Abstract
A series of phenyl-2, 2′-bichalcophene diamidines 1a-h were synthesized from the corresponding dinitriles either via a direct reaction with LiN(TMS)2, followed by deprotection with ethanolic HCl or through the bis-O-acetoxyamidoxime followed by hydrogenation in acetic acid and EtOH over Pd-C. These diamidines show a wide range of DNA affinities as judged from their ΔTm values which are remarkably sensitive to replacement of a furan unit with a thiophene one. These differences are explained in terms of the effect of subtle changes in geometry of the diamidines on binding efficacy. Five of the eight compounds were highly active (below 6 nM IC50) in vitro against Trypanosoma brucei rhodesiense (T. b. r.) and four gave IC50values less than 7 nM against Plasmodium falciparum (P. f.). Only one of the compounds was as effective as reference compounds in the T. b. r. mouse model for the acute phase of African trypanosomiasis.
Keywords: diamidines, bichalcophenes, Antiprotozoal agents, Stille coupling, Heck coupling
1. Introduction
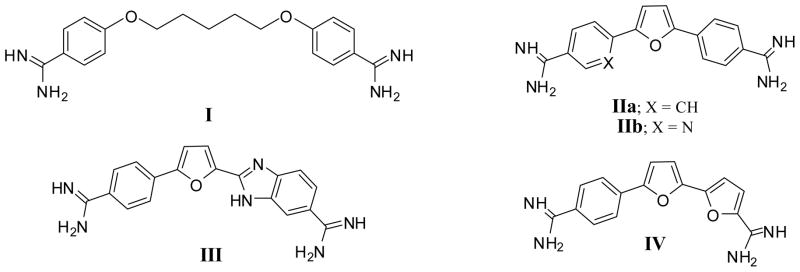
Aromatic diamidines exhibit broad-spectrum antimicrobial activity including effectiveness against the protozoan diseases caused by Trypanosoma sp and Plasmodium sp.1 The broad activity of the aromatic diamidines notwithstanding, pentamidine (I) is the only compound of this class to be used extensively in the clinic.1 Furamidine (IIa), a diamidino diphenylfuran, has been shown to be more potent and less toxic than pentamidine in murine models of trypanosomiasis.2 The oral prodrug of furamidine, 2,5-bis[4-(methoxyamidino)phenyl]furan (pafuramidine), showed promising results in Phase I and II clinical trials against both human African trypanosomiasis (HAT) and malaria.1 Unfortunately, in an additional safety study of pafuramidine paralleling the Phase III trials, liver and kidney toxicities in some volunteers were found and the development of pafuramidine was terminated.2 Many of the active aromatic diamidines for various structural classes have been shown to bind to the minor groove of DNA at AT rich sites.3–9 It has been assumed that the minor groove binding of these type of compounds leads to inhibition of one or more DNA dependant enzymes which gives rise to the anti-microbial effect.10–12 Recently, we have made compounds in which the phenyl group(s) of furamidine have been replaced with pyridyl group(s) (IIb). Several of these aza-analogues show in vivo activity which is superior to that of furamidine.13 More recently, one of these aza analogs was found to be effective in a mouse model for second stage African sleeping sickness.14 Furan units are often key structural elements in the aromatic frame work for the more effective diamidines.15–17 The furan analogue III has been found to bind to DNA in a unique stacked dimer array which has potential for development of new gene regulation molecules.18–21 Compound III has shown some activity in an immunosupressed rat model for Pneumocystis carinii pneumonia18 and in the STIB900 mouse model for acute stage African trypanosomiasis.22 The bichalcophene diamidine IV from our laboratory has been shown to recognize G-quadruplex DNA.23 More recently, the diverse modes of the interaction of IV with multi-stranded DNA structures has been reported.24
As part of a research program directed to drug discovery of antiprotozoal agents and due to the unusual DNA binding properties of III and IV we decided to prepare additional analogues of this series of bichalcophene diamidines in order to investigate structure-activity relationships (SAR) and their DNA binding profiles. We report here the synthesis of novel diamidino phenyl-bifuran derivatives and structural isosteres and their evaluation versus Trypanosoma brucei rhodesiense (T. b. r.) and Plasmodium falciparum (P. f.).
2. Results and Discussion
2.1. Chemistry
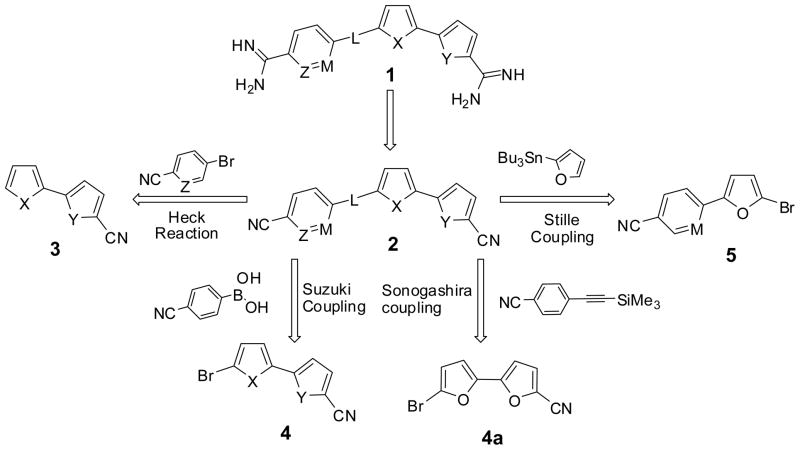
Our strategy for the synthesis of diamidines 1 is based upon the conversion of dinitriles either by direct reaction using lithium bis(trimethylsilyl)amide LiN(TMS)2 or through the bis-O-acetoxyamidoxime followed by hydrogenation in acetic acid. Retrosynthetic studies (Figure 2) for this strategy suggested that the triaryl structure 2 could be obtained by the application of palladium catalyzed reactions which permit the formation of C-C bond with full regioselectivity. The bichalcophenes 3 could be converted into dinitriles 2 using the Heck coupling reaction. The compounds 4 could be converted into the dinitriles 2 using a Suzuki coupling reaction. For SAR study, a two-carbon spacer could be inserted into dinitriles 2 using Sonogashira coupling27 by the reaction of 4a with trimethyl silyl acetylene derivative. Furthermore, Stille coupling is another Pd catalyzed reaction which is an alternative pathway to furnish certain analogues using compound 5.
Figure 2.
