INTRODUCTION
Despite all efforts towards its control and prevention, dental caries still remains a global health problem affecting all ages [1]. Although a decline in its prevalence has been witnessed in some developed countries [2], the level of this decline has been exaggerated [3, 4]. National Institutes of Health survey (1989) claims that 50% of 12-year old children in the US are free from tooth decay; however, in this survey, deciduous teeth were not examined and 85% of 17-year old children had one or more carious teeth [5].
Dental caries is an infectious disease, the development of which is a dynamic process involving alternating demineralization and remineralization, rather than unidirectional demineralization [6]. Demineralization of tooth structure is caused by the organic acids produced in dental plaque biofilm by the metabolic action of the cariogenic microorganisms on fermentable carbohydrates [7, 8, 9]. Formation of biofilm is a biological process associated with the attachment, detachment and proliferation of oral bacteria on the tooth surfaces. The dental biofilm is formed via adhesion of planktonic bacteria to a protein pellicle coating the tooth surfaces[10]. Many types of bacteria participate in the formation of the dental biofilm [10, 11]. More than five Streptococcus species and Actinomycesviscosus are regarded as early colonizers of tooth surfaces, while mutans streptococci such as Streptococcus sobrinus and Streptococcus mutans are considered late colonizers of the dental biofilm[12]. The inhibition of plaque biofilm formation is the key to successful control and prevention of dental caries. Previous antibacterial mouth rinses, which generally contain fluorides, alcohols, detergents and other antimicrobial substances, effectively reduce plaque formation. Synthetic antimicrobials used in tooth pastes and mouth rinses include povidone iodine products, chlorhexidine, cetylpyridinium chloride, triclosan and zinc citrate [13]. However, many of these substances cause unwarranted undesirable effects like vomiting, diarrhea and tooth staining.
Nanoemulsions (NE) are a unique class of disinfectants produced by mixing a water immiscible oil phase into an aqueous phase under high shear forces. This process yields a uniform population of droplets with mean diameters ranging from 200 to 400 nm. The emulsions have the appearance and consistency of whole milk. They are only kinetically stable [14]. NE have broad biocidal efficacy against bacteria, enveloped viruses, and fungi [15] by disrupting their outer membranes [16, 17]. The mixed immiscible preparations of soybean oil and water represent a new generation of disinfectants that selectively disrupt membranes of Gram-positive and Gram-negative bacteria [18], fungi in dilutions up to 1:1000 [15, 16] and enveloped virus [15, 19]. Therefore, the use of NE to control the adhesion and biofilm formation of these cariogenic bacteria on the tooth surface is a logical approach to prevent this common oral disease.
MATERIALS AND METHODS
Biofilm formation and emulsion preparation
Streptococcus mutans (ATCC 33402) grown in Brain Heart Infusion (BHI)(Difco Laboratories, Detroit, MI)supplemented with 2% sucrose was used for the biofilm and adherence assays. The oil-in-water nanoemulsion was composed of soybean oil (25% v/v of the total emulsion), cetylpyridiniumchloride (CPC)(1% w/v), and Triton X-100 (10% v/v). The ingredients were emulsified with a Microfluidizer (M-110L, Microfluidics, Newton, MA) at 20,000 psi and at room temperature. Two passes were carried out. The particle size was determined using a light scattering method (Dynamic Light Scattering, Brookhaven Instruments, Holtsville, NY) icrofluidizer emulsification resulted in a narrow distribution of droplets with a mean diameter of 308 nm (Fig. 1). This nanoemulsion was used for all experiments.
Fig. 1.
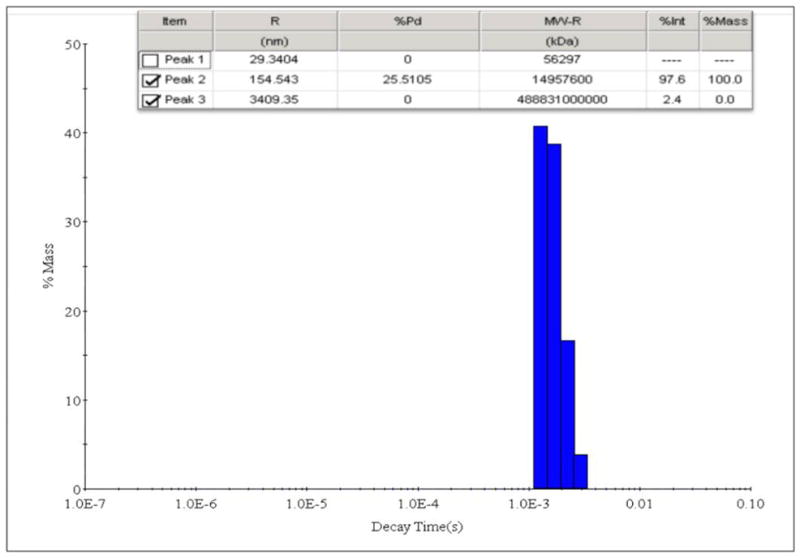
Light scattering image showing droplet size distribution of nanoemulsion
Determination of minimum inhibitory (MIC) and bactericidal (MBC) concentrations
The antimicrobial activity of NE was first evaluated by determining MIC and MBC. MIC was defined as the lowest concentration of the test agent that gives restricted growth and MBC was defined as the lowest concentration that allowed no visible growth on agar (99.9% killed). MBC concentrations were usually higher than the MIC. Chlorhexidinedigluconate 0.12% (v/v) (Sigma Aldrich), a potent anti-plaque agent, was used as positive control. NE was serially diluted in sterile BHI broth in microtitre wells. Each well was inoculated with 25 μl of standardized cell suspension (107 CFU/ml) and incubated at 37°C overnight. The highest dilution where no growth occurred was recorded as the MIC. For MBC testing, aliquots (10μl) of broth from wells containing no growth were plated onto BHI agar and again incubated overnight at 37°C. The highest dilution where there were no survivors was recorded as the MBC. In both of the above methods, controls for each organism were performed using sterile water in place of NE and the purity of cultures was confirmed by plating growth from wells.
