Abstract
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory demyelinating disease of the central nervous system (CNS) mediated by myelin-reactive CD4+ T cells. An unresolved issue that has important clinical implications concerns the cytokines produced by myelin-reactive T cells that determine their pathogenicity. Initially, IL-12 polarized, IFNγ producing Th1 cells were thought to be essential for the development of EAE. More recently, IL-23 polarized, IL-17 producing Th17 cells have been highlighted as critical encephalitogenic effectors. There is growing evidence that parallel autoimmune pathways can result in common clinical and histopathological endpoints. In the current study, we describe a form of EAE induced by the transfer of IL-23 modulated CD4+ T cells into IL-17 receptor (IL-17R) deficient hosts. We found that IL-23 stimulates myelin-reactive T cells to produce both IFNγ and IL-17. Surprisingly, in this model the development of EAE is IFNγ dependent. Our findings illustrate a novel mechanism by which IL-23 promotes encephalitogenicity and they further expand the spectrum of autoreactive T cells capable of mediating inflammatory demyelinating disease of the CNS.
Keywords: experimental autoimmune encephalomyelitis, autoimmune disease, neuroinflammation, T helper cells, cytokines
1. Introduction
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory demyelinating disease of the central nervous system (CNS) mediated by myelin-reactive CD4+ T cells. Although the immunopathogenesis of EAE has been the subject of extensive investigation, the mechanism by which autoreactive T cells initiate the disease process remains only partially understood. IFNγ producing Th1 cells and IL-17 producing Th17 cells infiltrate EAE lesions at high frequencies (Hofstetter and Forsthuber; Kroenke and Segal, 2007; Langrish et al., 2005; Momcilovic et al., 2008; Olsson, 1992). Numerous studies highlight the importance of myelin-reactive T cells of both lineages as disease effectors (Ando et al., 1989; Baron et al., 1993; Komiyama et al., 2006; Langrish et al., 2005; Olsson, 1992; Sedgwick et al., 1989). Furthermore, the Th1 polarizing factor, IL-12, and the Th17 polarizing factor, IL-23, directly enhance the encephalitogenicity of myelin-reactive T cells (Kroenke et al., 2008; Langrish et al., 2005; Segal and Shevach, 1996). However, studies using cytokine knock-out mice or wildtype (WT) mice treated with anti-cytokine neutralizing antibodies have demonstrated that neither IFNγ nor IL-17 are themselves essential for the induction of EAE by active immunization (Billiau et al., 1988; Duong et al., 1994; Duong et al., 1992; Ferber et al., 1996; Haak et al., 2009; Hofstetter et al., 2005; Willenborg et al., 1996). Indeed, IFNγ−/− and IFNγ receptor −/− (IFNγR −/−) mice both develop more severe disease than their WT counterparts (Ferber et al., 1996; Willenborg et al., 1996).
There are several potential explanations for why individual Th cytokines are dispensable in the development of EAE. Autoreactive T cells of multiple Th lineages accumulate in peripheral lymphoid tissues following immunization with myelin antigens (Hofstetter and Forsthuber; Kroenke and Segal, 2007). We have previously shown that IL-12 modulated Th1 cells and IL-23 modulated Th17 cells derived from myelin immunized mice are independently capable of transferring EAE to naive syngeneic hosts (Kroenke et al., 2008). Hence, one effector T cell subset could, theoretically, compensate for the deficiency of another. Furthermore, there is growing evidence that in the absence of their signature cytokine, Th polarized myelin-specific T cells can instigate CNS lesion formation via default mechanisms. For example, we recently reported that IL-12 stimulated IFNγ−/− cells induce atypical EAE in WT hosts through an IL-17 dependent pathway and conventional EAE in IL-17 receptor−/− hosts through a GM-CSF dependent pathway (Kroenke et al.).
In the studies describe here, we performed a detailed investigation of the conditions under which IL-23 modulated T cells mediate EAE. Unexpectedly, we found that IL-23 modulated T cells induce clinical EAE of comparable severity in IL-17 receptor (IL-17R) −/− and WT hosts. Our experiments showed that IL-23 stimulates myelin-reactive T cells to produce both IFNγ and IL-17, and that IFNγ contributes to the pathogenic process in this model of EAE. These findings illustrate a novel mechanism by which IL-23 promotes encephalitogenicity and they further expand the spectrum of autoreactive T cells capable of mediating inflammatory demyelinating disease of the CNS.