Retrosynthetic study of Diam id i n e s
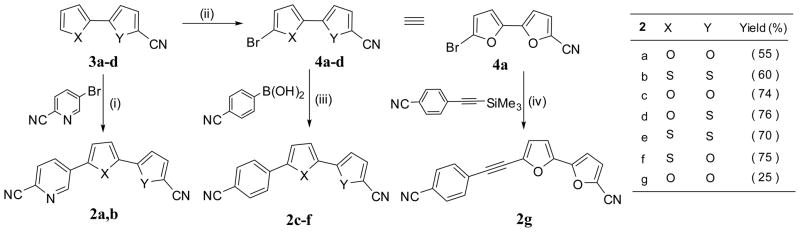
We initiated our study by the synthesis of dinitrile 2a which was obtained from 2,2′-bifuran-5-carbonitrile (3a), prepared according to our published procedure,25 via a Heck coupling reaction with the commercially available 5-bromopyridine-2-carbonitrile as shown in Scheme 1. The dinitrile 2b was prepared employing similar procedures used for 2a using 2, 2′-bithiophene-5-carbonitrile (3b). The synthesis of dinitriles 2c–f was accomplished in two steps. The regioselective bromination of 3a–d using N-bromosuccinamide (NBS) in DMF furnished bromo derivatives 4a–d. Suzuki coupling of 4a–d with p-cyanophenylboronic acid was accomplished in the presence of Pd (PPh3)4 as a catalyst, Na2CO3 as base and toluene as solvent.
Scheme 1.
Reagents and conditions:(i) DMF, KOAc, Pd(PPh3)4, 125–135 °C; (ii) NBS, DMF; (iii) Toluene, Pd(PPh3)4, Na2CO3, 80 °C; (iv) Pd(OAc)2, NaOAc, Bu4NCl, DMF, 70 °C.
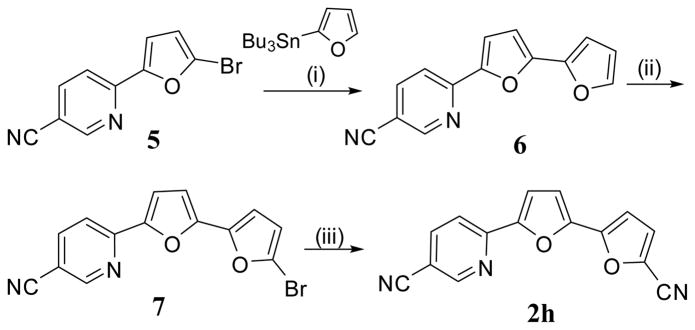
Compound 2h was obtained in three steps starting with a Stille coupling reaction of the readily available 6-(5-bromofuran-2-yl)nicotinonitrile 5 and 2-tributylstannylfuran to form the corresponding 6-(2,2′-bifuran-5-yl)-nicotinonitrile (6) (Scheme 2). Bromination of 6 with N-bromosuccinimide in DMF, furnished bromo-bifuran derivative 7 in a 58% yield. A subsequent cyanation reaction of compound 7 with Cu(I)CN in DMF gave the dinitrile 2h.
Scheme 2.
Reagents and conditions: (i) Pd(PPh3)4, 1,4-dioxane, 100–110 °C; (ii) NBS, DMF; (iii) Cu(I)CN, DMF.
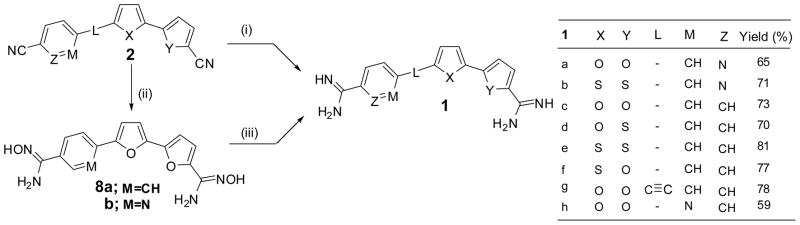
As outlined in Scheme 3, a series of diamidines 1a–h was obtained from the dinitriles 2a–h either by direct reaction using LiN(TMS)2 (cf. 1a–g) or through the bis-O-acetoxyamidoxime followed by hydrogenation in glacial acetic acid as in case of 1c,h. Thus, 5′-(2-amidinopyridin-5-yl)-2,2′-bifuran-5-carboxamidine (1a) was synthesized from the corresponding dinitrile 2a by treatment with LiN(TMS)2 followed by deprotection with ethanolic HCl(g). 6-(5′-Amidino-2, 2′-bifuran-5-yl)-nicotinamidine acetate salt (1h) was synthesized from 6-(5′-cyano-2, 2′bifuran-5-yl)-nicotinonitrile (2h), through the bis-O-acetoxyamidoxime followed by hydrogenation. The hydrochloride salts of the diamidines 1a–g were obtained by passing hydrogen chloride gas into ethanolic solutions of their free bases
Scheme 3.
Reagents and conditions: (i) a- LiN(TMS)2, THF, r.t., 12h; b- HCl(gas), dry EtOH, r.t. 12h. (ii) NH2OH.HCl/KO-t-Bu, DMSO; (iii) a- AcOH/Ac2O; b- H2/Pd-C, AcOH.
2.2. Biology
Aromatic diamidines are well known cationic molecules that have a strong and reversible interaction with DNA. Recent work has shown that both curved and linear molecules exhibit excellent biological activity against trypanosomes.22,26 The mechanism of action for antitrypanosomal activity has been suggested to involve the inhibition of DNA dependent enzymes or direct inhibition of transcription.1,9,10
Table 1 contains the DNA binding affinities for the new bichalcophene diamidines as well as the in vitro activities for these compounds against T. b. r. and P. f. For comparative purposes, similar data for furamidine and pentamidine are included. The use of the thermal melting increase ΔTm (Tm of complex – Tm of free DNA) is a rapid and reliable method for comparing binding affinities for a large number of diverse classes of diamidines. The complexes between poly (dA-dT) and the bichalcophene diamidines give an unexpected wide range (from 4 to 18°C) of ΔTm values. The ΔTm value (8.5°C) for the bifuran 1c is significantly lower than that of both pentamidine (12.6°C) and furamidine (25°C). Due to structural similarities this analysis will focus on comparisons between furamidine and the bichalcophene analogues. The large drop in ΔTm value on replacing one of the furamidine phenyl groups with a furan ring is probably due to a combination of factors presented by 1c including a more curved structure, a shorter distance between the two amidine units and a smaller stacking surface provided by the three aryl rings. The first two differences seem likely to be the more important contributors to the reduction in ΔTm value since the more curved structure would be expected to prevent optimal fit in the groove by pushing the central portion of the molecule away from the floor of the groove and the shorter distance between the amidine units could lead to a disadvantageous offset in H-bond indexing with the minor groove base pairs. Replacement of one of the furan rings with a thiophene ring (1d or 1f) results in an increase in the ΔTm by approximately 5°C. This result is consistent with the increased affinity of other thiophene based diamidines in comparison to furan counterparts.28 There is a small increase in bond angle for C-S-C in thiophene compared to that of C-O-C in furan attributable to the differences in van der Waals radii between S and O. This small angle difference when amplified to the terminal amidine units leads to a significant difference in the positions of the amidines in the two different molecules and likely leads to the increase in binding affinities for the thiophene analogues.28 The bithiophene analog 1e gives the highest ΔTm value (18.1 °C) for the bichalcophenes studied, consistent with the previous observations. Introduction of nitrogen into the phenyl ring in this system (1a, 1b, 1h) results in a 1.4 to 3.0 °C decrease in ΔTm values compared to that of their carbocycle analogues. This type decline has been noted previously for other diamidines and has been attributed to differences in hydration of the phenyl and pyridyl ring systems.13,29–31 The bifuran 1g in which a carbon-carbon triple bond has been inserted between the furan and the phenyl rings yields the bichalcophene compound with the lowest ΔTm value of those investigated. A similar reduction in binding affinity was noted in DAPI analogues in which a carbon-carbon triple bond had been inserted.30 The origin of the decline seems likely to be associated with poor stacking interactions of the triple bond and possible changes in H-bond indexing as a result of further separation of the amidine groups.