Kinetics of killing
Kinetics of killing assay was performed as previously described by Teixeira et al. [20]with some modification. Overnight bacterial cultures were added to 1, 4, 20 and 200 dilutions of the NE in microtiter plates. After the addition of each culture and at 1, 5, 15, 30 and 60 min, 1 ml samples of inoculated emulsions were immediately diluted in 9 ml of BHI. For viable counts, BHI broth samples were incubated at 37 °C for 24 h. The optical density of the resulting broth was read at 490 nm to measure killing effect of nanoemulsion. At least three independent experiments were carried out for each set of conditions. Chlorhexidinedigluconate 0.12% (v/v) was used as positive control. In each case, the mean optical density of the blank control was subtracted from the readings of all other samples.
Adherence Tests
A bacterial adherence assay was performed as previously described by Smullen et al., [21] with some modification. Overnight bacterial cultures containing 107 CFU/ml were added to NE in different dilutions (25, 50, 250 and 2500) in 5 ml test tubes. The tubes were incubated aerobically (to simulate conditions in the mouth) at 37°C for 24 h inclined plane position at 30°C. Attached bacteria were fixed with 5 ml of methanol per tube for 15 min. The tubes were then emptied and air dried. Each tube was then stained for 5 min with 5 ml of 1% (v/v) crystal violet. Excess stain was rinsed off by placing the tube under running tap water. The microplates were air dried and the dye bound to the adherent cells was removed with 5 ml of 33% (v/v) glacial acetic acid per tube. The optical density of the resulting solutions was read at 595 nm. Chlorhexidinedigluconate 0.12% (v/v) was used as positive control. In each case, the mean optical density of the blank control was subtracted from the readings of all other samples.
Biofilm studies
Biofilm formation in plastic microplates was performed as previously described by Stepanovic et al. [22]. A 20 μl portion of an overnight broth culture was added to each well of a 96-well tissue culture plate which was incubated aerobically, with mild agitation at 70 rpm, for 72 h at 37 °C. Every 12 h, the medium containing suspended bacterial cells was removed and an equal volume of fresh medium was added. Negative controls were obtained by incubating the microplates with media without inocula. At least three independent experiments were performed. After removing the supernatant media, the biofilms were treated with 1, 10, 50, 100, 200 and 250 μl emulsion, for 30 min at room temperature and without agitation. Following this, the emulsion was removed and the wells were gently washed twice with sterilized distilled water.
Quantification of viable cells in biofilms in plastic microplates was performed as previously described Stepanovic et al.[22]with some modification. Attached bacteria were fixed with 250 μl of methanol per well for 15 min. The microplates were then emptied and air dried. Each well was then stained for 5 min with 250 μl of 1% (v/v) crystal violet. Excess stain was rinsed off by placing the microplate under running tap water. The microplates were air dried and the dye bound to the adherent cells was removed with 250 μl of 33% (v/v) glacial acetic acid per well. The optical density of the resulting solutions was read at 595 nm on a microplate reader. Chlorhexidinedigluconate 0.12% (v/v) was used as positive control. In each case, the mean optical density of the blank control was subtracted from the readings of all other samples.
Live and dead staining
For the biofilm viability test, S. mutans was grown in 4-well Lab-Tek chamber slides with cover slide (NalgeNunc International, Naperville, IL) plates for 24 hrs with BHI broth and 2% sucrose supplementation. The grown biofilm was treated with NE for 1 min, 5 min and 1 hr. Following treatment, the biofilm was stained with L 7012 LIVE/DEAD® BacLight™ Bacterial Viability Kitfrom Molecular Probes Inc. (Eugene, OR) as described by Neu and Lawrence[23]. The live/dead stain, stored at −20 °C, was warmed to room temperature and centrifuged prior to use. The staining solution, containing the two components SYTO9 and propidium iodide, was mixed in equal quantities and applied to the wells for 15 min. The samples were immediately examined via an Olympus FV1000 confocal system on an IX81 microscope (Olympus Life Science, Center Valley, PA)at excitation wavelengths of 488 and 543 nm.
