Abstract
This study was undertaken to determine the dietary supplements of Zn containing diet on the antioxidant status in chickens experimentally infected with Eimeria acervulina. The antioxidant status was monitored via determination of MDA concentrations and erythrocyte SOD and CAT activities, as well as vitamin E, vitamin C, Cu, and Zn in liver, muscle, and serum. The results showed increased MDA (P < .05), CAT (P < .001), and decreased SOD (P < .001) in the infected birds. Significant changes in Cu and Zn concentrations and dramatically reduction of vitamin C and E concentrations in the infected chickens were found. The observed deviations in the studied enzymes and nonenzymatic parameters evidence the occurrence of oxidative stress following the infection and impaired antioxidant status of chickens, infected with Eimeria acervulina. Our results proved the ameliorating role of CuZn(OH)3Cl (0.170 g per kg food) against Eimeria acervulina-induced oxidative damage in infected chickens.
1. Introduction
It is widely accepted that the balance between the reactive oxygen species (ROS) in cells, tissues, and physiological fluids determines their red/ox status. Under usual conditions, the production of ROS and their elimination are in a dynamic equilibrium. This balance could be disturbed when the generation of ROS becomes higher than the protection capacity of systemic antioxidant defense. The impaired equilibrium in favour of oxidants is named oxidative stress and it is involved in the pathogenesis of numerous diseases, including parasitic infections [1–4]. Prime targets of ROS are the polyunsaturated fatty acids (PUFA) in the membrane lipids. This attack causes lipid peroxidation. Further, the decomposition of peroxidized lipids yields a wide variety of end-products, including malondialdehyde (MDA) that is widely used in practice as an indicator of free radical damages [5–7]. Antioxidant system comprising vitamins A, C, and E, and metal enzymes CuZn-superoxide dismutase (SOD) and catalase (CAT) have a cellular protective action against oxidative stress. Reduction of vitamin E, C, and A levels was established during eimeriosis [8–11]. Dietary trace elements/antioxidants can help maintain appropriate antioxidant balance in a lot of infections [12, 13]. Zinc has been shown to play a significant role as an antioxidant [14]. Burke and Fenton [15] have established that Zn deficiency causes increased lipid peroxidation in liver. The reduction of the trace elements/antioxidants such as Zn leads to a decrease in activity of antioxidant enzyme [16]. The mechanism of Zn action as an antioxidant manifests into acute and chronic effects [17]. Chronic effects involve exposure of an organism to Zn on a long-term basis, resulting in induction of some other substance that is the ultimate antioxidant, such as the metallothioneins. Acute effects involve two mechanisms: (1) protection of protein sulphydryl groups or (2) reduction of ●OH formation from H2O2 due to Zn antagonism to redoxactive transition metals, such as iron and copper [18]. Administration of pharmacological doses of Zn in vivo has shown to have a protective effect against general and liver-specific prooxidants. Hence Zn gained an increasing attention to be applied in diseases accompanied with ROS generation [14, 15, 19, 20].
In our previous studies, we observed an impaired blood antioxidant status in broiler chickens infected with Ascaridia galli [21], Eimeria tenella [22], Eimeria acervulina [23], and beneficial effect of 2Gly·ZnCl2·2H2O compound upon blood antioxidant status in broiler chickens experimentally infected with Eimeria acervulina [24].
Therefore, the activity of our investigation was oriented toward finding new sources of trace elements/antioxidants with regards to antioxidant requirements of the infected host and their possible use in the control of the parasitoses.
The aim of the present study was to determine the effect of Zn-Cu hydroxichloride-mixed crystals, (Cu0.78/Zn0.22)2(OH)3Cl, on antioxidant status in broiler chickens experimentally infected with Eimeria acervulina. For this purpose, we investigated blood MDA concentrations and erythrocyte SOD and CAT activities and vitamins E and C, copper and zinc in liver, muscles and serum from all experimental groups of chickens at the end of the experiment.
