Abstract
Serum interleukin-8 (IL-8) and interferon-alpha (IFN-α) levels have been estimated from a total of 88 individuals of which 19 were disease-free healthy individuals, and 69 were patients with thyroid diseases: goitre (N = 21), autoimmune diseases (N = 16), and carcinomas (N = 32). Both IL-8 and IFN-α were significantly higher in all the patients as compared to healthy individuals. Serum IL-8 levels showed significant positive correlation with disease stage in thyroid cancer patients. Higher serum IL-8 levels were associated with advanced disease stage while no significant correlation was observed between serum IFN-α levels and any of the clinicopathological parameters. IL-8 and IFN-α significantly correlated with each other in anaplastic carcinoma patients. Finally concluding, monitoring the serum IL-8 and IFN-α levels can help differentiate patients with thyroid diseases from healthy individuals, and IL-8 seems to have a role in the pathogenesis of thyroid diseases and may represent a target for innovative diagnostic and therapeutic strategies.
1. Introduction
Although thyroid problems are among the most common medical conditions, because their symptoms often appear gradually, they are commonly misdiagnosed. The three most common thyroid problems are the underactive thyroid, the overactive thyroid, and thyroid nodules [1]. Based on these problems, the disorders of the thyroid gland include: goitre, autoimmune thyroid diseases, as well as thyroid carcinoma.
Thyroid cancer is the fastest growing and most common cancer of the total endocrine malignancies, accounting for 94.5% of the total new endocrine cancers and 65.9% of the deaths due to endocrine cancers. It is the endocrine tumor that bears the highest incidence with 33 550 new cases per year in the US [2]. The vast majority of patients with thyroid diseases are curable using present treatment modalities. However, accumulating evidences indicate that follicular cell-derived thyroid cancer constitutes a biological continuum progressing from the highly curable well-differentiated thyroid cancer to the universally fatal anaplastic thyroid cancer [3, 4]. An association between thyroid cancer and a history of several benign diseases has been observed in most studies [5, 6]. However, the molecular mechanisms underlying thyroid cancer progression remain ill-defined. So it is important to decipher these mechanisms, since poorly differentiated and anaplastic carcinomas account for the majority of thyroid cancer-related deaths.
Moreover, accumulated epidemiologic studies support that chronic inflammatory diseases are frequently associated with increased risk of cancers [7–9]. It is estimated that underlying infections and inflammatory responses are linked to 15–20% of all deaths from cancer worldwide [7]. There are many triggers of chronic inflammation that increase the risk of developing cancer. Such triggers include microbial infections, autoimmune diseases, and inflammatory conditions of unknown origin. The hallmarks of cancer-related inflammation include the presence of inflammatory cells and inflammatory mediators (e.g., chemokines, cytokines, and prostaglandins) in tumor tissues, tissue remodeling and angiogenesis similar to that seen in chronic inflammatory responses, and tissue repair. Indeed, inflammatory cells and mediators are present in the microenvironment of most, if not all, tumors, irrespective of the trigger for development [10]. Cytokine shedding by tumor cells into the local microenvironment is an important modulator of tumorigenesis [11]. Thus, the relationship between the immune system and cancer is complex, and cytokines, chemokines, and growth factors in the tumor environment play a key role in this interaction [12].
Cytokines are a category of signaling proteins and glycoproteins that, like hormones and neurotransmitters, are used extensively in cellular communication, which mediate and regulate immunity, inflammation, and hematopoiesis. Thus, cytokines are a group of polypeptides produced mainly by inflammatory cells, but also by nonimmune cells, and have a key role in triggering and coordinating inflammatory and immune reactions [13].
However, the relationships between cytokines and cancer are multiple and bidirectional. On one hand, cytokines may directly influence carcinogenesis and metastasis by modifying the tumor phenotype while, on the other hand during tumor progression, modifications of the cytokine expression in the tumor environment may be induced by the tumor cells [14]. Thus, a better understanding of the basis of molecular talk between tumor cells and the immune system would be helpful in developing immunotherapeutic approaches.