Image analysis was conducted as described by Al-Ahmad et al. [24]. In order to quantify green (live) and red (dead) areas of the biofilms, a maximal projection of each image stack was built using the program LSM Image Browser (Zeiss, Oberkochen, Germany). Using the image analysis program Image J1.42q(Wayne Rasband National Institute of health, USA), the red and green projections were converted into merged black and white(B/W) images. In order to determine the area covered by all cells, live or dead, B/W intensity thresholds were manually set for each of the measured biofilm areas. The resulting green and red ratios were analyzed for their statistical significance.
Morphology by scanning electron microscopy
Scanning electron microscope (SEM) images were carried out on biofilm formed overnight in 6-well plates with glass slides in the bottom of each well. The biofilm examined was that with MBC concentration of NE for 30 min at room temperature. The emulsion containing medium was removed and the wells were gently washed twice with sterilized distilled water. Samples were placed in a fixative (4% formaldehyde [vol/vol], 1% glutaraldehyde [vol/vol] in PBS) until ready for scanning. The samples were rinsed in 0.1 M phosphate buffer (2 times, 3 min each) and then placed in 1% Zetterquist’s osmium for 30 min. The samples were subsequently dehydrated in a series of ethanol washes (70% for 10 min, 95% for 10 min, and 100% for 20 min), treated (2 times, 5 min each) with hexamethyldisilizane(Polysciences Inc., Warrington, PA), and finally air dried in a desiccator. The specimens were coated using a DESK IV Model Sputter Coater (Denton Vacuum, Moorestown, NJ) with a gold-palladium (40%/60%) target. After processing, samples were observed with a LEO 435VP Variable Pressure Digital Scanning Electron Microscope (LEO Electron Microscopy Ltd., Cambridge, England) in high vacuum mode at 20 kV [25].
Statistical analysis
Experiments were performed in triplicate and the means and standard deviations calculated. Statistical significance was determined using two-way ANOVA with replication using Microsoft Excel, with the level of significance (α) pre-chosen at 0.05.
RESULTS
Determination MIC and MBC
When Streptococcus mutans were exposed to logarithmic dilutions of NE, the agent demonstrated a dose-dependent antibacterial activity against S. mutans, with MIC and MBC of NE occurring at dilutions of 19683 and 2187 respectively. Inhibitory concentration of NE was 27-fold smaller than that of chlorhexidine (729).
Time kinetics
During the tested period of incubation with cell suspensions, NE considerably reduced the number of survivors, Fig. 2. In 1 min nanoemulsion reduced survivors by 71.9% while a 24.5% reduction was observed with chlorhexidine. Lower dilutions of NE resulted in the fewest survivors, with a dilution of 1 showing the highest activity. Percentage values were calculated based on the negative control.
Fig. 2.
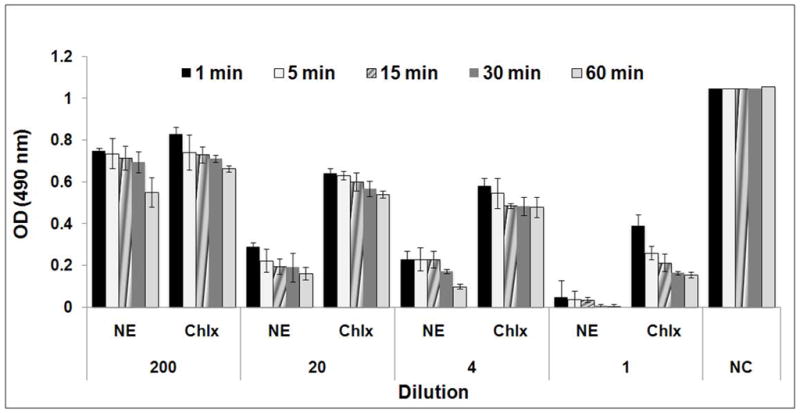
Effect of nanoemulsion on the survival of Streptococcus mutans (NE- Nanoemulsion; Chlx- Chlorhexidinedigluconate; NC- negative control (sterile distilled water))
Effect on inhibition of adherence of S. mutans to glass surfaces
When the inhibitory effect of NE on adherence of cells to the glass surfaces of test tubes was examined using growing cells of S. mutans, NE inhibited the adherence of the growing cells(Figure 3), and the inhibitory effectiveness was dependent on the dilution of NE. Furthermore, the inhibitory effect of NE on cellular adherence was significantly higher (P <0.03) than that of chlorhexidine at the 25 dilution, where an 89% adherence inhibition by NE was observed.
Fig. 3.