2. Materials and methods
2.1 Mice
8-12-wk-old WT and IFNγ-deficient C57BL/6 mice were obtained from NCI Frederick (Frederick, MD) or The Jackson Laboratory (Bar Harbor, ME). Breeding pairs of IL-17R−/− mice were obtained from J. Kolls (LSU) and bred in our facility. All mice were housed in microisolator cages. Animal protocols were approved by the University Committee on Use and Care of Animals.
2.2 Immunization and cell culture
Mice were injected subcutaneously with 100 µg MOG35–55 (MEVGWYRSP-FSRVVHLYRNGK, Biosynthesis, Lewisville, TX) in complete Freund’s adjuvant (Difco, Detroit, MI) at four sites over the flanks. Draining lymph nodes were harvested 12–14 days post-immunization, pooled, and passed through a 70-µm cell strainer (BD Falcon, Franklin Lakes, NJ). Lymph node cells were cultured in vitro with MOG35–55 and either recombinant mouse IL-23 (5 ng/mL) for Th17 polarization or no exogenous cytokines (for Th0 polarization).
2.3 Adoptive transfer and scoring of EAE
MOG-primed lymph node cells were collected after 96 hours of culture with antigen under Th17 polarizing condition. CD4+ T cells were enriched using negative selection columns (Cedarlane) to a purity of 88–95%, confirmed by flow cytometry. The isolated CD4+ T cells were suspended in sterile PBS and injected intraperitoneally into naïve syngeneic hosts (5 ×106 cells in 0.1 cc/host). Host mice were observed daily for signs of EAE. Those with conventional EAE were scored as described previously (Segal et al., 1998). For atypical EAE, the following scale was used: 0, asymptomatic; 1, slight listing/difficulty righting; 2, obvious imbalance but able to ambulate; 3, severely impaired balance/ambulation; 4, incapacitated due to inability to maintain upright posture/spinning. Mean clinical scores were compared using the student’s t-test.
2.4 ELISPOT assays
Purified CD4+ T cells were cultured with MOG35–55 (35 µg/ml) and T-depleted splenocytes for 24 hours in 96 well filtration plates (MAIP N4550; Millipore). Spots were counted using the CTL ImmunoSpot Analyzer (Cellular Technology) with ImmunoSpot software. Frequencies of cytokine producing cells were compared using the student’s t-test.
2.5 Quantitative RT-PCR
CNS tissues were harvested from 4–8 mice per group and homogenized in Trizol reagent (Invitrogen). RNA was isolated by phenol/chloroform extraction. Genomic DNA was removed by Turbo DNase (Ambion, Foster City, CA) and purity of RNA was confirmed by A260:A280 ratio. cDNA was then synthesized using a RT2 First Strand kit (SA Biosciences). RT-PCR was performed with primers and probes that were designed using Beacon Designer and synthesized by Integrated DNA Technologies. Samples were analyzed on a Bio-Rad iCycler PCR machine. All data were normalized to the geometric mean of GAPDH, HPRT1, and βactin, and are shown as fold increase over naïve spinal cords.
2.6 Histological studies
After perfusion, spinal cords were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin using standard protocols (Segal and Shevach, 1996).