Interleukin-8 (IL-8) is one of the best-characterized members of C-X-C subfamily of the chemotactic cytokines [15, 16]. It is a proinflammatory cytokine produced by macrophages and other cell types such as epithelial cells. As it serves as a chemical signal that attracts neutrophils at the site of inflammation, it is also called “neutrophil chemotactic factor”. Interleukin (IL)-8 is produced by a wide variety of normal cells as well as tumor cell and its principal role is in the initiation and amplification of acute inflammatory reactions. IL-8 has also been implicated in chronic inflammatory processes and diseases with a chronic inflammatory component such as cancer. IL-8 has been shown to contribute to human cancer progression through its potential functions as a mitogenic, angiogenic, and mitogenic factor [17, 18]. Recent studies have demonstrated that IL-8 regulates tumor cell growth and metastasis in melanoma [19], carcinoma of breast [20], stomach [21], pancreas [22], and liver [22, 23]. Elevated serum level of IL-8 was found to be a prognostic marker in soft tissue sarcoma [24], B-cell chronic lymphocytic leukemia [25], primary gastrointestinal non-Hodgkin's lymphoma [26], and malignant melanoma [27].
So, understanding the mechanisms of IL-8 expression can be helpful in designing potential therapeutic strategies of targeting IL-8 to control tumor growth and metastasis. On the other hand, according to current data, endocrine system is closely related to immune system, and interferons play an important role in this relationship. Interferons can inhibit cell proliferation or control apoptosis [28].
Interferon-alpha (IFN-α) is a small protein released by macrophages, lymphocytes, and tissue cells infected with a virus, it has pleiotropic properties and exerts a wide range of immunomodulatory activities [29]. It is predominantly characterized as an antiviral and antiproliferative agent which stimulates both macrophages and natural killer cells to elicit an antiviral response, and it is also active against tumors. Besides, a very important property of IFN-α is its ability to arrest the cell in the G1-G0 phase, which is what determines static effect of IFN-α on growth of many tumors [28]. Moreover, IFN-α is a potent activator of monocytic functions. But, since human monocytes are major producers of neutrophil chemotactic cytokine: IL-8, we have been tempted to study levels of both these cytokines (IL-8 and IFN-α) in sera of patients with thyroid disorders by ELISA. Moreover, it is much more convenient to quantitate serum levels of these cytokines than to evaluate their expression in tissue specimens as the former approach is noninvasive and reproducible. Furthermore, it does not require tumor tissue specimens.
So, the aim of this study was to explore the occurrence of interleukin-8 and interferon-alpha in sera of patients with various thyroid diseases (goiter, autoimmune disorders, and thyroid carcinoma) and to correlate the results with clinicopathological parameters in thyroid cancer patients.
2. Materials and Methods
Our study included a group of 88 individuals, out of which 69 were patients with thyroid disorders (goiter: N = 21, autoimmune thyroid diseases: N = 16, and thyroid carcinoma: N = 32) and 19 were age-matched disease-free healthy individuals (Table 1). The mean age of healthy individuals included in the study was 30.57 years (range: 18–56 years). Goiter occurred in patients at an earlier age (mean age: 34.23 years, range: 18–58 years) while the occurrence of autoimmune thyroid disease (mean age: 42.81 years, range: 26–61 years; P = .04) and thyroid carcinoma (mean age: 43.96 years, range: 18–78 years; P = .034) was found in more elderly people. But as in the American Joint Committee on Cancer (AJCC) TNM staging system, the patients are staged on the basis of their age (<45/≥45 years), we have also grouped our patients into a younger (<45 years) and an older group (≥45 years) (Table 1).
Table 1.
Characterization of patients with thyroid diseases and healthy individuals.
| Subjects | N (%) | Gender | Age | ||
|---|---|---|---|---|---|
| Male | Female | <45 years | ≥45 years | ||
| Healthy individuals | 19 | 6 | 13 | 15 | 4 |
| Total Patients | 69 | 16 | 53 | 40 | 29 |
| Goiter | 21 (30.43) | 3 | 18 | 14 | 7 |
| Autoimmune diseases | 16 (23.18) | 4 | 12 | 9 | 7 |
| Graves' disease | 12 (17.38) | 4 | 8 | 7 | 5 |
| Hashimoto's disorder | 4 (5.79) | — | 4 | 2 | 2 |
| Thyroid carcinoma | 32 (46.37) | 9 | 23 | 17 | 15 |
| Papillary | 18 (26.07) | 3 | 15 | 12 | 6 |
| Follicular | 7 (10.14) | 2 | 5 | 2 | 5 |
| Medullary | 4 (5.79) | 2 | 2 | 2 | 2 |
| Anaplastic | 3 (4.34) | 2 | 1 | 1 | 2 |
Fasting blood samples were collected in the morning from disease-free healthy individuals and patients with thyroid diseases in vacuettes with gel for serum separation. All patients with thyroid disorders were never previously treated with chemotherapy, surgery, or radiotherapy at the time of blood collection. Moreover, none of these patients were ever before diagnosed with any other autoimmune disease, no one of them was taking immunosuppressive or immunomodulant drugs. Prior to pretherapeutic blood collection, written consent of the patients was taken. Serums were separated from all blood samples after centrifugation and were stored at −80°C until analysis. IL-8 and IFN-α were determined from the serum samples using commercially available enzyme immunoassay (EIA) kits from Immunotech (A Beckman Coulter Company, France) following the manufacturer's instructions. The detailed clinical and histopathological characteristics of all patients were noted from the case files maintained at Gujarat Cancer and Research Institute (Table 2).