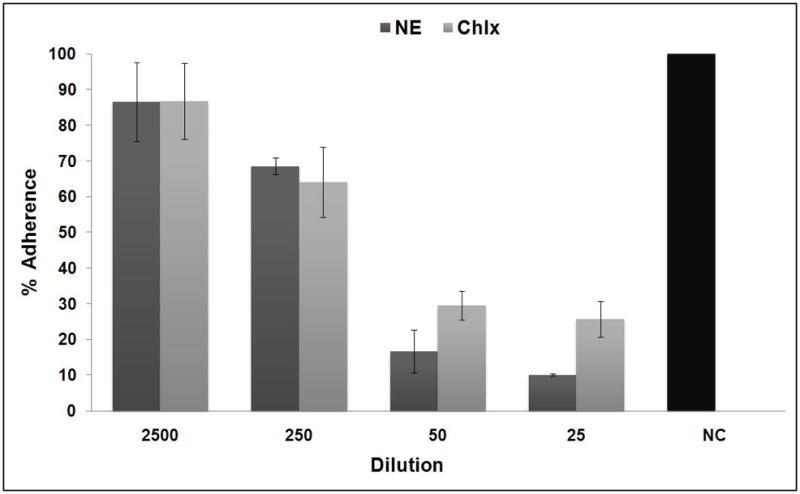
Inhibitory effect of nanoemulsion on glass surface adherence of Streptococcus mutans (NE- Nanoemulsion; Chlx- Chlorhexidinedigluconate; NC- negative control (sterile distilled water))
Biofilm studies
NE markedly inhibited Streptococcus mutans biofilm formation at quantities as low as 100, 200 and 250 μl, respectively (p < 0.05). Highest inhibition of biofilm formation was observed at 250 μl level (Fig. 4).
Fig. 4.
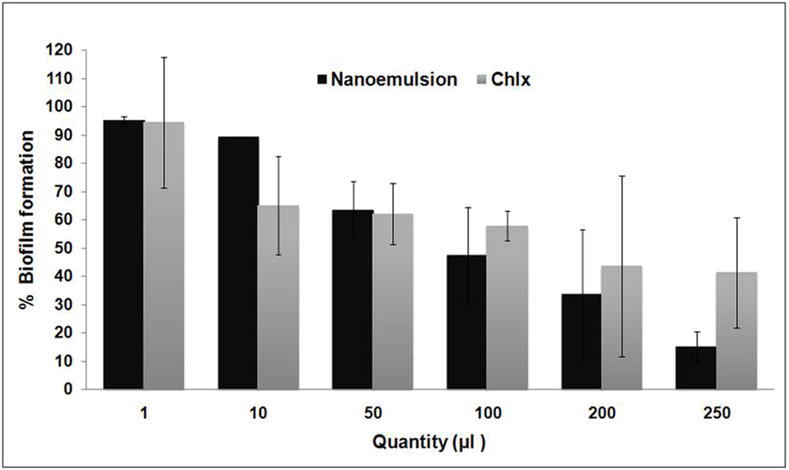
Inhibitory effect of nanoemulsion on growth of Streptococcus mutans biofilms(NE- Nanoemulsion; Chlx- Chlorhexidinedigluconate)
Live and dead staining
The effect of NE on S. mutans biofilm stained with LIVE/DEAD® BacLight™ Bacterial Viability Kitare shown in Figs. 5 and 6. In confocal micrographs (Fig. 6)of 24 hr sold S. mutans biofilm treated with NE, 0.2% CHLX and no treatment, red fluorescence indicates dead cells and green florescence live cells. The images reflect different green and red florescence intensities, and NE increased the dead cell area significantly (p < 0.05). The reduction of the green intensity caused by NE was comparable with the effect of chlorhexidinedigluconate, but was significantly higher than the reduction caused by the negative control (p < 0.05).
Fig. 5.
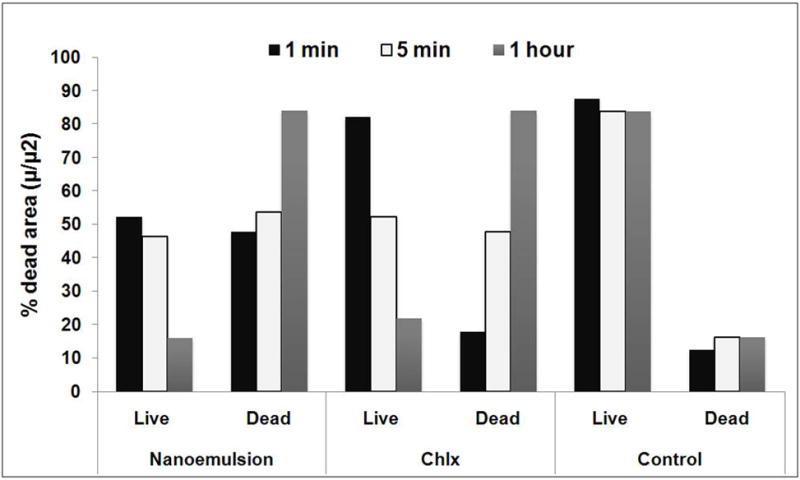
Nanoemulsionexposure to S. mutans biofilms-viability as measured with BacLight Live/Dead stain (Chlx- Chlorhexidinedigluconate; control- no treatment)
Fig. 6.