3. Results
3.1 IL-17R −/− mice are susceptible to EAE induced by the adoptive transfer of IL-23 modulated MOG-specific T cells
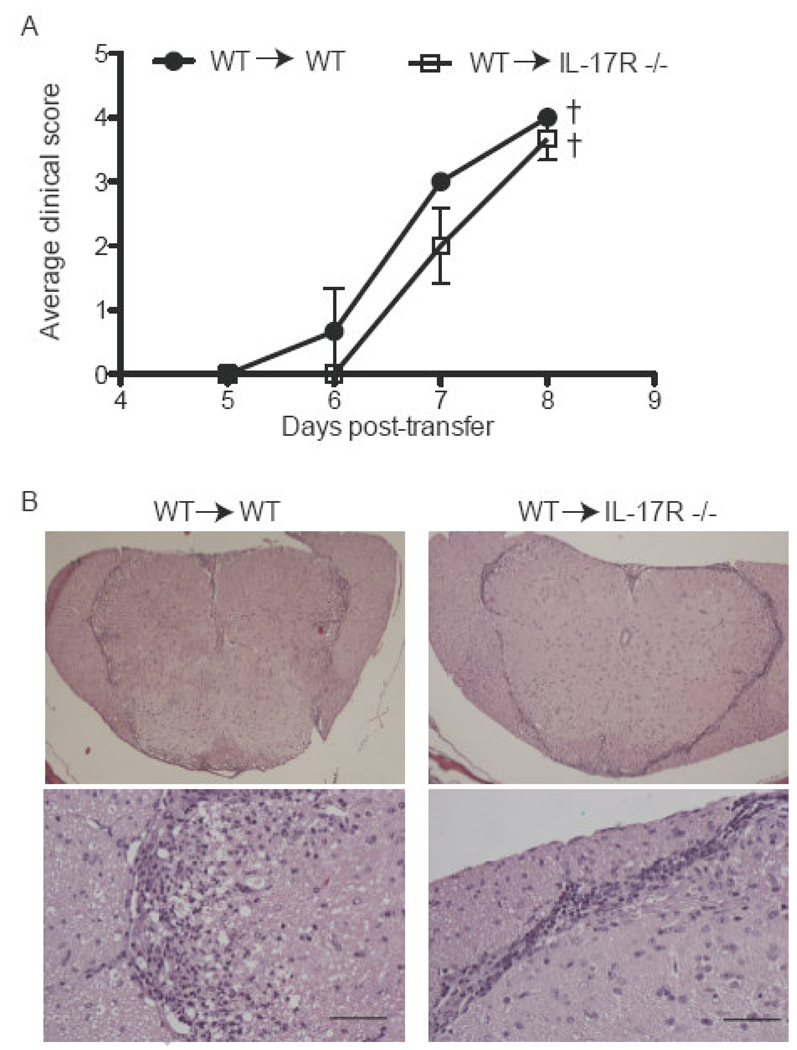
IL-23 deficient mice are completely resistant to EAE (Cua et al., 2003). Since IL-23 supports the accumulation of Th17 cells in vivo, it has been proposed to promote EAE through an IL-17 dependent pathway (Langrish et al., 2005). Mice deficient in either IL-17 or the Th17 polarizing factor, RORγt, remain susceptible to disease induction, even though they experience a relatively mild course (Haak et al., 2009; Komiyama et al., 2006; Yang et al., 2008). Similarly, we have found that treating mice that have received IL-23 modulated effector T cells with an anti-IL-17 antibody is only partially effective in suppressing EAE (Kroenke et al., 2008). It is not known whether this antibody penetrates the CNS and/ or other tissues in sufficient quantities to neutralize all local IL-17. In order to more definitively determine whether IL-23 modulated T cells can induce EAE independent of IL-17 signaling, we compared their pathogenicity in IL-17R−/− and WT hosts. Lymph node cells harvested from MOG immunized C57BL/6 mice that were reactivated with antigen and recombinant IL-23 in vitro transferred EAE to 100% of WT hosts (Fig. 1A). IL-23 stimulation was critical for the encephalitogenicity of the donor cells since MOG35–55 primed lymph node cells did not transfer disease following culture with antigen under neutral conditions (data not shown). Surprisingly, IL-17R−/− recipients of IL-23 modulated cells experienced EAE at a comparable incidence and severity to their WT counterparts (Fig. 1A). Histological analyses revealed spinal cord inflammation in both groups. Infiltrating cells were confined to the subpial white matter in IL-17R−/− hosts, but penetrated deeper into the parenchyma in WT hosts, even in mice with the same clinical score (Fig. 1B). This observation is consistent with an earlier study showing that IL-17 producing, myelin-reactive effector Tcells induce infiltrates that extend along venules into the white matter whereas non-IL-17 producing cells of the same antigenic specificity induce infiltrates that are primarily located in the meninges (Kroenke et al., 2008).
Figure 1. EAE mediated by IL-23 polarized cells is independent of IL-17 signaling.
Lymph nodes were harvested from WT mice that had been immunized 10 days earlier with MOG35–55+CFA. (A) Whole lymph node cells were stimulated with MOG35–55 and IL-23 for 4 days. Afterwards CD4+ T cells were isolated and transferred to either WT or IL-17R −/− naïve recipients (5×106 cells/host i.p.). (B) WT (left) or IL-17R −/− (right) mice that received Th17 polarized CD4+ T cells were sacrificed at peak disease. CNS specimens were harvested, fixed, sectioned, and stained with H&E. Upper panels, magnification 4×; lower panels, 40×. Bar 50µm. Data are representative of 4 independent experiments with 5–7 mice/group.