Table 2.
Clinicopathological parameters of thyroid cancer patients.
| Parameters | N (%) | Parameters | N (%) |
|---|---|---|---|
| Age | Multifocality | ||
| <45 years | 17 (53.10) | Present | 14 (43.70) |
| ≥45 years | 15 (46.90) | Absent | 18 (56.30) |
| Gender | Bilaterality | ||
| Male | 09 (28.10) | Unilateral | 23 (71.90) |
| Female | 23 (71.90) | Bilateral | 09 (28.10) |
| Tumor size | Haemorrhagic area | ||
| T1 + T2 | 16 (50.00) | Present | 07 (21.90) |
| T3 + T4 | 16 (50.00) | Absent | 25 (78.10) |
| Lymph node metastasis | Necrosis | ||
| Present | 18 (56.30) | Present | 03 (9.40) |
| Absent | 14 (43.70) | Absent | 29 (90.60) |
| Distant metastasis | Calcification | ||
| Present | 21 (65.60) | Present | 19 (59.40) |
| Absent | 11 (34.30) | Absent | 13 (40.60) |
| Stage | Sclerosis | ||
| Early stage (Stage I & II) | 15 (46.90) | Present | 04 (12.50) |
| Advanced stage (Stage III & IV) | 17 (53.10) | Absent | 28 (87.50) |
| Lymphatic permeation | Extrathyroidal extension | ||
| Present | 04 (12.50) | Present | 13 (40.60) |
| Absent | 28 (87.50) | Absent | 19 (59.40) |
| Vascular permeation | Fibrosis | ||
| Present | 08 (25.00) | Present | 08 (25.00) |
| Absent | 24 (75.00) | Absent | 24 (75.00) |
| Capsular invasion | Inflammation | ||
| Present | 13 (40.60) | Present | 14 (43.70) |
| Absent | 19 (59.40) | Absent | 18 (56.30) |
| Encapsulation | Differentiation | ||
| Well-encapsulated | 27 (84.40) | Well | 22 (68.75) |
| Not encapsulated | 05 (15.60) | Moderate/Poor | 10 (31.25) |
2.1. Statistical Analysis
The results were presented as mean ± standard error of mean (M ± S.E.). Mann-Whitney U test was performed to assess the differences in serum IL-8 and IFN-α levels between healthy individuals and patients with thyroid diseases. Receiver's operating characteristic (ROC) curves were also constructed to determine the discriminating efficacy of IL-8 and IFN-α between healthy individuals and patients with thyroid diseases. P values <.05 were considered statistically significant. Also, in thyroid cancer patients, the association between IL-8 and IFN-α levels and clinicopathological parameters was analyzed by Mann-Whitney U test, and the spearman's correlation was used to describe independent relationship between serum IL-8 and IFN-α levels.
3. Results and Discussion
Serum IL-8 and IFN-α levels were significantly elevated in all patients with thyroid disorders (goitre, autoimmune thyroid disorders, and thyroid carcinoma) as compared to healthy individuals. IL-8 and IFN-α levels in thyroid carcinoma patients with different histopathological subgroups and stages have also been compared to that of healthy individuals. Statistically significant higher levels of both these cytokines were found in patients with papillary and follicular carcinoma while, in medullary carcinoma patients, IFN-α and, in anaplastic carcinoma patients, IL-8 levels were found to be significantly increased as compared to healthy individuals. Early-stage thyroid carcinoma patients exhibited significant higher levels of IFN-α, but not of IL-8, while IL-8 and not IFN-α levels were found significantly elevated in advanced-stage thyroid carcinoma patients in comparison to healthy individuals (Tables 3 and 4).