2. Materials and Methods
2.1. Compounds Tested
Zn-Cu hydroxichloride-mixed crystals, (Cu0.78/Zn0.22)2(OH)3Cl, were synthesized by method of continuous coprecipitation under standard conditions with pH = 7 [25]. Diluted solutions of zinc and copper chloride and sodium hydroxide were used. Crystals were highly soluble in mineral acids but not in water.
2.2. Animal Studies and Treatment Schedules
The study was performed on 60 clinically healthy 20-day-old broiler chickens, Cobb 500 hybrids, weighing 288.0–411.0 g. Up to the age of 11 days, they were housed in cages on slat floors under conditions excluding an additional Eimeria infection and received a standard diet without antibiotics or coccidiostatics. At the age of 12 days, three groups of 20 birds each were formed. The first experimental group was healthy untreated and uninfected (negative controls). The second and the third experimental groups were infected three times with 3 × 105 sporulated Eimeria acervulina oocysts, at 2-day intervals (at 12th, 14th, 16th day), using an ingluvial tube [26]. The third experimental group was treated with double basic salt CuZn(OH)3Cl- 0.170 g per kg food. It was given starting lasting 10 days (2 days before infection and 8 after infection).
The experiment was approved by the Committee on Animal Experimentation at Trakia University, Stara Zagora, Bulgaria and was performed according to the recommendations of Directive 86/609/EC of November 24, 1986.
2.2.1. Infectious Material
Eimeria acervulina oocysts were obtained from naturally infected chickens, passed through 2-week-old broiler chickens, and stored in 2.5% potassium bichromate solution in refrigerator (4°C).
2.3. Analyses of MDA, SOD, and CAT in Blood
Blood for biochemical analyses (2 mL) for MDA, SOD, and CAT assays was sampled from v. subcutanea ulnaris or v. brachialis at postinfection day 8 (of every chicken from the experimental groups). Ethylenediaminetetraacetic acid (EDTA) was used as anticoagulant.
Peripheral Blood Processing —
Collected blood was centrifuged at 3000 g for 15 min and plasma was separated. Then, the plasma was deproteinized with 25% trichloroacetic acid by continuous mixing for 5 min and centrifugation at 2000 g for 15 min. The deproteinized plasma was used for lipid peroxidation products determination.
2.3.1. Determination of Products of Lipid Peroxidation
The total amount of lipid peroxidation products in plasma was assayed using the thiobarbituric acid (TBA) method, measuring spectrophotometrically malondialdehyde (MDA) reactive products at 532 nm [27].
Erythrocyte Processing —
The erythrocyte pellet was washed three times with saline and lysed. The hemoglobin was separated by precipitation with ethanol/chloroform mixture. The mixture was continuously shaken for 5 min and centrifuged at 2500 g for 20 min. The obtained supernatants were used for determination of enzyme activity.
2.3.2. Determination of Superoxide Dismutase (SOD) Activities
CuZn-SOD activity was determined as described by Sun et al. [28] with minor modifications. Briefly, the xanthine/xanthine oxidase system was used to generate the superoxide anion-radical (O2●−)x. This anion reduces nitroblue tetrazolium (NBT) to formazan, which is monitored at 560 nm. SOD in the sample sremoves the O2●− and inhibits the reduction. The level of this reduction is used as a measure of SOD activity. One unit of enzymatic activity is defined as the amount of enzyme causing 50% inhibition of the reduction of NBT to formazan observed. Results were expressed as units per g haemoglobin (U/gHb).
2.3.3. Determination of Catalase (CAT) Activities
CAT activity was assessed in the erythrocyte lysats by the method described by Beers and Sizer [29]. Briefly, hydrogen peroxide (30 Mm) was used as a substrate, and the decrease in H2O2 concentration at 22°C in phosphate buffer (50 mM, pH 7.0) was followed spectroscopically at 240 nm for 1 min. Results are presented as units per g haemoglobin (U/gHb). One unit of CAT activity is defined as the amount of enzyme that degrades 1 μmol H2O2 per minute.