3.2. IFNγ facilitates the induction of EAE by IL-23 polarized T cells in IL-17R−/− hosts
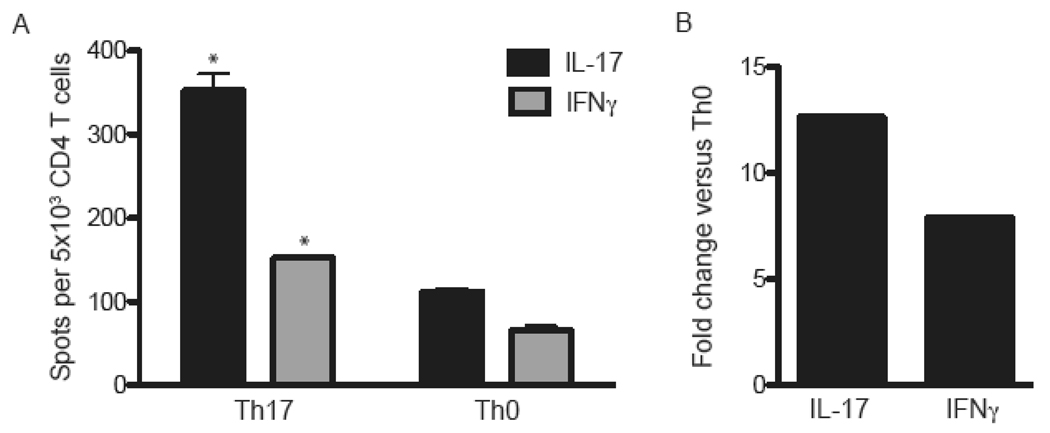
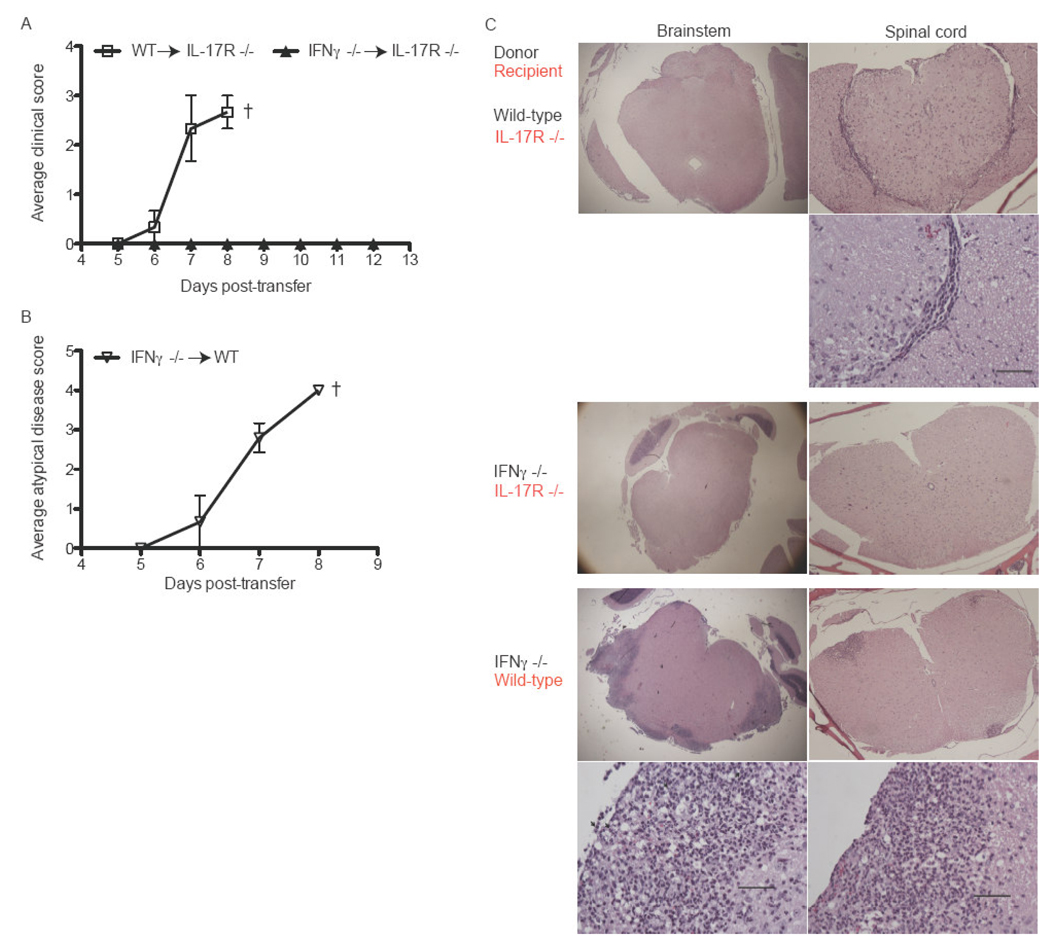
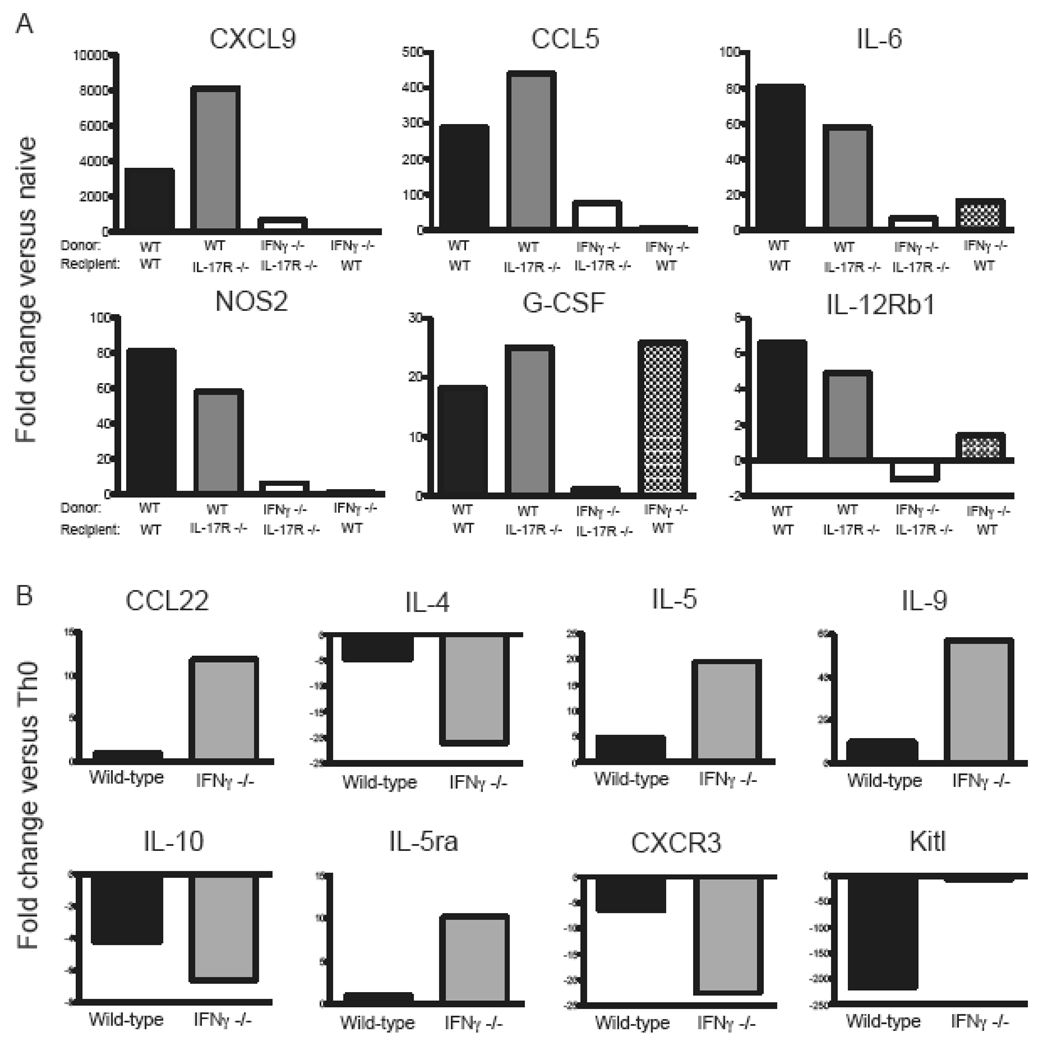
Analysis of the cytokine profiles of MOG-specific donor cells revealed that IL-23 augments the production of IFNγ as well as IL-17 (Fig. 2 A, B). Flow cytometric assays demonstrated that the vast majority of IL-23 stimulated T cells were either IFNγ or IL-17 single producers; IFNγ/IL-17 double producers comprised less than 1% of cytokine producing cells (unpublished data). Few MOG-primed cells produced IL-4 in response to antigenic challenge, either in the presence or absence of IL-23 (data not shown). Based on these results, we questioned whether IFNγ contributes to EAE induced via the injection of IL-23 modulated T cells into IL-17R−/− hosts. To test that hypothesis, we primed lymph node cells in IFNγ−/− mice with MOG35–55 in CFA and then stimulated them with IL-23 and peptide for 4 days ex vivo. These IL-23 modulated IFNγ−/− cells produced large quantities of IL-17 and TNFα (unpublished data). They transferred an atypical form of EAE to WT hosts, demonstrating that they possess intrinsic encephalitogenic properties (Fig. 3B). However, the same cells were innocuous in IL-17R−/− hosts (Fig. 3A). Histological studies showed that WT hosts developed infiltrates in the brainstem and spinal cord, while CNS sections from IL-17R−/− hosts were free of any pathological changes (Fig. 3C, middle and lower panels). IL-23 polarized WT, but not IFNγ−/−, cells upregulated CXCL9, CCL5, IL-6, iNOS and G-CSF in the spinal cords of IL-17R−/− as well as wildtype hosts (Fig. 4A). IFNγ−/− donor cells induced granulocyte colony stimulating factor (G-CSF) in WT but not IL-17R−/− mice, suggesting that neutrophils may play a role in atypical disease.