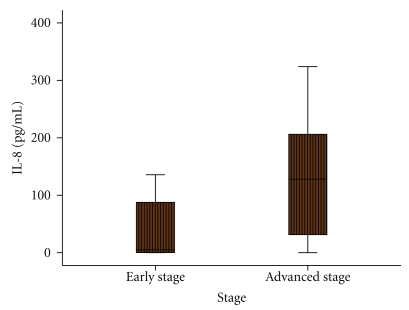
Figure 3.
Association between serum IL-8 levels and histopathological stage of thyroid carcinoma patients.
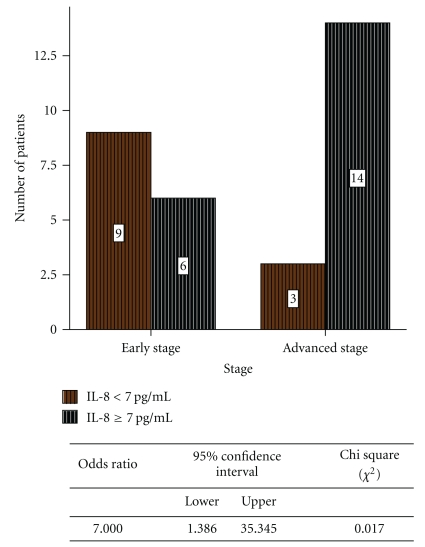
Figure 4.
Relative risk of IL-8 in early- and advanced-stage thyroid carcinoma patients.
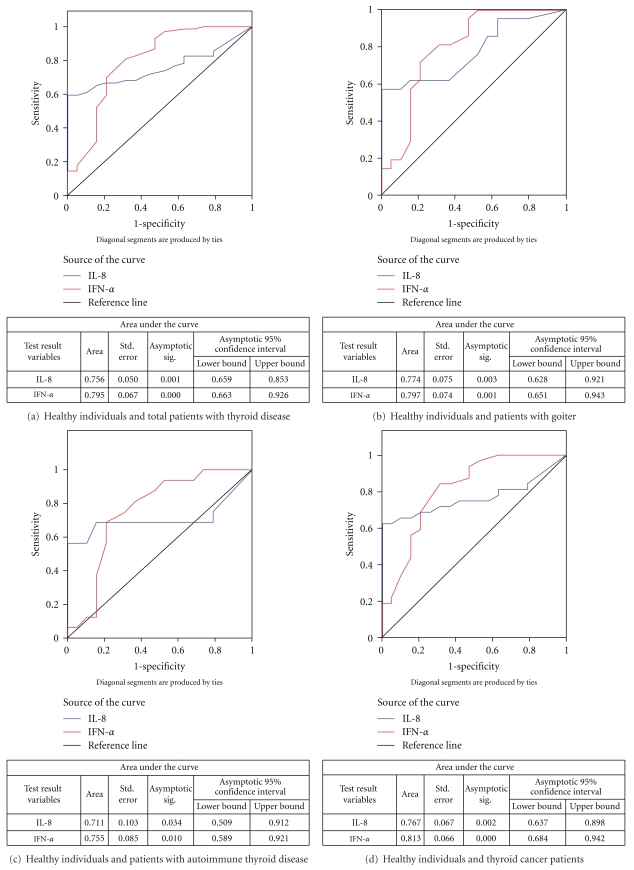
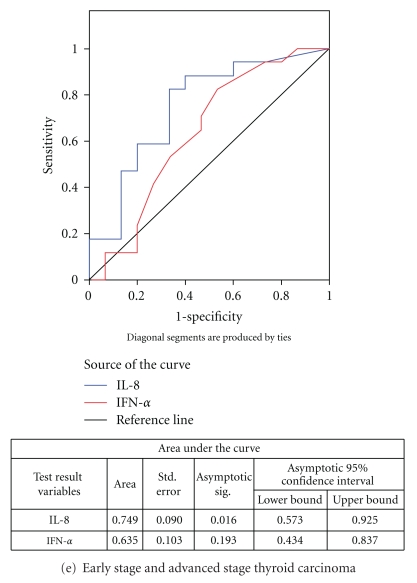
ROC curve (Figure 1(a)) indicates that both IL-8 and IFN-α exhibited a good discriminatory efficacy between healthy individuals and total patients with thyroid diseases (IL-8: AUC-0.756; IFN-α: AUC-0.795). Moreover, the ROC curves for both the cytokines between healthy individuals and individual groups of patients, that is, goiter, autoimmune disease, and thyroid cancer, revealed that both IL-8 as well as IFN-α showed good sensitivity and specificity to discriminate between healthy individuals and patients having different thyroid diseases (goiter- IL-8: AUC-0.774, IFN-α: AUC-0.797; autoimmune thyroid disease- IL-8: AUC-0.711, IFN-α: AUC-0.755, and thyroid cancer- IL-8: AUC-0.767, IFN-α: AUC-0.813) (Figures 1(b)–1(d)). Only IL-8 could significantly discriminate between early- and advanced-stage thyroid cancer patients (Figure 1(e)).