Table 1.
DNA affinities, in vitro anti-protozoan data and in vivo anti-trypanosomal activity of bichalcophene diamidines.
| Compound No | Substitution | DNA affinities and in vitro Anti-protozoan Data | In vivo anti-trypanosomal activity In the STIB900 Mouse Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | L | M | Z | ΔTma (°C) | Cytotoxicity b | T. b. rhodesiensec | P. falciparumc | Dosef (mg/kg) | Curesg | Survival (days)h | |||
| IC50 (μM) | IC50 (nM) | SITd | IC50 (nM) | SIpe | ||||||||||
| Pentamidine | 12.6 | 6 | 2.2 | 15533 | 6.4 | 991 | 5 | 2/4 | >45 | |||||
| Furamidine | 25.0 | 6.4 | 4.5 | 2093 | 15.5 | 486 | 5 | 1/4 | >46 | |||||
| 1a | O | O | - | CH | N | 5.5 | 38.5 | 6 | 6416 | 1 | 38500 | 5 | 0/4 | 41.75 |
| 1b | S | S | - | CH | N | 16.6 | 4.0 | 4 | 1000 | 2.3 | 1739 | 5 | 0/4 | 49 |
| 1c | O | O | - | CH | CH | 8.5 | 37.8 | 12 | 3150 | 41.5 | 910 | 20 | 2/4 | >45.5 |
| 1d | O | S | - | CH | CH | 14.1 | 10.9 | 6 | 1816 | 17.4 | 626 | 5 | 0/4 | 16.75 |
| 1e | S | S | - | CH | CH | 18.1 | 6.0 | 2 | 3000 | 10.4 | 576 | 5 | 0/4 | 26.25 |
| 1f | S | O | - | CH | CH | 13.1 | 29.3 | 2 | 14900 | 0.9 | 32555 | 5 | 2/4 | >46.5 |
| 1g | O | O | C≡C | CH | CH | 4.0 | 63.0 | 42 | 1500 | 14.1 | 4468 | 5 | 0/4 | 15.25 |
| 1h | O | O | - | N | CH | 6.0 | 26.9 | 97 | 277 | 6.4 | 4203 | 5 | 0/4 | 25.5 |
Increase in thermal melting of Poly d(A-T)n.32
Cytotoxicity was evaluated using cultured L6 rat myoblast cells using an Alamar Blue assay.33
The T.b.r.(Trypanosoma brucei rhodesiense) strain was STIB900, and the P. f. (Plasmodium falciparum) strain was K1. The values are the average of duplicate determination.33
Selectivity index for T. b. r. (SIT) expressed as the ratio: IC50(L6)/IC50(T. b. r.).
Selectivity index for P. f. (SIP) expressed as the ratio: IC50(L6)/IC50(P. f.).
Intraperitoneal administration.14
Number of mice that survive and are parasite free for 60 days.14
Average days of survival; untreated controls expired between day 7 and 10 post infection. 14
The IC50 values for the bichalcophene diamidines against T. b. r. range from 2 nM to 97 nM. Five (1a, 1b, 1d, 1e, 1f) of the eight compounds studied give IC50 values of 6 nM or less which are all in the range of the values of pentamidine and furamidine. These compounds were quite effective against P. f. with IC50 values ranging from of 0.9 to 41 nM. Against this organism four compounds (1a 1b, 1f, 1h) gave IC50 values less than 7 nM. On comparison of the T. b. r. activity of non-pyridyl containing analogues (1c–g) the thiophene compounds (1b, 1e) are the most effective. A similar trend is also seen for the pyridyl analogues (1a, 1b, 1h); however, the difference in IC50 values for 1a and 1b is within experimental error. There is not an obvious SAR pattern for the P. f. activity. As we have noted previously, there is no direct correlation between DNA binding affinity and antiparasitic activity.1 While some DNA affinity seems essential, transport of diamidines into the parasite plays and important role.1 There does appear to be a rough correlation between DNA affinity and cytotoxicity.31 Nevertheless, the selectivity of these compounds for the parasitic organisms is generally quite high as judged from their cytotoxicity to cultured L6 rat myoblast cells shown in Table 1. The selectivity indices range from 277 to 38500.
Given the in vitro selectivity and activity of the bichalcophenes they were evaluated in the stringent T. b. r. mouse model for the acute phase of African trypanosomiasis.14 The compounds were evaluated by daily intraperitoneal dosage of 5 mg/kg (except for 1c which was tested in an earlier protocol at 20 mg/kg) for four consecutive days. The results for these studies are included in Table 1. At the low dosage of 5 mg/kg only 1f provided cures (2/4) which represents activity comparable to that of the reference compounds pentamidine and furamidine. Compound 1f merits further evaluation in other models for HAT.
2.3. Conclusion
A new series of bichalcophene diamidines has been prepared in good yields. These analogues show a wide range of DNA affinities as judged from their ΔTm values which are remarkably sensitive to replacement of a furan unit with a thiophene one. These differences are explained in terms of the effect of subtle changes in geometry of the diamidines on binding efficacy. Five of the eight compounds were highly active (below 6 nM IC50) in vitro against T. b. r. and four gave IC50values less than 7 nM against P. f. Only one of the compounds was as effective as reference compounds in the T. b. r. mouse model for the acute phase of African trypanosomiasis.
3. Experimental Section
3.1. Biology
3.1.1. ΔTm Measurements
Thermal melting experiments were conducted with a Cary 300 spectrophotometer. Cuvettes for the experiment are mounted in a thermal block and the solution temperatures are monitored by a thermistor in the reference cuvette. Temperatures were maintained under computer control and are increased at 0.5 °C/min. The experiments were conducted in 1 cm path length quartz cuvettes in CAC 10 buffer (cacodylic acid 10 mM, EDTA 1 mM, NaCl 100 mM with NaOH added to give pH 7.0). The concentrations of DNA were determined by measuring the absorbance at 260 nm. A ratio of 0.3 mol compound per mole of DNA was used for the complex and DNA with no compound was used as a control.26, 32
3.1.2. In vitro activity determination
In vitro assays with Trypanosoma b. rhodesiense STIB 900 and Plasmodium falciparum K1 strain and cell toxicity assays using cultured L6 rat myoblast cells were carried out as previously described.33
3.1.3. STIB900 Acute Mouse Model of Trypanosomiasis
Experiments were performed as previously reported14 with minor modifications. Briefly, female NMRI mice were infected intraperitoneally (ip) with 2 × 104 STIB900 bloodstream forms. Groups of four mice were treated ip with the tested dications on 4 consecutive days from day 3 through 6 post infection. A control group was infected but was not treated. The tail blood of all mice was checked for parasitemia until 60 days post infection. Surviving and parasite free mice at day 60 were considered cured and then euthanized. Death of animals (including the aparasitemic mice, >60) was recorded to calculate the mean survival time in days.