2.4. Determination of the Levels of Cu, Zn, and Vitamins E and C
The levels of Cu and Zn and these of vitamins E and C were detected in the livers, serum, and breast musculature at the end of the experiment (8 days after the infection). The content of Cu and Zn was determined using an atomic absorption spectrophotometry, Varian Spectr. AA 220, Madrid [30]. Vitamin E concentration was detected fluorometrically [31] and vitamin C concentration spectrophotometrically [32].
2.5. Haemoglobin Concentrations
Haemoglobin concentrations of lysates were determined spectrophotometrically at 546 nm by the cyanmethemoglobin method of Mahoney et al. [33].
2.6. Statistical Analysis
The results are reported as means ± SD for the experimental groups of chickens. Statistical analysis was performed with Student's t-test and multiple regression analysis. P < .05 was considered statistically significant.
3. Results
The blood MDA concentrations and the activities of antioxidant enzymes SOD and CAT in studied birds are presented in Figure 1.
Figure 1.
Blood MDA concentrations and erythrocyte SOD and CAT activities in chickens.
The data showed a statistically significant increase of MDA concentrations—a marker of radical-induced damage, in chickens infected with E. acervulina versus the healthy birds (2.76 μmol/L versus 2.55 μmol/L, P < .05, Figure 1). The results of lipid peroxidation products measured by the formation of MDA in plasma in groups of chickens infected with E. acervulina and treated with double basic salt were found to be not significantly different in comparison to the healthy controls (2.65 μmol/L versus 2.55 μmol/L, P > .05, Figure 1) and were significantly reduced, compared to MDA of positive control group (2.65 μmol/L versus 2.76 μmol/L, P < .05, Figure 1). SOD activities were significantly lower in infected chickens than in negative controls (2759.4 U/gHb versus 3486.5 U/gHb, P < .001, Figure 1). The erythrocyte SOD activity in chickens infected with E. acervulina and treated with double basic salt was found to be increased, as compared to the infected chickens (2900 U/gHb versus 2759 U/gHb, P < .001, Figure 1) and lower to the healthy birds (3486 U/gHb, Figure 1). A significant increase of CAT activity was observed in infected, compared to healthy birds (2092.0 U/gHb versus 1218.4 U/gHb, P < .001, Figure 1). The supplementation of the basic salt restored the levels of CAT in lysate of infected with E. acervulina and treated with CuZn(OH)3Cl broiler chickens (1515 U/gHb versus 1218 U/gHb, P > .05, Figure 1).
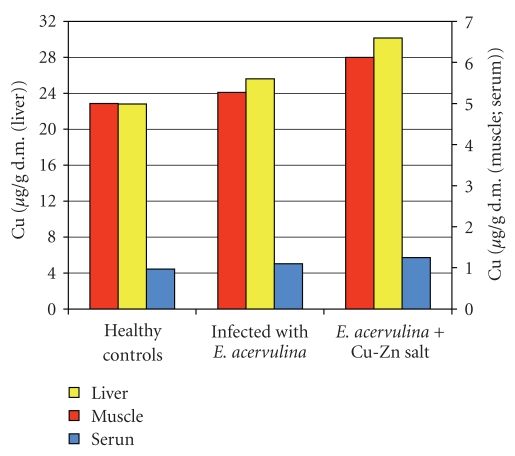
The results showed that E. acervulina infection increased the liver Cu level (Figure 2).
Figure 2.
Cu level in broiler chickens.
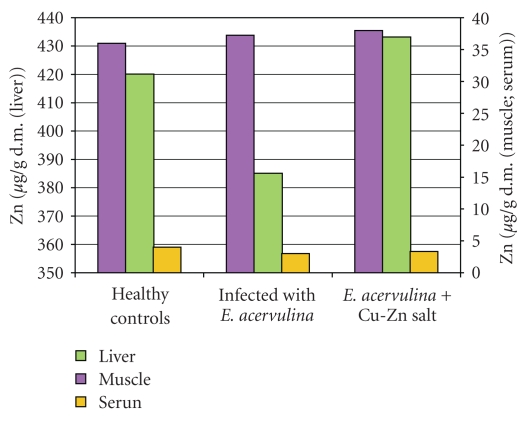
The levels of Cu and Zn in the musculature were slightly reduced as well as in the serum (Figures 2 and 3) and reduced the liver Zn level (Figure 3).