Figure 1.
ROC curves for IL-8 and IFN-α.
Similar to the present study, Limkov et al. have demonstrated statistically significant differences in IL-8 levels thyroid disease patients and reference normal group [30]. Bossowski and Urban and Siddiqi et al. have also observed significantly elevated levels of IL-8 in patients with Graves' disease and nodular goiter as compared to the respective healthy control groups [31, 32]. In contrast to these of studies, Krassas and colleagues found that IL-8 levels were not elevated in Graves' disease, toxic nodular goiter and Hashimoto's thyroiditis [33]. A study by Lee et al. has shown increased levels of cytokines including IL-8 and IL-10 in patients who frequently developed euthyroid sick syndromes followed by allogeneic bone marrow transplantation [34]. Antonelli et al. observed increased CXCL10 levels in autoimmune thyroiditis [35]. Also, they have demonstrated elevated circulating levels of CXCL10 and IFN-gamma in patients with Graves' disease, particularly in those with Graves' ophthalmopathy [36]. Increased IL-8 levels have also been observed in patients with other malignancies. In their study, Yi. Ren et al. also found that the level of serum IL-8 was markedly elevated in most patients with HCC as compared with healthy subjects [37]. In fact, elevated serum level of IL-8 was found to be a prognostic marker in soft tissue sarcoma [38], B-cell chronic lymphocytic leukemia [39], primary gastrointestinal non-Hodgkin's lymphoma [40], and malignant melanoma [41]. So, high serum levels of both the cytokines in thyroid carcinoma patients may be caused by an excessive production in tumor cells and subsequent release into the circulation.
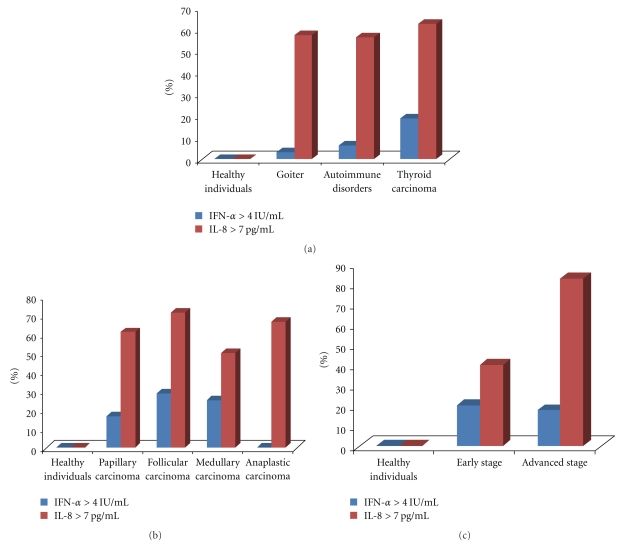
The incidence of patients with thyroid diseases having higher levels of IL-8 and IFN-α than those of healthy individuals has been shown in Figures 2(a)–2(c). The levels of IL-8 > 7.00 ng/ml (maximum level of IL-8 in healthy individuals) were found in 57.10% of patients with goitre, 56.30% patients having autoimmune diseases and in 62.50% thyroid carcinoma patients. While levels of IFN-α > 4.00 ng/ml (maximum level in healthy individuals) were observed in only 14.30% patients with goitre, 6.30% patients with autoimmune diseases and in 18.80% thyroid carcinoma patients.
Figure 2.
(a) Incidence of IL-8 and IFN-α levels in patients with thyroid diseases as compared to healthy individuals. (b) Incidence of IL-8 and IFN-α levels in thyroid carcinoma patients as compared to healthy individuals. (c) Incidence of IL-8 and IFN-α levels in early- and advanced-stage thyroid carcinoma patients as compared to healthy individuals.
The incidence of thyroid carcinoma patients having higher levels of IL-8 as compared to that of healthy individuals increased with the increase in disease stage. That is, 40% of early-stage thyroid carcinoma patients as compared to 82.40% advanced-stage thyroid carcinoma patients had higher IL-8 levels than the maximum range found in healthy individuals.