3.2. Chemistry
Melting points were recorded using a Thomas-Hoover (Uni-Melt) capillary melting point apparatus and are uncorrected. TLC analysis was carried out on silica gel 60 F254 precoated aluminum sheets and detected under UV light. 1H and 13C NMR spectra were recorded employing a Varian Unity Plus 300 spectrometer and a Bruker Avance 400 MHz spectrometer, and chemical shifts (δ) are in ppm relative to TMS as internal standard. Mass spectra were recorded on a VG analytical 70-SE spectrometer. Elemental analyses were obtained from Atlantic Microlab Inc. (Norcross, GA) and are within ±0.4 of the theoretical values. The compounds reported as salts frequently analyzed correctly for fractional moles by water and/or ethanol of solvation. In each case proton NMR showed the presence of indicated solvent (s). All chemicals and solvents were purchased from Aldrich Chemical Co., Fisher Scientific or Frontier.
General procedure of Heck reaction (2a, b)
A mixture of 2,2′-bifuran-5-carbonitrile 3a or 2,2′-bithiophene-5-carbonitrile 3b (10 mmol), 5-bromopyridine-2-carbonitrile (1.82 g, 10 mmol), Pd(PPh3)4(0) (400 mg) and KOAc (5 g, 50 mmol) in dry DMF (10 mL) was heated under nitrogen at 130–135 °C for overnight. The reaction mixture then poured onto cold-water. The precipitate which formed was collected, recrystallized from DMF.
5′-(2-Cyanopyridin-5-yl)-2,2′-bifuran-5-carbonitrile (2a)
Yellow solid, yield 55%, mp 213–214 °C. 1H NMR (DMSO-d6); δ 7.25 (d, J = 3.6 Hz, 1H), 7.27 (d, J = 3.6 Hz, 1H), 7.59 (d, J = 3.6 Hz, 1H), 7.80 (d, J = 3.6 Hz, 1H), 8.13 (d, J = 8.0 Hz, 1H), 8.41 (dd, J = 8.0, 2.4 Hz, 1H), 9.22 (d, J = 2.4 Hz, 1H). 13C NMR (DMSO-d6); δ 149.9, 149.1, 146.5, 145.1, 131.7, 130.7, 129.3, 128.3, 125.6, 124.4, 117.6, 113.1, 112.1, 111.8, 108.5. MS (EI) m/e (rel.int.); 262 (M+ +1, 100), 207 (18), 179 (24), 148 (80). Anal. Calcd for C15H7N3O2: C, 68.96; H, 2.70. Found: C, 68.72.; H, 2.79.
5′-(2-Cyanopyridin-5-yl)-2,2′-bithiophene-5-carbonitrile (2b)
Yellow solid, yield 60%, mp 257–258.5 °C. 1H NMR (DMSO-d6); δ 7.59 (d, J = 4.0 Hz, 1H), 7.72 (d, J = 4.0 Hz, 1H), 7.95 (d, J = 4.0 Hz, 1H), 8.00 (d, J = 4.0 Hz, 1H), 8.11 (d, J = 8.0 Hz, 1H), 8.34 (dd, J = 8.0, 2.4 Hz, 1H), 9.14 (d, J = 2.4 Hz, 1H). 13C NMR (DMSO-d6); δ 147.6, 143.0, 140.4, 138.9, 136.3, 133.6, 132.2, 131.0, 129.3, 129.0, 128.5, 125.4, 117.5, 114.1, 107.0. MS (EI) m/e (rel.int.); 293 (M+, 10), 152 (12), 133 (15), 69 (100). Anal. Calcd for C15H7N3S2: C, 61.41; H, 2.41. Found: C, 61.54; H, 2.47.
General procedure of Suzuki coupling (2c–f)
To a stirred solution of 5′-bromo-2,2′-bifuran-5-carbonitrile25 4a (4.74 g, 20 mmol), and Pd(PPh3)4(0) (600 mg) in toluene (40 mL) under a nitrogen atmosphere was added 20 mL of a 1.5 M aqueous solution of Na2CO3 followed by 4-cyanophenylboronic acid (3.21 g, 22 mmol) in 5 mL of methanol. The vigorously stirred mixture was warmed to 80 °C for 16 h. The solvent was evaporated, the precipitate was partitioned between CH2Cl2 (300 mL) and aqueous solution containing 15 mL of concentrated ammonia. The organic layer was dried (Na2SO4), and then concentrated to dryness under reduced pressure.
5′-(4-Cyanophenyl)-2,2′-bifuran-5-carbonitrile (2c)
Yellow solid, yield 74%, mp 194–195 °C (DMF).25 1H NMR (DMSO-d6) δ 7.17 (d, J = 3.6 Hz, 1H), 7.21 (d, J = 3.6 Hz, 1H), 7.43 (d, J = 3.6 Hz, 1H), 7.76 (d, J = 3.6 Hz, 1H), 7.91 (d, J = 8.4 Hz, 2H), 7.99 (d, J = 8.4 Hz, 2H).
5-[5-(4-Cyanophenyl)-furan-2-yl]-thiophene-2-carbonitrile (2d)
Yellowish green solid, yield 76%, mp 187–188 °C.25 1H NMR (DMSO-d6); δ 7.22 (d, J = 3.6 Hz, 1H), 7.35 (d, J = 3.6 Hz, 1H), 7.62 (d, J = 3.9 Hz, 1H), 7.85–7.94 (m, 5H).
5′-(4-Cyanophenyl)-2,2′-bithiophene-5-carbonitrile (2e)
Yellow solid, yield 70%, mp 231–233 °C.25 1H NMR (DMSO-d6) δ 7.50 (d, J = 3.9 Hz, 1H), 7.59 (d, J = 3.9 Hz, 1H), 7.73 (d, J = 3.9 Hz, 1H), 7.84–7.87 (m, 4H), 7.91 (d, J = 3.9 Hz, 1H).
5-[5-(4-Cyanophenyl)-thiophen-2-yl]-furan-2-carbonitrile (2f)
Yellow solid, yield 75%, mp 216.5–218 °C.25 1H NMR (DMSO-d6) δ 7.09 (d, J = 3.9 Hz, 1H), 7.64–7.66 (m, 2H), 7.75 (d, J = 3.9 Hz, 1H), 7.84–7.88 (m, 4H).