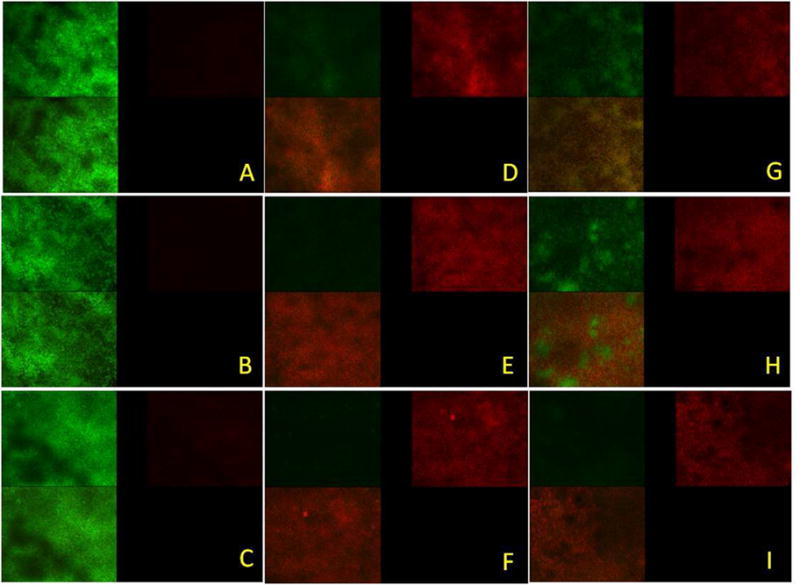
Confocal micrographs of Streptococcus mutans biofilms- control (no treatment) 1 min (A), 5 min (B) and 1 hour (C), treated with nanoemulsion 1 min (D), 5 min (E) and 1 hour (F), treated with Chlorhexidinedigluconate min (G), 5 min (H) and 1 hour (I). Each micrograph represents 3 optical sections- green, red and combined green and red from two channel images.
Morphology by scanning electron microscopy
When scanning electron microscope (SEM) examination was carried out to check for possible morphological changes in S. mutans caused by NE treatment, significant morphological changes were observed in the cells treated with nanoemulsion (Fig. 7B and B1) compared to intact cells (Fig. 7A). After NE treatment, the cell surface was remarkably disintegrated. Irregular boundaries were observed and margins of cell walls were unclear, apparently a direct effect of exposure to NE.
Fig. 7.
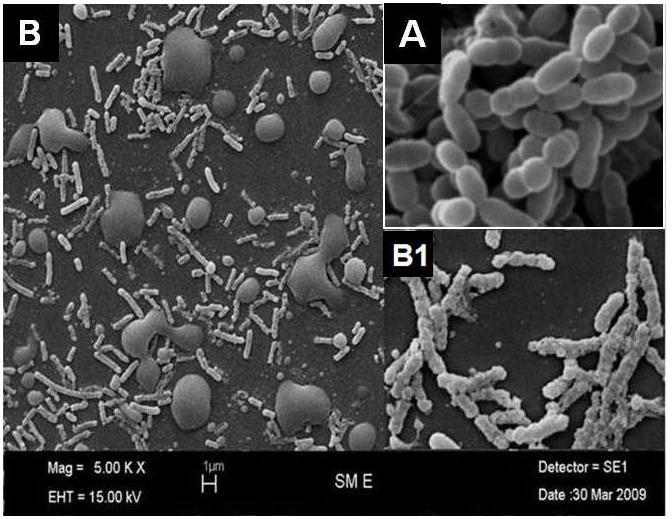
Scanning electron microscope (SEM) of A- control (no treatment) and B -nanoemulsion treated S. mutans with higher magnification inset. Note oil droplets in B1 from NE.