Association of the serum IL-8 and IFN-α levels with different clinicopathological parameters have been studied by Mann-Whitney U test. It revealed that elevated serum IL-8 levels were significantly associated with advanced-disease stage (Figure 3). In fact, serum IL-8 levels were significantly increased in patients with advanced stage compared to those with early-stage disease (445.12 ± 214.30 versus 101.56 ± 53.89; P = .016).
Moreover, Spearman rank's correlation analysis revealed significant positive relationships between IL-8 levels and disease stage (r = 0.437, P = .012). IFN-α levels were not significantly associated nor did they exhibit any significant relationship with any of the clinicopathological parameters. Yi Ren et al. demonstrated that high serum IL-8 level correlated with large tumor volume and advanced tumor stage in patients with hepatocellular carcinoma [37]. Increased IL-8 expression has been found in various tumors and in some studies IL-8 serum and/or tissue levels correlate with tumor progression and metastasis [42–45]. So, this finding additionally supports the role of IL-8 in the progression of thyroid carcinoma.
The relative risk study indicated that 14/17 advanced-stage thyroid carcinoma patients and 6/15 early-stage thyroid carcinoma patients had elevated IL-8 levels (>7.00 pg/ml) (Figure 4), which indicates IL-8 as a risk factor in development of advanced-stage thyroid cancer.
4. Conclusion
Finally concluding, as both serum IL-8 and IFN-α levels were significantly higher, not only in thyroid cancer patients, but also in patients having goitre and autoimmune diseases, they can be used for differentiating such patients, having any disease of thyroid gland, from that of healthy individuals. Since such conditions often constitute prevalent pre-existing disease states in the pathogenesis of thyroid cancer, these results strongly suggest an association between IL-8 and IFN-α upregulation and development of thyroid carcinoma.
Moreover, IL-8 could efficiently discriminate between early- and advanced-stage disease. Also, the major findings demonstrate that IL-8 levels, were significantly positively related to the disease stage and the elevated IL-8 levels were predominantly associated with the advanced stage of the disease whereas IFN-α levels did not show any significant correlation or association with any of the histopathological parameters of thyroid carcinoma patients. Thus, IL-8 seems to have a role in thyroid cancer pathogenesis, and measurement of preoperative serum IL-8 levels might be more useful and feasible in the clinical setting to predict tumor stage where it may represent a putative target for innovative diagnostic and therapeutic strategies. However, further studies including more number of patients and evaluating the postoperative changes in serum IL-8 levels may provide additional predictive value on tumor recurrence and prognosis.
Table 3.
Significance of IL-8 levels in patients with thyroid diseases as compared to healthy individuals.
| Subjects | Mean ± S.E (pg/ml) | Median | Minimum | Maximum | P value |
|---|---|---|---|---|---|
| Healthy individuals | 3.15 ± 0.58 | 2.97 | 0.00 | 7.00 | |
| Total patients | 231.67 ± 72.51 | .001 | |||
| Goitre | 187.16 ± 93.88 | 8.41 | 0.00 | 1899.06 | .002 |
| Autoimmune disorders | 185.30 ± 167.84 | 7.92 | 0.00 | 2700.00 | .034 |
| Graves' disease | 20.21 ± 10.93 | .085 | |||
| Hashimoto disorder | 680.57 ± 673.14 | .097 | |||
| Thyroid Carcinoma | 284.08 ± 118.97 | 69.35 | 0.00 | 3200.00 | .001 |
| Papillary carcinoma | 116.34 ± 44.36 | .006 | |||
| Follicular carcinoma | 514.13 ± 295.64 | .030 | |||
| Medullary carcinoma | 828.96 ± 790.79 | .456 | |||
| Anaplastic carcinoma | 27.17 ± 19.50 | .003 | |||
| Early stage (Stage I & II) | 101.56 ± 53.89 | .286 | |||
| Advanced stage (Stage III & IV) | 445.12 ± 214.30 | <.001 |
Table 4.