Figure 2. IL-23 induces IFNγ production by CD4+ T cells.
(A) Lymph node cells were harvested from MOG immunized C57BL/6 mice and polarized in vitro under Th17 or Th0 conditions. Following a 4 day incubation, CD4+ T cells were purified and restimulated with MOG35–55 and naïve T-depleted splenocytes in ELISPOT assays. (B) CD4 T cells were isolated from the draining lymph nodes of MOG-immunized mice and stimulated under Th17 or Th0 conditions. At 96 hours, RNA was isolated from the cells in each group and reverse transcribed into cDNA. IL-17 and IFNγ transcripts were analyzed by qPCR and normalized to the geometric mean of GAPDH, βactin, and HPRT. The data shown represent the ratio of normalized cytokine mRNA expression in Th17 over Th0 CD4+ T cells. Data are representative of 2–3 independent experiments. * P<.0001 Th17 versus Th0
Figure 3. IFNγ contributes to EAE mediated by IL-23 polarized cells.
Lymph nodes were harvested from WT or IFNγ −/− mice that had been immunized 10 days earlier with MOG35–55+CFA. Whole lymph node cells were stimulated with MOG35–55 and IL-23 for 4 days before 5×106 purified CD4+ T cells were transferred to naive IL-17R −/− (A) or wild-type (B) hosts. (C) WT or IFNγ −/− donor cells were challenged with antigen and IL-23 before transfer to WT or IL-17R −/− hosts. Mice were sacrificed at peak disease. CNS specimens were fixed, sectioned, and stained with H&E. In some cases, the same sections was photographed at low (4×) and high (40×) magnification. Bar, 50µm. Data are representative of 3 independent experiments with 5 mice/group.
Figure 4. Gene expression in donor CD4+ T cells and peak EAE.
(A) RNA was isolated from spinal cords of mice at peak EAE (n=3/ group). qPCR was performed to measure expression of a panel of proinflammatory molecules. Transcript levels were normalized to the geometric mean of GAPDH, βactin, and HPRT. Data are shown as fold change over naïve spinal cords. (B) RNA was isolated from WT of IFNγ−/− MOG-specific CD4+ T cells after polarization under Th17 or Th0 polarizing conditions. qPCR was performed, and data was normalized to the geometric mean of GAPDH, βactin, and HPRT. Data is shown as the ratio of normalized mRNA expression in Th17 over Th0 CD4+ T cells.
3.3 IL-23 modulated IFNγ−/− and WT donor T cells express distinct mRNA profiles
In order to gain further insight into the mechanism of action by which IL-23 modulated, MOG-specific T cells induce EAE in IL-17R−/− mice (Fig. 1B), we analyzed the expression of candidate immune related genes in WT and IFNγ−/− donor T cells by microarray analysis. Genes of interest were then quantified by real-time RT-PCR. IL-23 polarized IFNγ−/− deficient T cells expressed elevated levels of IL-5 and IL-9 in comparison to WT cells cultured under the same conditions (Fig. 4B). In addition, the WT cells expressed relatively low levels of stem cell growth factor (Kitl).
4. Discussion
IL-23 deficient mice fail to generate Th17 responses and are completely resistant to EAE following immunization with myelin antigens (Cua et al., 2003). In contrast, mice deficient in IL-17, IL-17R or RORγt (the transcription factor that orchestrates Th17 differentiation), are only partially resistant (Haak et al., 2009; Komiyama et al., 2006; Yang et al., 2008). Furthermore, treatment of adoptive recipients of IL-23 modulated, myelin-reactive T cells with an anti-IL-17 neutralizing antibody suppresses, but does not abrogate, EAE (Kroenke et al., 2008). Collectively, these data indicate that IL-23 promotes inflammatory demyelination through both IL-17 independent and IL-17 dependent, mechanisms. Our observation that IL-23 can enhance the pathogenicity of myelin-specific T cells via induction of IFNγ clarifies one of these IL-17 independent pathways.