DISCUSSION
The application of nanoemulsion (NE)as an antimicrobial agent is a new and promising innovation [14, 26, 27, 28]. The investigation into the use of nanoemulsion as antimicrobial agent was prompted by the known problem of development of antimicrobial resistant strains experienced with the use of existing agents due to the widespread, and sometimes inappropriate, use of antibiotics, disinfectants and antiseptics [20]. These drawbacks justify further research and development of new antimicrobial agents targeting specific pathogens while being safe for the host. Since the mechanism of action of NE appears to be the non-specific disruption of bacterial cell membranes, NEs would not result in the development of resistant strains. Due to their intrinsic features, NE can be further diluted in aqueous solutions and stored at a broad range of temperatures for up to 2 years. Nanoemulsion has been reported to have extensive bactericidal, sporicidal and virucidal effects [16, 19, 28, 30, 31, 32]. NE at biocidal concentrations is non-toxic in short term application to skin, mucous membranes, and the gastrointestinal tract [16]. However, the application of nanoemulsion for the control of cariogenic biofilm has been widely investigated. In this study, we showed that one NE has antimicrobial activity against cariogenic planktonic and biofilm Streptococcus mutans. The results show that NE inhibited S. mutans with MIC and MBC occurring at dilutions of 19683 and 2187. Myc et al., [33] found that the MIC of a different nanoemulsion never exceeded 0.1% of NE. When compared with earlier reports our NE showed very high activity [15, 20, 34]. The nanoemulsion activity was 27-fold higher than that of Chlorhexidine, which is currently the most potent antimicrobial agent against oral biofilm. However, chlorhexidine is limited in its applications in oral care as it may produce detrimental side effects such as tooth discoloration [35, 36] and has the drawback of unpleasant taste.
Although time kinetics assays showed NE to reduce survivors by at least 71.9% in 1 min, and not complete elimination, one dilution of nanoemulsion showed 99% killing in 1 hour. Our results are in agreement with previous findings that emulsion had bactericidal properties against Gram-positive and enteric pathogen species [15, 18, 20]. In dental caries process, adherence of S. mutans to the tooth surface is the initial stage [37].Inhibition of adherence is essential for the prevention of dental caries [38]. The anti-cariogenic potential of NE may be attributed to their ability to inhibit the attachment of bacteria. NE is significantly effective in inhibiting the growth of the S. mutans(89%)by inhibition of adherence to glass surfaces.
Dental biofilm formation is mediated by the attachment, detachment and accumulation of oral microbial flora on the tooth surface. Initial adherence and colonization thus form the key events in biofilm formation [38,39]. The capacity of NE to remove biofilms of S. mutans was evaluated. Generally, exposure to emulsion for 30 min resulted in maximum reductions of biofilms and effectively reduced the numbers of the planktonic form. However, complete elimination of biofilm did not occur, possibly due to the short exposure time. Further work needs to be carried out to investigate the mechanisms of the interactions between emulsions, cells in suspension and biofilms [20].
In addition, Al-Adham et al. [40] found that one microemulsion was highly effective against biofilms of P. aeruginosa and S. aureus. Also, our previous work proved that nanoemulsion has high effect on controlling the cariogenic plaque formation and dental caries [41]. Some authors report that biofilm microorganisms are very resistant to typical cleaning agents, yet are susceptible to nanoemulsions [42, 43].
It is doubtful that the differentiation of viable and nonviable biofilm regions observed in this study is an artifact of BacLight staining. No problems have been reported with the uptake of LIVE/DEAD stains by oral bacteria or by biofilms [44]. Our results of disruption of biofilms by NE are in agreement with those previously reported by Al-Adham et al. [45] for P. aeruginosa and S. aureus by microemulsion. With NE treatment, cell surfaces were remarkably disintegrated and margins of cell walls were unclear, and probably reflect disruption of the cell wall that results in non-viability. A similar result was reported for a NE on Candida albicans[33, 46, 47].
In conclusion, nanoemulsion with droplets of mean diameter 308 nm showed remarkable inhibitory effects on the growth of cariogenic S. mutans at very high dilution. NE effectively inhibited adherence of S. mutans to glass surfaces and subsequent biofilm formation. The anti-adherence, anti-biofilm and morphological disruption effects of NE suggest that this material could be useful for the development of promising anti-cariogenic agents. Further studies are necessary to elucidate detailed mechanisms of action against bacteria and to assess their clinical relevance.
Acknowledgments
Sincere appreciation is extended to the authorities of University of Texas Health Science center at San Antonio, San Antonio, TX, USA and National Institute of Health (NIH/NIDCR K08DE018003)for providing fellowship and facilities to perform this research. We would also like to acknowledge the NIH supported UTHSCSA imaging core facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karthikeyan Ramalingam, Email: ramalingamk@uthscsa.edu, Department of Comprehensive Dentistry, MC 7919, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900, USA, Tel: 1 210 567 4584 Fax: 1 210 567 4587 Cell: 1 210 649 5976.
Bennett T. Amaechi, Email: Amaechi@uthscsa.edu, Associate Professor and Director of Cariology, Department of Comprehensive Dentistry, MC 7919, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900, USA, Tel: 1 210 567 3185 Fax: 1 210 567 4587 Cell: 1 210 834 7675.
H Ralph Rawls, Email: RAWLS@UTHSCSA.EDU, Professor in Restorative dentistry, Department of Comprehensive Dentistry, MC 7919, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900, USA, phone: 210-567-6871 Fax: 210-567-3669 (Fax).
A Lee Valerie, Email: leev@uthscsa.edu, Assistant Professor in Restorative dentistry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900, USA, Phone: 210-567-6871 Fax: 210-567-3669 (Fax).