Significance of IFN-α levels in patients with thyroid diseases as compared to healthy individuals.
| Subjects | Mean ± S.E (IU/ml) | Median | Minimum | Maximum | P value |
|---|---|---|---|---|---|
| Healthy individuals | 2.41 ± 0.18 | 2.26 | 0.50 | 4.00 | |
| Total patients | 3.82 ± 0.50 | .002 | |||
| Goitre | 3.40 ± 0.30 | 3.14 | 2.27 | 9.07 | .001 |
| Autoimmune disorders | 2.97 ± 0.12 | 3.02 | 2.01 | 4.16 | .009 |
| Graves' disease | 2.91 ± 0.15 | .028 | |||
| Hashimoto disorder | 3.14 ± 0.21 | .054 | |||
| Thyroid carcinoma | 4.51 ± 1.06 | 3.21 | 2.14 | 36.67 | <.001 |
| Papillary carcinoma | 5.10 ± 1.87 | .003 | |||
| Follicular carcinoma | 3.48 ± 0.31 | .007 | |||
| Medullary carcinoma | 4.72 ± 1.29 | .006 | |||
| Anaplastic carcinoma | 3.10 ± 0.27 | .087 | |||
| Early stage (Stage I & II) | 5.37 ± 2.24 | .007 | |||
| Advanced stage (Stage III & IV) | 3.76 ± 0.36 | <.001 |
Acknowledgments
This paper was financially supported by the Directorate of Medical Education and Researches (DMER-Gujarat state) and Gujarat Cancer Society (GCS), and it was approved by the GCRI/GCS ethics committee.
References
- 1. http://www.endocrinologist.com/thyroid.htm.
- 2.Blesa JMG, Pulido EG, Pulla MP, et al. Old and new insights in the treatment of thyroid carcinoma. Journal of Thyroid Research. 2010;2010:16 pages. doi: 10.4061/2010/279468. Article ID 279468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66(2):321–330. doi: 10.1002/1097-0142(19900715)66:2<321::aid-cncr2820660221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic thyroid carcinoma: a study of 70 cases. American Journal of Clinical Pathology. 1985;83(2):135–158. doi: 10.1093/ajcp/83.2.135. [DOI] [PubMed] [Google Scholar]
- 5.Ron E. Epidemiology of thyroid cancer. In: Schottenfeld D, Fraumeni JR Jr., editors. Cancer Epidemiology and Prevention. Oxford, UK: Oxford University Press; 1996. pp. 1000–1021. [Google Scholar]
- 6.Franceschi S, Preston-Martin S, Dal Maso L, et al. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes and Control. 1999;10(6):583–595. doi: 10.1023/a:1008907227706. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Seminars in Cancer Biology. 2004;14(6):433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels in patients with advanced gastrointestinal malignancies. Cancer. 1999;86(10):1936–1943. [PubMed] [Google Scholar]
- 12.Shurin MR, Shurin GV, Lokshin A, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer and Metastasis Reviews. 2006;25(3):333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 13.Ajjan RA, Weetman AP. Cytokines in thyroid autoimmunity. Autoimmunity. 2003;36(6-7):351–359. doi: 10.1080/08916930310001603046. [DOI] [PubMed] [Google Scholar]
- 14.Tartour E, Fridman WH. Cytokines and cancer. International Reviews of Immunology. 1998;16(5-6):683–704. doi: 10.3109/08830189809043014. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. Journal of Clinical Investigation. 1989;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Advances in Immunology. 1994;55:97–179. [PubMed] [Google Scholar]
- 17.Xie K. Interleukin-8 and human cancer biology. Cytokine and Growth Factor Reviews. 2001;12(4):375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 18.Iwahashi N, Murakami H, Nimura Y, Takahashi M. Activation of RET tyrosine kinase regulates interleukin-8 production by multiple signaling pathways. Biochemical and Biophysical Research Communications. 2002;294(3):642–649. doi: 10.1016/S0006-291X(02)00528-4. [DOI] [PubMed] [Google Scholar]
- 19.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Research. 1994;54(12):3242–3247. [PubMed] [Google Scholar]
- 20.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. International Journal of Cancer. 1997;72(6):937–941. doi: 10.1002/(sici)1097-0215(19970917)72:6<937::aid-ijc3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Kitadai Y, Haruma K, Sumii K, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. American Journal of Pathology. 1998;152(1):93–100. [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunology Immunotherapy. 1998;47(1):47–57. doi: 10.1007/s002620050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiba J, Yano H, Ogasawara S, Higaki K, Kojiro M. Expression and function of interleukin-8 in human hepatocellular carcinoma. International journal of oncology. 2001;18(2):257–264. doi: 10.3892/ijo.18.2.257. [DOI] [PubMed] [Google Scholar]