There are conflicting reports regarding the role of IFNγ in EAE, although most studies demonstrate that it has a protective effect. Both IFNγ−/− and IFNγ receptor−/− mice experience a severe form of clinical EAE associated with pronounced inflammatory infiltrates that extend into the brain parenchyma and even into grey matter (Ferber et al., 1996; Willenborg et al., 1996). Furthermore, several studies have shown that treatment of myelin immunized mice with anti-IFNγ neutralizing antibodies exacerbates EAE (Billiau et al., 1988; Duong et al., 1994; Duong et al., 1992). Some investigators have speculated that IFNγ regulates EAE by suppressing proliferation and driving the activation induced cell death of CNS infiltrating lymphocytes (Chu et al., 2000; Refaeli et al., 2002; Sabatino et al., 2008). Alternatively, the secretion of ELR-negative CXC chemokines by glial cells in response to IFNγ could lead to the sequestration of effector T cells within the perivascular and meningeal spaces and/or to the recruitment of regulatory T cells to the CNS (Muller et al., 2007; Oo et al.). Conversely, other groups have reported that when given intrathecally, IFNγ potentiates the ability of antibodies against myelin proteins to mediate demyelination and induces perivascular infiltrates similar in pattern to those observed during EAE (Simmons and Willenborg, 1990; Vass et al., 1992). Our current study provides another instance wherein IFNγ actually contributes to the development of demyelinating lesions.
In our model, the disease promoting activity of IFNγ is only realized in the context of deficient IL-17 signaling and thus can be interpreted as representing a compensatory response. The data in Figure 4B demonstrate that IFNγ suppresses the transcription of genes encoding CCL22, IL-5, IL-9 and Kitl in MOG-reactive T cells. Each of these molecules is induced by infection with parasites. There is a considerable body of evidence that, in the setting of parasite infections, autoreactive T cell cells are skewed from a pathogenic to an innocuous phenotype in a process termed immune deviation. Immune deviation has been invoked to explain the observation that parasite infected MS patients and myelin-immunized mice have a reduced number of exacerbations and altered immune reactivity compared with their uninfected counterparts (Correale and Farez, 2007; Gruden-Movsesijan et al., 2008; Sewell et al., 2003). Based on our data, we speculate that IFNγ might promote EAE in IL-17R−/− mice by preventing myelin-specific T cells from deviating towards a non-encephalitogenic lineage. Alternatively, IFNγ could support neuroinflammation by stimulating cerebrovascular endothelial cells and astrocytes to upregulate MHC Class II and adhesion molecules and/or by activating macrophages and microglia to secrete toxic factors, such as matrix metalloproteinases and reactive oxygen species (Beller, 1984; Dasilva and Yong, 2008; Fierz et al., 1985; McCarron et al., 1986).
The IL-12/IFNγ and IL-23/IL-17 pathways interact in complex ways that vary depending on the cell types and environmental conditions involved. In many experimental systems, they are mutually antagonistic. For example, IFNγ directly suppresses Th17 differentiation (Harrington et al., 2005). Conversely, IL-23 can antagonize IFNγ production by T cells in response to IL-12 (Sieve et al.). Our data demonstrates that the IL-12/IFNγ and IL-23/IL-17 pathways can also act in synergy. Consistent with our findings in the mouse, IL-23 has been shown to stimulate human T cells to secrete IFNγ (Oppmann et al., 2000). Lovett-Racke and colleagues have reported that Tbet binds the IL-23 receptor promoter in myelin-specific T cells and initiates transcription of IL-23 receptor gene (Gocke et al., 2007). This observation, in combination with our findings, suggests that IL-23 and IFNγ may be involved in a positive feedback loop. Hence, IFNγ induces Tbet in MOG-reactive T cells, resulting in upregulation of the IL-23 receptor and increased sensitivity to IL-23. IL-23 then induces IFNγ production, thereby initiating a self-amplifying cycle. It is also possible that IL-23 acts on non-T cells within the lymph node cell population (such as dendritic cells) to elicit IFNγ production by T cells and encephalitogenicity via an indirect pathway.