References
- 1.Mandel I, Bowen WH, Tabak LA, editors. Cariology for the Nineties. New York: University of Rochester Press; 1993. [Google Scholar]
- 2.Glass RL. Conference on Declining Prevalence of Dental Caries. J Dent Res. 1982;61(Spec Iss):1304. [Google Scholar]
- 3.National Institutes of Health, Oral health of United States adults. National findings NIH publication no 87-2868. Washington DC: US Government Printing Office; 1987. The national survey of oral health in U.S. employed adults and seniors 1985–1986. [Google Scholar]
- 4.National Institutes of Health, Oral health of United States children. National and regional findings NIH publication no 89-2247. Washington DC: US Government Printing Office; 1989. The national survey of dental caries in U.S. school children 1986–1987. [Google Scholar]
- 5.Edelstein BL, Douglass CW. Dispelling the myth that 50 percent of U.S. schoolchildren have never had a cavity. Public Health Report. 1995;110:522–530. [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson C, Shore RC, Bonass WA, Brookes SJ, Boteva E, Kirkham J. Identification of human serum albumin in human caries lesions of enamel: the role of putative inhibitors of remineralization. Caries Res. 1998;32:193–199. doi: 10.1159/000016452. [DOI] [PubMed] [Google Scholar]
- 7.Twetman S, Lindqvist L. Effect of salivary lysozyme on glucose incorporation and acid production in Streptococcus mutans. Caries Res. 1985;19:414–421. doi: 10.1159/000260876. [DOI] [PubMed] [Google Scholar]
- 8.Kohler B, Birkhed D, Olsson S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1995;29:402–406. doi: 10.1159/000262099. [DOI] [PubMed] [Google Scholar]
- 9.Wiater A, Choma A, Szczodrak J. Insoluble glucans synthesized by cariogenic streptococci: a structural study. J Basic Microbiol. 1999;39:265–273. doi: 10.1002/(sici)1521-4028(199909)39:4<265::aid-jobm265>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg D. Studying plaque biofilms on various dental surfaces. In: An YH, Friedman RJ, editors. Handbook of Bacterial Adhesion Principles Methods and Application. Totowa NJ: Humana Press; 2000. pp. 353–370. [Google Scholar]
- 11.Marsh PD, Bradshaw DJ. Microbial community of dental plaque. In: Newman HN, Wilson M, editors. Dental Plaque Revisited: Oral Biofilms in Health and Disease. UK Bioline; 1999. pp. 237–253. [Google Scholar]
- 12.Rozen R, Bachrach G, Bronshteyn M, Gedalia I, Steinberg D. The role of fructans on dental biofilm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomycesviscosus. FEMS Microbiol Lett. 2001;195:205–210. doi: 10.1111/j.1574-6968.2001.tb10522.x. [DOI] [PubMed] [Google Scholar]
- 13.Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003;9:23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Advanced Drug Delivery Reviews. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 15.Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR., Jr A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria enveloped viruses and fungi. Microbiological Research. 2001;156:1–7. doi: 10.1078/0944-5013-00069. [DOI] [PubMed] [Google Scholar]
- 16.Hamouda T, Hayes MM, Cao Z. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus spores. J Infect Dis. 1999;180:1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- 17.Wright DC. Antimicrobial oil-in-water emulsions. 5475667. US patent. 1996
- 18.Hamouda T, Baker JR., Jr Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. Journal of Applied Microbiology. 2000;89:397–403. doi: 10.1046/j.1365-2672.2000.01127.x. [DOI] [PubMed] [Google Scholar]
- 19.Donovan BW, Reuter JD, Cao Z, Myc A, Johnson KJ, Baker JR., Jr Prevention of murine influenza A virus pneumonitis by surfactant nano-emulsions. Antiviral Chemistry and Chemotherapy. 2001;11:41–49. doi: 10.1177/095632020001100104. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira PC, Leite GM, Domingues RJ, Paul JS, Gibbs A, Ferreira JP. Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. International Journal of Food Microbiology. 2007;118:15–19. doi: 10.1016/j.ijfoodmicro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Smullen J, Koutsou GA, Foster HA, Zumbe A, Storey DM. The Antibacterial Activity of Plant Extracts Containing Polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–349. doi: 10.1159/000104791. [DOI] [PubMed] [Google Scholar]
- 22.Stepanovic S, Cirkovic I, Ranin L, Svabic-Vlahovic M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in Applied Microbiology. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 23.Neu TR, Lawrence JR. Development and structure of microbial stream biofilms as studied by confocal laser scanning microscopy. FEMS MicrobEcol. 1997;24:11–25. [Google Scholar]
- 24.Al-Ahmad A, Wunder A, Auschill TM, Follo M, Braun G, Hellwig E. The in vivo dynamics of Streptococcus spp., Actinomycesnaeslundii, Fusobacteriumnucleatum and Veillonella spp. in dental plaque biofilm as analyzed by five-color multiplex fluorescence in situ hybridization. J Med Microbiol. 2007;56:681–7. doi: 10.1099/jmm.0.47094-0. [DOI] [PubMed] [Google Scholar]