Figure 3.
Zn level in broiler chickens.
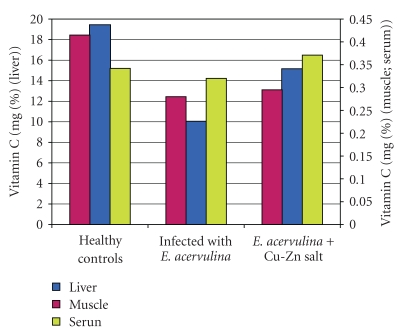
The contents of vitamin C (10.05 mg% versus 19.45 mg%, P < .05, Figure 4) and vitamin E (2.40 mg% versus 4.20 mg%, P < .001, Figure 5) were decreased in the liver but they did not change in the musculature in the infected chickens, as compared to the healthy broiler chickens (P > .05, Figure 5 and P > .01, Figure 4).
Figure 4.
Content of vitamin C in broiler chickens.
Figure 5.
Content of vitamin E in broiler chickens.
There was a significant decrease in the concentration of vitamin E (0.80 mg% versus 1.10 mg%, Figure 5, P < .01, Figure 5) and no significant change in the vitamin C (P > .05, Figure 4) levels in the serum in the infected chickens, compared to the healthy controls.
The supplementation of the basic salt restored the levels of both vitamins to the controls, increased significantly the Cu level under the control (P < .05, Figure 2), and restored the Zn level (P > .05, Figure 3) in the infected and treated chicks. The vitamin C (P < .05, Figure 4) and vitamin E (P < .05, Figure 5) levels as well as those of Cu (P < .05, Figure 2) and Zn (P < .05, Figure 3) in serum from the infected and uninfected chickens were significantly increased after the treatment.
SD, %, values for the parameters Cu, Zn, Vitamin E, and Vitamin C are present in Table 1.
Table 1.
SD, %, values for the parameters MDA, SOD, CAT, Cu, Zn, and Vitamins E and C.
| Groups | Cu | Zn | Vitamin E | Vitamin C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA | SOD | CAT | liver | muscle | serum | liver | muscle | serum | liver | muscle | serum | liver | muscle | serum | |
| Healthy controls | 0.07 | 63.60 | 117.60 | 7.100 | 0.800 | 0.350 | 55.600 | 7.600 | 0.990 | 0.330 | 0.250 | 0.300 | 3.330 | 0.055 | 0.099 |
| Infected with E. acervulina | 0.041 | 106.203 | 115.203 | 8.140 | 0.990 | 0.330 | 90.450 | 4.100 | 0.990 | 0.750 | 0.140 | 0.040 | 3.300 | 0.042 | 0.092 |
| E. acervulina + Cu-Zn salt | 0.05* | 211.00*** | 152.00*** | 5.000 | 1.450 | 0.100 | 81.000 | 1.200 | 1.100 | 1.120 | 0.210 | 0.090 | 2.900 | 0.080 | 0.073 |
1 P < .05; 2P < .01; 3P < .001 versus healthy (negative) controls
*P < .05; **P < .01; ***P < .001 versus infected (positive) controls.