Many studies have attempted to identify the specific cytokine(s) produced by myelin-specific T cells that determine encephalitogenicity. However, a review of the literature reveals inconsistent findings. Hence, the relative importance of IL-17 and IFNγ varies with mouse strain, encephalitogen and stage of disease (Billiau et al., 1988; Duong et al., 1994; Ferber et al., 1996; Haak et al., 2009; Komiyama et al., 2006; Kroenke et al., 2008; Segal and Shevach, 1996; Simmons and Willenborg, 1990; Vass et al., 1992; Willenborg et al., 1996). The current study joins several recent publications in demonstrating that parallel autoimmune pathways can result in common histological and clinical endpoints. Here, we demonstrate that conventional EAE can be driven by IFNγ when IL-17 signaling is disrupted. Conversely, IL-17 drives atypical disease in the absence of IFNγ. In conclusion, EAE represents a heterogeneous syndrome, that can be caused by qualitatively distinct types of immune effectors, rather than a single disease entity. If the same holds true in human MS, then differences in the type of effector cell and cytokines that drive pathogenesis might underlie the diversity in clinical course and lesion distribution as well as in responses to immunomodulatory agents.
Acknowledgements
This work was supported by grants from the National Multiple Sclerosis Society (CA 1037A1/1 and RG 3866-A-3) and the National Institutes of Health (R01NS057670). We thank Dr. David Irani for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller DI. Functional significance of the regulation of macrophage Ia expression. Eur J Immunol. 1984;14:138–143. doi: 10.1002/eji.1830140207. [DOI] [PubMed] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dasilva AG, Yong VW. Expression and regulation of matrix metalloproteinase-12 in experimental autoimmune encephalomyelitis and by bone marrow derived macrophages in vitro. J Neuroimmunol. 2008;199:24–34. doi: 10.1016/j.jneuroim.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J Neuroimmunol. 1994;53:101–107. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Duong TT, St Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–115. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Fierz W, Endler B, Reske K, Wekerle H, Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985;134:3785–3793. [PubMed] [Google Scholar]
- Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp Parasitol. 2008;118:641–647. doi: 10.1016/j.exppara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuroinflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Forsthuber TG. Kinetics of IL-17- and interferon-gamma-producing PLPp-specific CD4 T cells in EAE induced by coinjection of PLPp/IFA with pertussis toxin in SJL mice. Neurosci Lett. 2010;476:150–155. doi: 10.1016/j.neulet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur J Immunol. 2010;40:2340–2348. doi: 10.1002/eji.201040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Segal BM. Th17 and Th1 responses directed against the immunizing epitope, as opposed to secondary epitopes, dominate the autoimmune repertoire during relapses of experimental autoimmune encephalomyelitis. J Neurosci Res. 2007;85:1685–1693. doi: 10.1002/jnr.21291. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron RM, Spatz M, Kempski O, Hogan RN, Muehl L, McFarlin DE. Interaction between myelin basic protein-sensitized T lymphocytes and murine cerebral vascular endothelial cells. J Immunol. 1986;137:3428–3435. [PubMed] [Google Scholar]
- Momcilovic M, Miljkovic Z, Popadic D, Miljkovic D, Mostarica-Stojkovic M. Kinetics of IFN-gamma and IL-17 expression and production in active experimental autoimmune encephalomyelitis in Dark Agouti rats. Neurosci Lett. 2008;447:148–152. doi: 10.1016/j.neulet.2008.09.082. [DOI] [PubMed] [Google Scholar]
- Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, Campbell IL. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, Shetty S, Harki J, Shaw JC, Eksteen B, Hubscher SG, Walker LS, Adams DH. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino JJ, Jr, Shires J, Altman JD, Ford ML, Evavold BD. Loss of IFN-gamma enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J Immunol. 2008;180:4451–4457. doi: 10.4049/jimmunol.180.7.4451. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, MacPhee IA, Puklavec M. Isolation of encephalitogenic CD4+ T cell clones in the rat. Cloning methodology and interferon-gamma secretion. J Immunol Methods. 1989;121:185–196. doi: 10.1016/0022-1759(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184:771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Meeks KD, Lee S, Berg RE. A novel immunoregulatory function for IL-23: Inhibition of IL-12-dependent IFN-gamma production. Eur J Immunol. doi: 10.1002/eji.200939759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RD, Willenborg DO. Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J Neurol Sci. 1990;100:37–42. doi: 10.1016/0022-510x(90)90010-k. [DOI] [PubMed] [Google Scholar]
- Vass K, Heininger K, Schafer B, Linington C, Lassmann H. Interferon-gamma potentiates antibody-mediated demyelination in vivo. Ann Neurol. 1992;32:198–206. doi: 10.1002/ana.410320212. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]