- 25.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans Biofilm Formation by Farnesol, a Quorum-Sensing Molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azemar N. The role of microemulsions in detergency processes. In: Solans C, Kunieda H, editors. Industrial Applications of Microemulsions. Science series New York: Marcel Dekker; 1997. [Google Scholar]
- 27.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Critical Reviews in Therapeutic Drug Carrier Systems. 1999;16:461–521. [PubMed] [Google Scholar]
- 28.Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Nanoemulsions: a new vehicle for skincare products. Advances in Colloid and Interface Science. 2004;108–109:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Baker JR, Wright DC, Hayes MM, Hamouda T, Brisker JM. Methods of inactivating bacteria including bacterial spores. 6015832. US Patent. 2000
- 30.Hamouda T, Wright DC, Brisker JM, Baker JR., Jr Microbiocidal effects of liposome-like microemulsions on pathogenic Gram negative bacteria. Paper presented at: American Society for Microbiology 98th General Meeting; 1998; Atlanta, Georgia. [Google Scholar]
- 31.Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR., Jr A novel surfactant nanoemulsion with a unique nonirritant topical antimicrobial activity against bacteria enveloped viruses and fungi. International Microbiol Res. 2000;156:1–7. doi: 10.1078/0944-5013-00069. [DOI] [PubMed] [Google Scholar]
- 32.Myc A, Anderson MJ, Wright DC, Brisker J, Baker JR., Jr Inhibitory effect of non-phospholipid liposomes and nanoemulsions on influenza A virus infectivity. Paper presented at 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1998; San Diego, California. [Google Scholar]
- 33.Myc A, Vanhecke T, Landers JL, Hamouda T, Baker JR. The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia. 2001;155:195–201. doi: 10.1023/a:1021129710316. [DOI] [PubMed] [Google Scholar]
- 34.Chepurnov AA, Bakulina LF, Dadaeva AA, Ustinova EN, Chepurnova TS, Baker JR., Jr Inactivation of Ebola virus with a surfactant nanoemulsion. Acta Tropica. 2003;87:315–320. doi: 10.1016/s0001-706x(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 35.Marsh PD. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–8. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 36.Daneshmand N, Jorgensen MG, Nowzari H. Initial effect of controlled release chlorhexidine on subgingival microorganisms. J Periodontal Res. 2002;37:375–9. doi: 10.1034/j.1600-0765.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto M, Minami T, Sasaki H, Sobue S, Hamada S, Ooshima T. Inhibitory effects of oolong tea extract on caries-inducing properties of mutans streptococci. Caries Res. 1999;33:441–445. doi: 10.1159/000016549. [DOI] [PubMed] [Google Scholar]
- 38.Schilling KM, Bowen WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infection and Immunity. 1992;60:284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. Journal of Dental Research. 2005;84:1016–1020. doi: 10.1177/154405910508401109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Adham ISI, Al-Hmoud ND, Khalil E, Kierans M, Collier PJ. Microemulsions are highly effective anti-biofilm agents. Applied Microbiology. 2003;36:97–100. doi: 10.1046/j.1472-765x.2003.01266.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee VA, Karthikeyan R, Rawls HR, Amaechi BT. Anti-cariogenic effect of a cetylpyridinum chloride containing nanoemulsion. Journal of Dentistry. 2010;38:742–749. doi: 10.1016/j.jdent.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chmielewski RAN, Frank JF. Biofilm formation and control in food processing facilities. Comprehensive Reviews in Food Science and Food Safety. 2003;2:22–33. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 43.Moretro T, Langsrud S. Listeria monocytogenes: biofilm formation and persistence in food processing environments. Biofilms. 2004;1:107–121. [Google Scholar]
- 44.Hope CK, Wilson M. Analysis of the Effects of Chlorhexidine on Oral Biofilm Vitality and Structure Based on Viability Profiling and an Indicator of Membrane Integrity. Antimicrob Agents Chemother. 2004;48:1461–1468. doi: 10.1128/AAC.48.5.1461-1468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Adham ISI, Khalil E, Al-Hmoud ND, Kierans M, Collier PJ. Microemulsions are membrane-active, antimicrobial, self-preserving systems. Journal of Applied Microbiology. 2000;89:32–39. doi: 10.1046/j.1365-2672.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- 46.Park Y, Simionato MR, Sekiya K. Short fimbriae of Porphyromonasgingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005;73:3983–3989. doi: 10.1128/IAI.73.7.3983-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwan H, Jae-Youn C, NamIn B, Jung-Hee P. Isopanduratin A from Kaempferiapandurataas an active antibacterial agent against cariogenic Streptococcus mutans. International Journal of Antimicrob Agents. 2004;23:377–81. doi: 10.1016/j.ijantimicag.2003.08.011. [DOI] [PubMed] [Google Scholar]