5′-[(4-Cyanophenyl)ethynyl]-2,2′-bifuran-5-carbonitrile (2g)
A mixture of 4-[(trimethylsilyl)ethynyl]benzonitrile (1.99 g, 10 mmol), 5′-bromo-2,2′-bifuran-5-carbonitrile (4a) (1.19 g, 5 mmol), palladium acetate (56 mg, 0.25 mmol), tri(o-tolyl)phosphine (152 mg, 0.5 mmol), Bu4NCl (1.39 g, 5 mmol), and sodium acetate (1.64 g, 20 mmol) in DMF (20 mL) was heated at 70 °C for 3 hr. The reaction mixture was poured onto water and extracted with ethyl acetate 200 mL (3X). The extract was evaporated, and the residue was chromatographed on silica gel using hexanes/EtOAc (95:5) as an eluent to furnish compound 2g as yellow solid in 25% yield, mp 172–173 °C. 1H NMR (DMSO-d6); δ 7.16 (d, J = 3.6 Hz, 1H), 7.21 (d, J = 3.6 Hz, 1H), 7.23 (d, J = 3.6 Hz, 1H), 7.76–7.79 (m, 3H), 7.93 (d, J = 8.0 Hz, 2H). 13C NMR (DMSO-d6); δ 148.7, 145.1, 136.1, 132.7, 131.9, 125.6, 125.5, 124.5, 119.8, 118.3, 111.7, 111.2, 108.6, 93.8, 82.5. MS (EI) m/e (rel.int.); 284 (M+, 10), 228 (35), 201 (40), 164 (60), 64 (100). Anal. Calcd for C18H8N2O2: C, 76.05; H, 2.83. Found: C, 75.80; H, 2.96.
6-(2,2′-Bifuran-5-yl)-nicotinonitrile (6)
A mixture of 6-(5-bromofuran-2-yl)nicotinonitrile13 5 (2.48 g, 10 mmol), 2-n-tributyltin furan (3.58 g, 10 mmol), and tetrakis(triphenylphosphine) palladium (300 mg) in dry dioxane (20 mL) was heated under nitrogen at 100–110 °C for 24 h. The solvent was evaporated under reduced pressure and the resulting residue was dissolved in ethyl acetate. This solution was passed through celite to remove Pd. The solution was evaporated, and the residue was chromatographed on silica gel using hexanes/EtOAc (70:30) as an eluent to furnish compound 6 as a golden yellow solid in 78% yield, mp 169–170 °C. 1H NMR (CDCl3); δ 6.52 (dd, J = 3.6, 1.8 Hz, 1H), 6.72–6.76 (m, 2H), 7.31 (d, J = 3.6 Hz, 1H), 7.49 (d, J = 1.8 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.95 (dd, J = 8.4, 2.1 Hz, 1H), 8.80 (d, J = 2.1 Hz, 1H). 13C NMR (CDCl3); δ 152.5, 151.3, 151.0, 148.7, 145.5, 142.9, 139.6, 117.6, 117.0, 114.3, 111.7, 108.1, 107.1, 106.6. Anal. Calcd for C14H8N2O2: C, 71.18; H, 3.41; N, 11.85. Found. C, 70.83; H, 3.61; N, 11.84.
6-(5′-Bromo-2,2′-bifuran-5-yl)-nicotinonitrile (7)
To a solution of 6 (1.41 g, 6 mmol) in DMF (20 mL) was added portionwise N-bromosuccinimide (1.07 g, 6 mmol) with stirring. The reaction mixture was stirred overnight, then poured onto cold water. The precipitate which formed was collected, washed with water and dried to give 7 as a golden yellow solid in 58% (EtOH), mp 143–145 °C. 1H NMR (CDCl3); δ 6.44 (d, J = 3.6 Hz, 1H), 6.70 (d, J = 3.6 Hz, 1H), 6.75 (d, J = 3.6 Hz, 1H), 7.30 (d, J = 3.6 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.95 (dd, J = 8.4, 2.1 Hz, 1H), 8.80 (d, J = 2.1 Hz, 1H). 13C NMR (CDCl3); δ 152.5, 151.2, 151.1, 147.5, 147.3, 139.7, 122.8, 117.7, 117.0, 114.3, 113.5, 109.3, 108.6, 106.9. MS (EI) m/e (rel.int.); 314 (M+, 60), 285 (10), 235 (20), 207 (100), 179 (10). HRMS calcd for C14H7BrN2O2: 313.9690. Observed 313.9661.
6-(5′-Cyano-2,2′-bifuran-5-yl)-nicotinonitrile (2h)
A mixture of 7 (942 mg, 3 mmol) and Cu(1)CN (540 mg, 6 mmol) in dry DMF (25 mL) was refluxed for 48 h. The reaction mixture was poured onto water/ammonia and extracted with methylene chloride. The extract was washed with water and brine, dried over Na2SO4, then passed on silica gel to give compound 2h as yellow solid in 27% yield, mp 209–210.5 °C. 1H NMR (DMSO-d6); δ 7.23 (d, J = 3.6 Hz, 1H), 7.27 (d, J = 3.6 Hz, 1H), 7.52 (d, J = 3.6 Hz, 1H), 7.79 (d, J = 3.6 Hz, 1H), 8.04 (d, J = 8.4 Hz, 1H), 8.39 (dd, J = 8.4, 2.4 Hz, 1H), 9.03 (d, J = 2.4 Hz, 1H). Anal. Calcd for C15H7N3O2: C, 68.96; H, 2.70. Found: C, 68.81; H, 2.64.
General procedure of diamidines synthesis (Method A)
The dinitrile 2a (0.87 g, 3.34 mmol), suspended in freshly distilled THF (10 mL), was treated with LiN(TMS)2 (1M solution in THF, 8 mL, 8 mmol) and the reaction was allowed to stir overnight. The reaction mixture was then cooled to 0 °C to which was added HCl saturated ethanol (40 mL) whereupon a precipitate started forming. The mixture was left to run overnight whereafter it was diluted with ether and the resultant solid was collected by filtration. The diamidine was purified by neutralization with 1N NaOH followed by filtration of the resultant solid and washing with water (3X 10 mL). Finally, the free base was stirred with ethanolic HCl overnight, diluted with ether, and the solid formed was filtered and dried to give the diamidine 1a hydrochloride salt
5′-(2-Amidinopyridin-5-yl)-2,2′-bifuran-5-carboxamidine hydrochloride (1a)
Yellow solid, yield 65%, mp >320 °C. 1H NMR (DMSO-d6); δ 7.30 (d, J = 2.4 Hz, 1H), 7.38 (d, J = 2.4 Hz, 1H), 7.67 (d, J = 2.4 Hz, 1H), 8.05 (d, J = 2.4 Hz, 1H), 8.51–8.57 (m, 2H), 9.26 (s, 2H), 9.29 (s, 1H), 9.53 (s, 2H), 9.60 (s, 2H), 9.69 (s, 2H). 13C NMR (DMSO-d6); δ 161.4, 153.3, 150.3, 148.8, 145.4, 145.0, 142.2, 140.5, 132.1, 129.1, 123.8, 120.9, 113.0, 112.4, 109.5. MS (ESI) m/e (rel.int.); 296 (M+ + 1, 100), 208 (7), 148 (52). HRMS calcd for C15H14N5O2: 296.1147. Observed: 296.1144. Anal. Calcd for C15H13N5O2-2.0HCl-1.25H2O: C, 46.10; H, 4.51; N, 17.92. Found: C, 46.01; H, 4.44; N, 17.63.