4. Discussion
The production of ROS as by-products of metabolism that have the potential to damage or destroy cellular structures is in a dynamic equilibrium under normal conditions in living organisms. It has been demonstrated that concentrations of ROS are increased in many parasitoses [1, 22, 23, 34–36]. The observed deviations in the studied enzymes and nonenzymatic parameters evidence the occurrence of oxidative stress following the infection and impaired the ecological oxidative balance (EOB) between antioxidants and pro-oxidants of chickens, infected with E. acervulina. In a state of impaired EOB and oxidative stress, biological systems are not protected against the oxidative radical challenge that could result in toxic damage or death of the aerobic organisms [37]. The deviations in the antioxidant status of Eimeria acervulina-infected birds, compared to healthy controls, allowed us to extend the studies upon the mechanism of avian eimeriosis, and how oxidative stress in broiler chickens could be reduced using a substances with proved antioxidant properties. Superoxide dismutase is involved in the antioxidant defense system in a first attempt (or approach) to control and eliminate the toxic ROS [38]. According to Amstad et al. [39], the decrease in the activities of antioxidant enzymes could have a negative impact on cellular resistance against the oxidant-induced damage of cell genome and cell killing. On the other hand Speranza, et al. [40] and Popova and Popov [41] reported that the antioxidant enzyme CAT was important for adaptation of cells to oxidative stress and preserved cells via degradation of the reactive hydrogen peroxide. In the present study, the increases of plasma MDA concentrations and the marked reduction of the blood SOD activity in E. acervulina infected birds evidenced the occurrence of an oxidative stress due to infection and the impairment of antioxidant/pro-oxidant equilibrium in favour of pro-oxidants. The number of facts evidencing the existence of a changed expression of the principal antioxidant enzymes in various diseases is increasing, but the reports are rather conflicting [12, 42–44]. The concomitant increase of CAT activity (Figure 1) would be compensatory mechanism in infected birds against E. acervulina-induced oxidative damage. Similar increased CAT activity was found in birds infected with E. tenella [22]. The application of Cu-Zn basic salt restored the CAT enzyme antioxidant defense system in chickens infected with E. acervulina, but SOD activities were significantly different compared to negative controls. Probably Cu-Zn basic salt produced ROS and this finding was compromised by reduction of the SOD activity in chickens of this group compared to healthy birds (P < .05, Figure 1). The impaired enzyme antioxidant system may favour accumulation of ROS, which probably induced E. acervulina infection, too. Free radicals including ROS are known to be toxic to some parasites [45]. A more logical interpretation of increased both CAT activity and ROS production, after the salt application, is difficult to be done now. It is envisaged that ROS may also be useful in combating other kinds of skin infections and Cu-Zn basic salt to minimize the possible negative effects of E. acervulina. This would agree with our observation of decreased levels of MDA—marker of oxidative stress, in plasma of chickens treated with CuZn(OH)3Cl.
Vitamin E is one of the antioxidants widely used in poultry diets and has been proposed as a major antioxidant in plasma membranes of all cells and subcellular organs, functioning as a chain-breaker and free radical scavenger. Poultry cannot synthesize vitamin E and its concentration is reduced under stress conditions. Vitamin C and E concentrations were dramatically reduced in infected chickens (liver-P < .05, serum-P < .01, and muscle-P < .05, Figure 4 and liver-P < .001, serum-P < .01 and muscle-P < .05, resp. Figure 5). Higher vitamin E reduction and that of vitamin C are comparable with that established in chickens infected with Eimeria sp. [24]. Antioxidant imbalance, increase CAT and decrease SOD, vitamins C and E levels in blood of chickens due to the eimeriosis were based on the liver hypovitaminoses