- 24.Seo H-J, Park K-K, Han SS, et al. Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. International Journal of Cancer. 2002;100(4):463–471. doi: 10.1002/ijc.10496. [DOI] [PubMed] [Google Scholar]
- 25.Molica S, Vitelli G, Levato D, Levato L, Dattilo A, Gandolfo GM. Clinico-biological implications of increased serum levels of interleukin-8 in B-cell chronic lymphocytic leukemia. Haematologica. 1999;84(3):208–211. [PubMed] [Google Scholar]
- 26.Retzlaff S, Padró T, Koch P, et al. Interleukin 8 and Flt3 ligand as markers of advanced disease in primary gastrointestinal non-Hodgkin’s lymphoma. Oncology reports. 2002;9(3):525–527. [PubMed] [Google Scholar]
- 27.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. Journal of Clinical Oncology. 2001;19(2):577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 28.Bazhanova ED. Participation of interferon-alpha in regulation of apoptosis. Journal of Evolutionary Biochemistry and Physiology. 2005;41(2):127–133. [Google Scholar]
- 29.Giosuè S, Casarini M, Alemanno L, et al. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine. 1998;158(4):1156–1162. doi: 10.1164/ajrccm.158.4.9803065. [DOI] [PubMed] [Google Scholar]
- 30.Linkov F, Ferris RL, Yurkovetsky Z, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics—Clinical Applications. 2008;2(12):1575–1585. doi: 10.1002/prca.200780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossowski A, Urban M. Serum levels of cytokines in children and adolescents with Graves’ disease and non-toxic nodular goiter. Journal of Pediatric Endocrinology and Metabolism. 2001;14(6):741–747. doi: 10.1515/jpem.2001.14.6.741. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqi A, Monson JP, Wood DF, Besser GM, Burrin JM. Serum cytokines in thyrotoxicosis. Journal of Clinical Endocrinology and Metabolism. 1999;84(2):435–439. doi: 10.1210/jcem.84.2.5436. [DOI] [PubMed] [Google Scholar]
- 33.Krassas GE, Bougoulia M, Koliakos G. Serum interleukin-8 levels in thyroid diseases. Thyroid. 2000;10(5):445–446. doi: 10.1089/thy.2000.10.445. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Suh JY, Kim SW, et al. Circulating IL-8 and IL-10 in euthyroid sick syndromes following bone marrow transplantation. Journal of Korean medical science. 2002;17(6):755–760. doi: 10.3346/jkms.2002.17.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonelli A, Rotondi M, Fallahi P, et al. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5496–5499. doi: 10.1210/jc.2004-0977. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli A, Rotondi M, Ferrari SM, et al. Interferon-γ-inducible α-chemokine CXCL10 involvement in Graves’ ophthalmopathy: modulation by peroxisome proliferator-activated receptor-γ agonists. Journal of Clinical Endocrinology and Metabolism. 2006;91(2):614–620. doi: 10.1210/jc.2005-1689. [DOI] [PubMed] [Google Scholar]
- 37.Ren YI, Poon RTP, Tsui HT, et al. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clinical Cancer Research. 2003;9(16):5996–6001. [PubMed] [Google Scholar]
- 38.Seo H-J, Park K-K, Han SS, et al. Cytokine serum levels in soft tissue sarcoma patients: correlations with clinico-pathological features and prognosis. International Journal of Cancer. 2002;100(4):463–471. doi: 10.1002/ijc.10496. [DOI] [PubMed] [Google Scholar]
- 39.Molica S, Vitelli G, Levato D, Levato L, Dattilo A, Gandolfo GM. Clinico-biological implications of increased serum levels of interleukin-8 in B-cell chronic lymphocytic leukemia. Haematologica. 1999;84(3):208–211. [PubMed] [Google Scholar]
- 40.Retzlaff S, Padró T, Koch P, et al. Interleukin 8 and Flt3 ligand as markers of advanced disease in primary gastrointestinal non-Hodgkin’s lymphoma. Oncology Reports. 2002;9(3):525–527. [PubMed] [Google Scholar]
- 41.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. Journal of Clinical Oncology. 2001;19(2):577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 42.Uehara H, Troncoso P, Johnston D, et al. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate. 2005;64(1):40–49. doi: 10.1002/pros.20223. [DOI] [PubMed] [Google Scholar]
- 43.Lee KH, Bae SH, Lee JL, et al. Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology. 2004;66(3):210–217. doi: 10.1159/000077997. [DOI] [PubMed] [Google Scholar]
- 44.Chung YC, Chang YF. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003;50(54):1910–1913. [PubMed] [Google Scholar]
- 45.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Frontiers in Bioscience. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]