5′-(2-Amidinopyridin-5-yl)-2,2′-bithiophene-5-carboxamidine hydrochloride (1b)
The same procedure described for preparation of 1a was used starting with the dinitrile 2b. Orange solid, yield 71%, mp 318–321 °C. 1H NMR (D2O/DMSO-d6); δ 7.55 (d, J = 4.0 Hz, 1H), 7.60 (d, J = 4.0 Hz, 1H), 7.82 (d, J = 4.0 Hz, 1H), 7.94 (d, J = 4.0 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.30 (dd, J = 8.4, 2.0 Hz, 1H), 9.06 (d, J = 2.0 Hz, 1H). 13C NMR (D2O/DMSO-d6); δ 161.5, 158.4, 146.6, 143.7, 142.4, 139.4, 137.0, 135.8, 134.3, 133.4, 129.2, 128.8, 127.2, 126.3, 123.9. MS (ESI) m/e (rel.int.); 328 (M+ + 1, 75), 224 (10), 203 (7), 164 (100). HRMS calcd. for C15H14N5S2: 328.0691. Observed: 328.0696. Anal. Calcd for C15H13N5S2-2.0HCl-0.5H2O-0.25EtOH: C, 44.23; H, 4.19; N, 16.64. Found: C, 44.18; H, 4.13; N, 16.61.
5′-(4-Amidinophenyl)-2,2′-bifuran-5-carboxamidine hydrochloride (1c)
The same procedure described for preparation of 1a was used starting with the dinitrile 2c. Yellow solid, yield 73%, mp > 300 °C. 1H NMR (D2O/DMSO-d6); δ 7.16 (d, J = 3.6 Hz, 1H), 7.23 (d, J = 3.6 Hz, 1H), 7.36 (d, J = 3.6 Hz, 1H), 7.75 (d, J = 3.6 Hz, 1H), 7.85 (d, J = 7.8 Hz, 2H), 8.01 (d, J = 7.8 Hz, 2H). Anal. Calcd for C16H14N4O2-2.0HCl-1.0H2O: C, 49.88; H, 4.70; N, 14.54. Found. C, 49.92; H, 4.50; N, 14.41.
5-[5-(4-Amidinophenyl)-furan-2-yl]-thiophene-2-carboxamidine hydrochloride (1d)
The same procedure described for preparation of 1a was used starting with the dinitrile 2d. Brown solid, yield 70%, mp 250–252 °C. 1H NMR (D2O/DMSO-d6); δ 7.14 (d, J = 3.6 Hz, 1H), 7.25 (d, J = 3.6 Hz, 1H), 7.58 (d, J = 3.6 Hz, 1H), 7.79 (d, J = 7.8 Hz, 2H), 7.85–7.93 (m, 3H). 13C NMR (D2O/DMSO-d6); δ 165.9, 159.2, 153.2, 148.8, 140.2, 136.1, 134.8, 129.8, 127.6, 127.5, 125.5, 125.0, 112.9, 112.7. MS (ESI) m/e (rel.int.); 311 (M+ +1, 92), 156 (100). HRMS calcd for C16H15N4OS: 311.0967. Observed: 311.0977. Anal. Calcd for C16H14N4OS-2.0HCl-1.5H2O-0.25EtOH: C, 46.97; H, 4.89; N, 13.28. Found: C, 46.82; H, 4.69; N, 13.13.
5′-(4-Amidinophenyl)-2,2′-bithiophene-5-carboxamidine hydrochloride (1e)
The same procedure described for preparation of 1a was used starting with the dinitrile 2e. Redish brown solid, yield 81%, mp 271–273 °C. 1H NMR (D2O/DMSO-d6); δ 7.49 (d, J = 3.6 Hz, 1H), 7.53 (d, J = 3.6 Hz, 1H), 7.67 (d, J = 3.6 Hz, 1H), 7.84 (s, 4H), 7.94 (d, J = 3.6 Hz, 1H). 13C NMR (D2O/DMSO-d6); δ 165.5, 158.7, 144.1, 143.3, 138.4, 136.1, 136.0, 129.8, 129.0, 128.1, 127.5, 127.4, 126.4, 126.2. MS (ESI) m/e (rel.int.); 327 (M+ +1, 100), 208 (17), 164 (90). HRMS calcd. for C16H15N4S2: 327.0738. Observed: 327.0739. Anal. Calcd for C16H14N4S2-2.0HCl-1.5H2O: C, 45.06; H, 4.49; N, 13.13. Found: C, 45.00; H, 4.38; N, 13.03.
5-[5-(4-Amidinophenyl)-thiophen-2-yl]-furan-2-carboxamidine hydrochloride (1f)
The same procedure described for preparation of 1a was used starting with the dinitrile 2f. Yellow solid, yield 77%, mp 248–249.5 °C. 1H NMR (D2O/DMSO-d6); δ 7.19 (d, J = 3.6 Hz, 1H), 7.78–7.82 (m, 3H), 7.91–7.96 ((m, 4H). 13C NMR (D2O/DMSO-d6); δ 165.5, 153.8, 153.4, 143.7, 140.2, 138.5, 131.9, 129.6, 128.9, 127.4, 126.4, 126.0, 123.8, 121.8. MS (ESI) m/e (rel.int.); 311 (M+ +1, 100), 156 (87). HRMS calcd. for C16H15N4OS: 311.0967. Observed: 311.0966. Anal. Calcd for C16H14N4OS-2.0HCl-1.5H2O: C, 46.83; H, 4.67; N, 13.65. Found: C, 46.90; H, 4.66; N, 13.30.
5′-[(4-Amidinophenyl)ethynyl]-2,2′-bifuran-5-amidine hydrochloride (1g)
The same procedure described for preparation of 1a was used starting with the dinitrile 2g. Yellow solid, yield 78%, mp >320 °C. 1H NMR (DMSO-d6); δ 7.19 (d, J = 3.6 Hz, 1H), 7.25 (d, J = 3.6 Hz, 1H), 7.32 (d, J = 3.6 Hz, 1H), 7.83 (d, J = 8.4 Hz, 2H), 7.93 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 3.6 Hz, 1H), 9.26 (s, 2H), 9.40 (s, 2H), 9.58, 9.61 (2s, 4H). 13C NMR (DMSO-d6); δ 164.9, 153.2, 148.3, 145.4, 140.5, 136.2, 131.4, 128.7, 128.4, 125.9, 120.9, 119.7, 111.5, 109.6, 94.1, 81.95. MS (ESI) m/e (rel.int.); 319 (M+ + 1, 100), 160 (75). HRMS calcd for C18H15N4O2: 319.1195. Observed 319.1206. Anal. Calcd for C18H14N4O2-2.0HCl-1.25H2O: C, 52.24; H, 4.51; N, 13.54. Found: C, 52.50; H, 4.54; N, 13.23.