C and E and reduced Zn level (Figures 3, 4, 5). The antioxidant imbalance, comprising decreased levels only of vitamins/antioxidants (vitamin E, C, retinal, and carotene), has been found in parasitize goats (34). Significant changes in activity/concentrations of antioxidant parameters in acute phase of fascioliasis in rats were observed by Kolodziejczyk et al. [46]. The authors established decreased activity of liver enzymes SOD, GSH-Px and GSSG-R, as well as a reduction of vitamin C, E, A and glutathatione levels. Vitamin E plays the most important role in the antioxidant system because it is an excellent biological chain-breaking antioxidant that protects cells and tissue from lipoperoxidative damage induced by free radicals. Vitamin C enhances antioxidant activity of vitamin E by reducing the tocopheroxyl radicals back to their active form of vitamin E or by sparing available vitamin E [9, 13]. Regarding antioxidant property there is a synergistic effect of vitamins C and E on the antioxidant defense system in infected with E. acervulina chickens. Vitamins A, C, and E, Zn, and Cu act as a coordinated and balanced system to protect tissues from damage by reactive oxygen species and each relies on the action of the others [13]. There was a little information about the effect of trace elements supplementation on the antioxidant status in parasitoses. Recently, a positive effect of Zn-Cu mixed basic salt on the antioxidant imbalance in chicks infected with Ascaridia galli [21] and in rabbits infected with Fasciola hepatica [47] was established. The authors investigated the levels of vitamins C and E, the levels of Zn and Cu, as well as SOD-activity in liver of hosts (chickens and rabbits). Developed hypovitaminoses C and E and reduced Zn and Cu levels in infected chickens were restored by Zn-Cu salt supplementation. The differences in the rates of depletion of Zn, as well as vitamins C and E, depended on the parasite and host species, the parasite localization, their life cycle, the biological role, and the possible store of the elements in the host organism. The Cu level in infected with Eimeria acervulina and treated chickens was higher, than that in the healthy controls (P < .001 for liver, P < .01 for muscle and P < .05 for serum, Figure 2), but without any toxic signs. Additional studies are required to establish the optimum Cu : Zn ratio for mixed Zn-Cu crystals for an application in eimeriosis without any copper accumulation.
5. Conclusion
The observed deviations in the studied enzymes and nonenzymatic parameters evidence the occurrence of oxidative stress following the infection and impaired the EOB between antioxidants and pro-oxidants of chickens, infected with Eimeria acervulina. Our results proved the ameliorating role of CuZn(OH)3Cl (0.170 g per kg food) against Eimeria acervulina-induced oxidative damage in infected chickens.
References
- 1.Allen PC. Production of free radical species during Eimeria maxima infection in chichens. Poultry Science. 1997;76(6):814–821. doi: 10.1093/ps/76.6.814. [DOI] [PubMed] [Google Scholar]
- 2.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radical Biology and Medicine. 2000;28(3):463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 3.Costantini D, Møller AP. Does immune response cause oxidative stress in birds? A meta-analysis. Comparative Biochemistry and Physiology A. 2009;153(3):339–344. doi: 10.1016/j.cbpa.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Veterinary Medicine International. 2011;2011:7 pages. doi: 10.4061/2011/686137. Article ID 686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks DB, Marks AD, Smith CM. Oxygenmetabolism and oxygen toxycity. In: Velker J, editor. Basic Medical Biochemistry. A Clinical Approach. Baltimore, Md, USA: Williams & Wilkins; 1996. pp. 327–340. [Google Scholar]
- 6.Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clinica Chimica Acta. 2001;306(1-2):1–17. doi: 10.1016/s0009-8981(01)00393-x. [DOI] [PubMed] [Google Scholar]
- 7.Dröge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 8.Lauridsen C, Jensen C, Jakobsen K, et al. The influence of vitamin C on the antioxidative status of chickens in vivo, at slaughter and on the oxidative stability of broiler meat products. Acta Agriculturae Scandinavica A. 1997;47(3):187–196. [Google Scholar]
