitative assessment of myocardial capillarydensity. (A, B) Myocardial capillary density in the infarctborder zone (A) and in the nonischemic zone (B)revealed no significant difference among mice that were givenvehicle, expanded untreated VSEL-SCs, and expanded preincubatedVSEL-SCs. Data are means ± SEM. n = 7--11 mice/group.
Fig. S3. Quantitative assessment of cardiomyocyte cellcycle activity. (A, B) The percentage of myocyte nucleiexpressing Ki67 in the infarct border zone (A) and in thenonischemic zone (B) revealed no significant differenceamong mice that were given vehicle, expanded untreated VSEL-SCs,and expanded preincubated VSEL-SCs. Data are means ± SEM. n = 7--11 mice/group.
References
- 1.Abdel-latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Int Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Ratajczak MZ, Kucia M, Reca R, et al. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 3.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 4.Zuba-Surma EK, Kucia M, Abdel-Latif A, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood following myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuba-Surma EK, Kucia M, Dawn B, et al. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–73. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawn B, Tiwari S, Kucia MJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–55. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Wu WJ, Qiu Y, et al. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–87. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawn B, Guo Y, Rezazadeh A, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Li B, Wang X, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–9. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anversa P, Olivetti G. The cardiovascular system. In: Page E, Fozzard HA, Solaro RJ, editors. The heart. 1st ed. New York, NY: Oxford University Press; 2002. pp. 75–144. [Google Scholar]
- 12.Anversa P, Beghi C, Kikkawa Y, et al. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ Res. 1986;58:26–37. doi: 10.1161/01.res.58.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–71. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawn B, Zuba-Surma EK, Abdel-Latif A, et al. Cardiac stem cell therapy for myocardial re-generation. A clinical perspective. Minerva Cardioangiol. 2005;53:549–64. [PubMed] [Google Scholar]
- 15.Anversa P, Melissari M, Beghi C, et al. Structural compensatory mechanisms in rat heart in early spontaneous hypertension. Am J Physiol. 1984;246:H739–46. doi: 10.1152/ajpheart.1984.246.6.H739. [DOI] [PubMed] [Google Scholar]
- 16.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 18.Schuster MD, Kocher AA, Seki T, et al. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol. 2004;287:H525–32. doi: 10.1152/ajpheart.00058.2004. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 20.Dow J, Simkhovich BZ, Kedes L, et al. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67:301–7. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 22.Muslin AJ, Williams LT. Well-defined growth factors promote cardiac development in axolotl mesodermal explants. Development. 1991;112:1095–101. doi: 10.1242/dev.112.4.1095. [DOI] [PubMed] [Google Scholar]
- 23.Spirito P, Fu YM, Yu ZX, et al. Immunohistochemical localization of basic and acidic fibroblast growth factors in the developing rat heart. Circulation. 1991;84:322–32. doi: 10.1161/01.cir.84.1.322. [DOI] [PubMed] [Google Scholar]
- 24.Bassas L, Lesniak MA, Serrano J, et al. Developmental regulation of insulin and type I insulin-like growth factor receptors and absence of type II receptors in chicken embryo tissues. Diabetes. 1988;37:637–44. doi: 10.2337/diab.37.5.637. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Latif A, Zuba-Surma EK, Case J, et al. TGF-beta1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Res Cardiol. 2008;103:514–24. doi: 10.1007/s00395-008-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heng BC, Haider H, Sim EK, et al. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42. doi: 10.1016/j.cardiores.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Li TS, Hayashi M, Ito H, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–45. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 28.Bartunek J, Croissant JD, Wijns W, et al. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H1095–104. doi: 10.1152/ajpheart.01009.2005. [DOI] [PubMed] [Google Scholar]
- 29.Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 30.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawn B, Bolli R. Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circ Res. 2006;98:1002–13. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 33.Guan K, Hasenfuss G. Do stem cells in the heart truly differentiate into cardiomyocytes? J Mol Cell Cardiol. 2007;43:377–87. doi: 10.1016/j.yjmcc.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 34.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 35.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 36.Shin DM, Zuba-Surma EK, Wu W, et al. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23:2042–51. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphometric assessment of LV anatomy. Thefigure illustrates LV chamber diameter measured postmortemin hearts arrested in diastole. The differences in chamber diameteramong the three groups did not reach statistical significance. Dataare means ± SEM. n = 7--11 mice/group.
Fig. S2. Quantitative assessment of myocardial capillarydensity. (A, B) Myocardial capillary density in the infarctborder zone (A) and in the nonischemic zone (B)revealed no significant difference among mice that were givenvehicle, expanded untreated VSEL-SCs, and expanded preincubatedVSEL-SCs. Data are means ± SEM. n = 7--11 mice/group.
Fig. S3. Quantitative assessment of cardiomyocyte cellcycle activity. (A, B) The percentage of myocyte nucleiexpressing Ki67 in the infarct border zone (A) and in thenonischemic zone (B) revealed no significant differenceamong mice that were given vehicle, expanded untreated VSEL-SCs,and expanded preincubated VSEL-SCs. Data are means ± SEM. n = 7--11 mice/group.