General procedure of diamidines synthesis (Method B)
N-Hydroxy-5′-[4-(N-hydroxyamidino)-phenyl]-2,2′-bifuran-5-carboxamidine (8a)
A mixture of hydroxylamine hydrochloride (695 mg, 10 mmol, 10 equiv.) in anhydrous DMSO (8 mL) was cooled to 5 °C under nitrogen and potassium t-butoxide (1.12 g, 10 mmol, 10 equiv.) was added in portions. The mixture was stirred for 30 min. This mixture was added to the dinitrile derivative 2c (260 mg, 1 mmol, 1 equiv.). The reaction mixture was stirred overnight at room temperature. The reaction mixture was then poured slowly onto ice-water. The precipitate was filtered and washed with water and then ethanol to afford 8a (free base) in 93%, mp 205–206 °C. 1H NMR (DMSO-d6); δ 5.83 (br s, 4H), 6.86–6.89 (m, 3H), 7.14 (s, 1H), 7.75 (s, 4H), 9.70 (s, 1H), 9.75 (s, 1H). 13C NMR (DMSO-d6); δ 152.3, 150.3, 146.7, 145.0, 144.9, 144.1, 132.3, 129.9, 125.8, 123.1, 109.7, 108.5, 107.2. MS (FAB) m/e (rel.int.); 327 (M++1, 40), 307 (100), 299 (60), 273 (10), 220 (30). HRMS calcd for C16H15N4O4 ms 327.1093. Observed 327.1137.
N-Hydroxy-6-[5′-(N-hydroxyamidino)-2,2′-bifuran-5-yl]-nicotinamidine (8b)
The same procedure described for 8a was used starting with 2h. Yield 89%, mp 248–250 °C. 1H NMR (DMSO-d6); δ 5.88 (s, 2H), 6.04 (s, 2H), 6.92–6.96 (m, 3H), 7.29 (d, J = 3.6 Hz, 1H), 7.84 (d, J = 8.4 Hz, 1H), 8.11 (dd, J = 8.4, 2.1 Hz, 1H), 8.88 (d, J = 2.1 Hz, 1H), 9.80 (s, 1H), 9.92 (s, 1H). 13C NMR (DMSO-d6); δ 152.2, 148.7, 147.8, 147.0, 146.6, 146.0, 144.6, 144.0, 133.6, 127.3, 117.7, 111.3, 109.7, 108.5, 107.9. MS (EI) m/e (rel.int.);; 327 (M+, 15), 311 (5), 295 (10), 278 (85), 261 (100). HRMS calcd for C15H13N5O4: 327.0967. Observed 327.0974.
5′-(4-Amidinophenyl)-2,2′-bifuran-5-carboxamidine acetate (1c)
To a solution of the diamidoxime 8a (326 mg, 1 mmol) in glacial acetic acid (10 mL) was slowly added acetic anhydride (0.35 mL). After stirring for overnight TLC indicated complete acylation of the starting material, ethanol (10 mL) and 10% palladium on carbon (80 mg) were then added. The mixture was placed on Parr hydrogenation apparatus at 50 psi for 4 h at room temperature. The mixture was filtered through hyflo and the filter pad washed with water. The filtrate was evaporated under reduced pressure and the precipitate was collected and washed with ether to give 1c acetate salt in 67% yield, mp 240–242 °C. 1H NMR (D2O/DMSO-d6); δ 1.80 (s, 2 x CH3), 7.06 (d, J = 3.6 Hz, 1H), 7.11 (d, J = 3.6 Hz, 1H), 7.36 (d, J = 3.6 Hz, 1H), 7.39 (d, J = 3.6 Hz, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.98 (d, J = 8.4 Hz, 2H). Anal. Calcd for C16H14N4O2-2.0AcOH-2.4H2O: C, 52.48; H, 5.87; N, 12.23. Found. C, 52.28; H, 5.49; N, 11.81.
5′-(4-Amidinophenyl)-2,2′-bifuran-5-carboxamidine (1c)
It was prepared by dissolving the acetate salt 1c (50 mg) in water (5 mL) and by neutralization with 1N NaOH. The precipitate was filtered, dried to afford free amidine of 1c. mp 202–203.5 °C. 1H NMR (D2O/DMSO-d6); δ 6.93 (d, J = 3.6 Hz, 1H), 7.01 (d, J = 3.6 Hz, 1H), 7.11 (d, J = 3.6 Hz, 1H), 7.22 (d, J = 3.6 Hz, 1H), 7.83 (s, 4H). 13C NMR (D2O/DMSO-d6); δ 162.2, 153.9, 152.4, 147.4, 145.7, 145.0, 134.1, 131.1, 127.3, 123.1, 111.9, 109.4, 109.2, 107.7. MS (EI) m/e (rel.int.); 294 (M+, 50), 277 (100), 261 (25). HRMS calcd for C16H14N4O2: 294.1116. Observed: 294.1101.
6-(5′-Amidino-2,2′-bifuran-5-yl)-nicotinamidine acetate (1h)
The same procedure described for preparation of 1c was used starting with 8b. Yellow solid, yield 59%, mp 269–271 °C. 1H NMR (D2O/DMSO-d6); δ 7.15 (d, J = 3.6 Hz, 1H), 7.23 (d, J = 3.6 Hz, 1H), 7.47 (d, J = 3.6 Hz, 1H), 7.57 (d, J = 3.6 Hz, 1H), 8.04 (d, J = 8.4 Hz, 1H), 8.30 (d, J = 8.4 Hz, 1H), 8.99 (s, 1H). MS (EI) m/e (rel.int.); 296 (M++1, 100), 273 (12), 239 (40). HRMS calcd for C15H14N5O2: 296.1147. Observed 296.1189. Anal. Calcd for C15H13N5O2-2.0AcOH-2.65H2O-0.5EtOH: C, 49.41; H, 6.07; N, 14.40. Found. C, 49.72; H, 5.96; N, 14.02.
Figure 1.