- 9.Allen PC, Fetterer RH. Interaction of dietary vitamin E with Eimeria maxima infections in chickens. Poultry Science. 2002;81(1):41–48. doi: 10.1093/ps/81.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Effect of vitamin A deficiency on host intestinal immune response to Eimeria acervulina in broiler chickens. Poultry Science. 2002;81(10):1509–1515. doi: 10.1093/ps/81.10.1509. [DOI] [PubMed] [Google Scholar]
- 11.Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Intestinal immunomodulation by vitamin A deficiency and lactobacillus-based probiotic in Eimeria acervulina infected chickens broiler. Avian Diseases. 2003;47(4):1313–1320. doi: 10.1637/6079. [DOI] [PubMed] [Google Scholar]
- 12.Miller JK, Brzezinska-Slebodzinska E, Madsen FC. Oxidative stress, antioxidants, and animal function. Journal of Dairy Science. 1993;76(9):2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- 13.Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. British Journal of Nutrition. 2001;85(2):S67–S74. [PubMed] [Google Scholar]
- 14.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radical Biology and Medicine. 1990;8(3):281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 15.Burke JP, Fenton MR. Effect of a zinc-deficient diet on lipid peroxidation in liver and tumor subcellular membranes. Proceedings of the Society for Experimental Biology and Medicine. 1985;179(2):187–191. doi: 10.3181/00379727-179-42083. [DOI] [PubMed] [Google Scholar]
- 16.Bou R, Guardiola F, Barroeta AC, Codony R. Effect of dietary fat sources and zinc and selenium supplements on the composition and consumer acceptability of chicken meat. Poultry Science. 2005;84(7):1129–1140. doi: 10.1093/ps/84.7.1129. [DOI] [PubMed] [Google Scholar]
- 17.Powell SR. The antioxidant properties of zinc. Journal of Nutrition. 2000;130(5):1447–1454. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 18.Gabrashanska M, Teodorova SE, Anisimova M. Oxidative-antioxidant status of Fasciola hepatica-infected rats supplemented with zinc. A mathematical model for zinc bioaccumulation and host growth. Parasitology Research. 2008;104(1):69–78. doi: 10.1007/s00436-008-1160-8. [DOI] [PubMed] [Google Scholar]
- 19.Richards MP, Augustine PC. Serum and liver zinc, copper, and iron in chicks infected with Eimeria acervulina or Eimeria tenella. Biological Trace Element Research. 1988;17:207–219. doi: 10.1007/BF02795457. [DOI] [PubMed] [Google Scholar]
- 20.Georgieva N, Stoyanchev K, Bozakova N, Jotova I. Combined effects of muscular dystrophy, ecological stress and selenium on blood antioxidant status in broiler chickens. doi: 10.1007/s12011-010-8782-2. Biological Trace Element Research. In press. [DOI] [PubMed] [Google Scholar]
- 21.Gabrashanska M, Galvez Morros M, Tsocheva-Gaytandzieva N, Pollet S, Ermidou-Pollet S, Mitov M. Antioxidant status in Ascaridia galli infected chicks treated with zinc-copper double basic salts. In: Proceedings of the 4th International Symposium on Trace Elements: New Perspectives; October 2003; Athens, Greece. pp. 845–854. [Google Scholar]
- 22.Georgieva NV, Koinarski V, Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Veterinary Journal. 2006;172(3):488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Koinarski V, Georgieva N, Gadjeva V, Petkov P. Antioxidant status of broiler chickens, infected with Eimeria acervulina. Revue de Medecine Veterinaire. 2005;156(10):498–502. [Google Scholar]
- 24.Koinarski V, Gabrashanska M, Georgiva N, Petkov P. Anitioxidant parameters in Eimeria acervulina infected chicks after treatment with a new zinc compound. Bulletin of the Veterinary Institute in Pulawy. 2006;50(1):55–61. [Google Scholar]
- 25.Markov L. Synthesis and thermal decomposition of Me(II)-Co(II) hydroxonitrate mixed crystals with layer structure type (Me=Mg, Ni, Cu, Zn) Sofia, Bulgaria: Bulgarian Academy of Sciences; 1987. Ph.D. thesis. [Google Scholar]
- 26.Willis GM, Baker DH. Eimeria acervulina infection in the chicken: a model system for estimating nutrient requirements during coccidiosis. Poultry science. 1981;60(8):1884–1891. doi: 10.3382/ps.0601884. [DOI] [PubMed] [Google Scholar]
- 27.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Analytical Biochemistry. 1966;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 29.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. The Journal of biological chemistry. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 30.Anonnymous. Analytical methods for atomic absorption spectrophotometry. Norwalk. Conn, USA: Perkin-Elmer; 1982. [Google Scholar]
- 31.Bieri J, Tolliver T. On the occurance of alpha-tocopherol in rat tissues. Lipids. 1981;16:777–779. doi: 10.1007/BF02535351. [DOI] [PubMed] [Google Scholar]
- 32.Omaye ST, Turnbul JD, Saveberlich HE. Ascorbic acid analysis. II. Determination after derivatisation with 2,2-dinitrophenylhydrazine. Selected methods for determination of ascorbic acid in animal cells,tissues and fluids. Method in Enzymology, London. 1979;62:p. 7. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney JJ, Vreman HJ, Stevenson DK, Van Vessel, AL. Measurements of carboxyhaemoglobin by five spectrophotometers (cooximeters) in comparison with reference methods. Clinical Chemistry. 1993;39:1693–1700. [PubMed] [Google Scholar]
- 34.Dede S, Değer Y, Değer S, Kahraman T, Alkan M, Cemek M. Oxidation products of nitric oxide and the concentration of antioxidant vitamins in parasitise goats. Acta Veterinaria Brno. 2002;71(3):341–345. [Google Scholar]
- 35.Lillehoj HS, Li G. Nitric oxide production by macrophages stimulated with coccidia sporozoites, lipopolysaccharide, or interferon-γ, and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Diseases. 2004;48(2):244–253. doi: 10.1637/7054. [DOI] [PubMed] [Google Scholar]
- 36.Karamouz H, Shahriar H, Doust R. Response of male broiler to different levels of food industries residual oil on serum lipoproteins, lipid peroxidation and total antioxidant status. American-Eurasian Journal of Agricultural & Environmental Sciences. 2009;6(2):252–256. [Google Scholar]
- 37.Georgieva NV. Oxidative stress as a factor of disrupted ecological oxidative balance in biological systems—a review. Bulgarian Journal of Veterinary Medicine. 2005;8(1):1–11. [Google Scholar]
- 38.McCord JM. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983;94(3):412–414. [PubMed] [Google Scholar]
- 39.Amstad P, Moret R, Cerutti P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn- superoxide dismutase overproducers to oxidant stress. Journal of Biological Chemistry. 1994;269(3):1606–1609. [PubMed] [Google Scholar]
- 40.Speranza MJ, Bagley AC, Lynch RE. Cells enriched for catalase are sensitized to the toxicities of bleomycin, adriamycin, and paraquat. Journal of Biological Chemistry. 1993;268(25):19039–19043. [PubMed] [Google Scholar]
- 41.Popova MP, Popov CS. Effect of heavy metal salts on the activity of rat liver and kidney catalase and lysosomal hydrolases. Journal of Veterinary Medicine Series A. 1998;45(6-7):343–351. doi: 10.1111/j.1439-0442.1998.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 42.Challey J. The effect of caecal coccidiosis infections and experimental hemorhage upon adrenal ascorbic acid levels in the chickens. The Journal of Parasitology. 1960;46:727–731. [PubMed] [Google Scholar]
- 43.Baker AM, Oberley LW, Cohen MB. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate. 1997;32(4):229–233. doi: 10.1002/(sici)1097-0045(19970901)32:4<229::aid-pros1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Matés JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clinical Biochemistry. 1999;32(8):595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 45.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB Journal. 1995;9(2):200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 46.Kolodziejczyk L, Siemieniuk E, Skrzydlewska E, Machoy-Mokrzynska A. Antioxidant abilities of rat liver in acute fascioliasis. In: Proceedings of the 9th European Multicolloquium of Parasitology; July 2004; Valencia, Spain. p. 538. [Google Scholar]
- 47.Tsocheva-Gaytandzhieva N, Gabrashanska M, Galvez-Morros M, Soto de Zaldivar B, Pollet S, Ermidou-Pollet S. Liver antioxidant status under acute fascioliasis and (ZnxCu1−x)2Cl supplementation in rabbits. In: Proceedings of the 22nd Macro and Trace Elements Workshop, vol. 1; 2004; Jena, Germany. pp. 619–624. [Google Scholar]