Acknowledgments
This work was supported by NIH Grant AI64200, and an award from the Bill and Melinda Gates Foundation. Serry A. El Bialy was supported by a Fulbright Fellowship awarded by the Binational Fulbright Commission of Egypt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.a) Tidwell RR, Boykin DW. Dicationic DNA Minor Groove Binders as Antimicrobial Agents. In: Demeunynck M, Bailly C, Wilson WD, editors. Small Molecule DNA and RNA Binders: From Synthesis to Nucleic Acid Complexes. Vol. 2. Wiley-VCH; New York: 2003. pp. 414–460. [Google Scholar]; b) Wilson WD, Nguyen B, Tanious FA, Mathis A, Hall JE, Stephens CE, Boykin DW. Dications That Target the DNA Minor Groove: Compound Design and Preparation, DNA Interactions, Cellular Distribution and Biological Activity. Current Medicinal Chemistry-Anti-Cancer Agents. 2005;5:389. doi: 10.2174/1568011054222319. [DOI] [PubMed] [Google Scholar]; c) Soeiro MNC, de Souza EM, Stephens CE, Boykin DW. Expert Opin Invest Drugs. 2005;14:957. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]; d) Dardonville C. Expert Ther Pat. 2005;15:1241. [Google Scholar]; e) Werbovetz KA. Curr Opin Invest Drugs. 2006;7:147. [PubMed] [Google Scholar]; f) Paine MF, Wang MZ, Generaux CN, Boykin DW, Wilson WD, De Koning HP, Olson CA, Pohling G, Burri C, Brun R, Murilla GA, Thuita JK, Barrett MP, Tidwell RR. Expert Opin Invest Drugs. 2010;11:876. [PubMed] [Google Scholar]
- 2.Thuita JK, Karanja SM, Wenzler T, Mdachi RE, Ngotho JM, Kagira JM, Tidwell RR, Brun R. Acta Tropica. 2008;108:6. doi: 10.1016/j.actatropica.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Czarny A, Boykin DW, Wood A, Nunn CM, Neidle S, Zhao MRJ, Wilson WD. J Am Chem Soc. 1995;117:4716. [Google Scholar]
- 4.Laughton CA, Tanious FA, Nunn CM, Boykin DW, Wilson WD, Neidle S. Biochemistry. 1996;35:5655. doi: 10.1021/bi952162r. [DOI] [PubMed] [Google Scholar]
- 5.Trent JO, Clark GR, Kumar A, Wilson DW, Boykin DW, Hall JE, Tidwell RR, Blagburn BL, Neidle S. J Med Chem. 1996;39:4554. doi: 10.1021/jm9604484. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WD, Tanious FA, Ding D, Kumar A, Boykin DW, Colson P, Houssier C, Bailly C. J Am Chem Soc. 1998;120:10310. [Google Scholar]
- 7.Boykin DW, Kumar A, Spychala J, Zhou M, Lombardy R, Wilson WD, Dykstra CC, Jones SK, Hall JE, Tidwell RR, Laughton C, Nunn CM, Neidle S. J Med Chem. 1995;38:912. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 8.Boykin DW, Kumar A, Xiao G, Wilson WD, Bender BC, McCurdy DR, Hall JE, Tidwell RR. J Med Chem. 1998;41:124. doi: 10.1021/jm970570i. [DOI] [PubMed] [Google Scholar]
- 9.Francesconi I, Wilson WD, Tanious FA, Hall JE, Bender BC, Tidwell RR, McCurdy D, Boykin DW. J Med Chem. 1999;42:2260. doi: 10.1021/jm990071c. [DOI] [PubMed] [Google Scholar]
- 10.Dykstra CC, McClernon DR, Elwell LP, Tidwell RR. Antimicrob Agents Chemother. 1994;38:1890. doi: 10.1128/aac.38.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailly C, Dassonneville L, Carrascol C, Lucasl D, Kumar A, Boykin DW, Wilson WD. Anti-Cancer Drug Design. 1999;14:47. [PubMed] [Google Scholar]
- 12.Fitzgerald DJ, Anderson JN. J Biol Chem. 1999;274:27128. doi: 10.1074/jbc.274.38.27128. [DOI] [PubMed] [Google Scholar]
- 13.Ismail MA, Brun R, Easterbrook JD, Tanious FA, Wilson WD, Boykin DW. J Med Chem. 2003;46:4761. doi: 10.1021/jm0302602. [DOI] [PubMed] [Google Scholar]
- 14.Wenzler T, Boykin DW, Ismail MA, Hall EJ, Tidwell RR, Brun R. Antimicrob Agents Chemother. 2009;53:4185. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tidwell RR, Geratz JD, Dann O, Volz G, Zeh D, Loewe H. J Med Chem. 1978;21:613. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- 16.Del Poeta M, Schell WA, Dykstra CC, Jones SK, Tidwell RR, Czarny A, Bajic M, Bajic M, Kumar A, Boykin D, Perfect JR. Antimicrob Agents Chemother. 1998;42:2495. doi: 10.1128/aac.42.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Poeta M, Schell WA, Dykstra CC, Jones SK, Tidwell RR, Kumar A, Boykin DW, Perfect JR. Antimicrob Agents Chemother. 1998;42:2503. doi: 10.1128/aac.42.10.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins KT, Wilson WD, Bender BC, McCurdy DR, Hall JE, Tidwell RR, Kumar A, Bajic M, Boykin DW. J Med Chem. 1998;41:3872. doi: 10.1021/jm980230c. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Bailly C, Kumar A, Ding D, Bajic M, Boykin DW, Wilson WD. Proc Natl Acad USA. 2000;97:12. doi: 10.1073/pnas.97.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Carrasco C, Kumar A, Stephens CE, Bailly C, Boykin DW, Wilson WD. Biochemistry. 2001;40:2511. doi: 10.1021/bi002301r. [DOI] [PubMed] [Google Scholar]
- 21.Tanious FA, Wilson WD, Wang L, Kumar A, Boykin DW, Marty C, Baldeyrou B, Bailly C. Biochemistry. 2003;42:13576. doi: 10.1021/bi034852y. [DOI] [PubMed] [Google Scholar]
- 22.Ismail MA, Brun R, Wenzler T, Tanious FA, Wilson WD, Boykin DW. Bioorg and Med Chem. 2004;12:5405. doi: 10.1016/j.bmc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 23.(a) White EW, Tanious FA, Ismail MA, Reszka AP, Neidle S, Boykin DW, Wilson DW. Biophys Chem. 2007;126:140. doi: 10.1016/j.bpc.2006.06.006. [DOI] [PubMed] [Google Scholar]; (b) Tidwell RR, Boykin DW, Ismail MA, Wilson DW, White EAW, Kumar A, Nanjunda R. Eur Pat Appl. 2007:110. CODEN: EPXXDW EP1792613 A2 20070606. [Google Scholar]
- 24.Kaluzhny DN, Borisova OF, Shchyolkina AK. Biopolymers. 2010;93:8–20. doi: 10.1002/bip.21287. [DOI] [PubMed] [Google Scholar]
- 25.Ismail MA. J Chem Res. 2006:733. [Google Scholar]
- 26.Ismail MA, Arafa RK, Brun R, Wenzler T, Miao Y, Wilson WD, Generaux C, Bridges A, Hall JE, Boykin DW. J Med Chem. 2006;49:5324. doi: 10.1021/jm060470p. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen US, Pombo-Villar E. Tetrahedron. 2005;61:2697. [Google Scholar]
- 28.Mallena S, Lee MPH, Bailly C, Neidle S, Kumar A, Boykin DW, Wilson WD. J Am Chem Soc. 2004;126:13659. doi: 10.1021/ja048175m. [DOI] [PubMed] [Google Scholar]
- 29.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. Biochimie. 2008;90:999. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farahat AA, Kumar A, Say M, Barghash AEM, Goda FE, Eisa HM, Wenzler T, Brun R, Liu Y, Mickelson L, Wilson WD, Boykin DW. Bioorg and Med Chem. 2010;18:557. doi: 10.1016/j.bmc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 31.We appreciate a reviewer making this observation.
- 32.Branowska D, Farahat AA, Kumar A, Wenzler T, Brun R, Liu Y, Wilson WD, Boykin DW. Bioorg and Med Chem. 2010;18:3551. doi: 10.1016/j.bmc.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakunova SM, Bakunov SA, Patrick DA, Suresh Kumar EVK, Ohemeng KA, Bridges AS, Wenzler T, Barszcz T, Susan Jones SK, Werbovetz KA, Brun R, Tidwell RR. J Med Chem. 2009;52:2016. doi: 10.1021/jm801547t. [DOI] [PubMed] [Google Scholar]