Abstract
This article describes FLASH (Fast Lithographic Activation of Sheets), a rapid method for laboratory prototyping of microfluidic devices in paper. Paper-based microfluidic devices are emerging as a new technology for applications in diagnostics for the developing world, where low cost and simplicity are essential. FLASH is based on photolithography, but requires only a UV lamp and a hotplate; no clean-room or special facilities are required (FLASH patterning can even be performed in sunlight if a UV lamp and hotplate are unavailable). The method provides channels in paper with dimensions as small as 200 μm in width and 70 μm in height; the height is defined by the thickness of the paper. Photomasks for patterning paper-based microfluidic devices can be printed using an ink-jet printer or photocopier, or drawn by hand using a waterproof black pen. FLASH provides a straightforward method for prototyping paper-based microfluidic devices in regions where the technological support for conventional photolithography is not available.
Introduction
This article describes a rapid, simple, and inexpensive method—which we term FLASH (Fast Lithographic Activation of Sheets)—for laboratory prototyping of microfluidic, paper-based analytical devices (μPADs). The method produces μPADs in <30 min (from design to completion) using an ink-jet printer, a source of UV light (a UV lamp or sunlight), and a hot plate (when the UV lamp is used). The method is compatible with small pieces of paper (i.e., 0.5 in2) as well as large (8.5 in × 11 in), and produces hydrophilic features in paper with dimensions as small as 200 μm that are demarcated by well-defined hydrophobic barriers of photoresist (as small as 200 μm); the hydrophobic barriers extend through the thickness of paper (in the z-direction), and produce microfluidic channels that are capable of distributing fluids by capillary action.
Paper microfluidic devices are a promising technology for applications in which low cost and simplicity of fabrication must be combined with complex microfluidic function.1,2 These applications include diagnostic devices for the developing world, devices for use in drug development, and tools for monitoring the quality of water and the environment.3-5 These devices—μPADS—combine many of the useful characteristics of microfluidic devices made out of poly(dimethyl siloxane) and glass (e.g., they use microliter volumes of fluids and measure multiple assays simultaneously)6 with the capabilities of dipsticks and lateral-flow assays (e.g., they wick fluids by capillary action, adsorb reagents, filter samples, and are easy to dispose of by incineration).5,7 Paper-based microfluidic devices are thus a new class of microfluidic systems that generate high-technology function from low-technology materials.
This paper describes a method for prototyping these paper-based devices rapidly in a way that is accessible to a wide variety of laboratories for minimal cost. FLASH patterning involves: (i) impregnating paper with photoresist (we use a home-made version of SU-8 photoresist that costs ~$0.06 g−1); (ii) drying the paper to remove propylene glycol monomethyl ether acetate (PGMEA) from the paper (PGMEA is the solvent in the photoresist formulation); (iii) covering one face of the photoresist-impregnated paper with an adhesive transparency film and the other face with black construction paper; (iv) printing a pattern onto the transparency film using an ink jet printer, a photocopying machine, or a pen; (v) exposing the paper to UV light using a UV lamp (here, an Intelliray 600 lamp) or sunlight; (vi) removing the transparency and black paper backing; (vii) baking the paper to polymerize the photoresist (no baking step is required if sunlight is used for the exposure step); and (viii) removing unpolymerized resist (the sections not exposed to UV light) by washing the paper with acetone and isopropyl rubbing alcohol (70% propan-2-ol, 30% water), both of which are available in hardware stores.
Steps 1–3 of this process can be performed independently of steps 4–7, and provide a material we term FLASH paper (transparency-covered, photoresist-impregnated paper). FLASH paper can be prepared in bulk quantities and stored in the absence of light for >6 months before use, and, we presume, could be shipped to other locations for patterning devices. The photoresist in FLASH paper is dry and there are no odors. We demonstrate FLASH using three types of paper: Whatman Chromatography paper No. 1, ITW Technicloth, and Scott hard roll paper towel, but other types of paper can be used as well.
Experimental design
Strategy for patterning hydrophilic and hydrophobic channels in paper
Microfluidic channels in paper require that the patterned hydrophobic polymer extend through the entire thickness of the paper (otherwise the aqueous fluid escapes the channel and spreads through the device). The requirement that the channel-forming polymer extend through the full thickness of the paper is a constraint that limits the methods available for patterning paper on a laboratory scale: most printing methods using standard inks, for example, are not suitable for making channels in paper because inks are designed to remain on the surface of paper, not to absorb into it. Another limitation is the structure of paper itself: paper is composed of intertwined fibers that are oriented in the x,y-plane of a sheet of paper, and that are stacked on top of one another in the z-direction.8 The result of this arrangement is that spreading of liquids is faster in the x-, y-plane than in the z-direction; this anisotropy in rates of spreading leads to blurring of the patterns. Appropriate choices of monomers, polymers, and solvents are important to overcome these characteristics of paper, and to enable the patterning of distinct features that pass through the entire thickness of paper.
We use photolithography to pattern paper. The problems with creating well-defined patterns in paper can be overcome by impregnating an entire sheet of paper with photoresist. The photoresist is selectively polymerized in the paper by exposure to UV light through a transparency mask (black ink printed on the transparency film). The unexposed photoresist is removed by washing, and the remaining polymerized photopolymer extends through the thickness of the paper.
Choice of photoresist
Paper-based microfluidic devices (for the applications we are now developing) contain hydrophilic channels with feature sizes >100 μm, and more commonly, feature sizes ranging from 250–2000 μm (these dimensions allow visual readout of colorimetric assays carried out on the paper).1,2 Commercial photoresists are designed to produce features on silicon wafers with sizes <1 μm and with well-defined edges and thicknesses.9,10 In paper, the thickness of the features is defined by the thickness of the paper, and expensive commercial photoresists are unnecessary for this application. Instead, we formulated an epoxy-based negative photoresist from commercially available reagents for ~$65 kg−1 (approximately 6 g or $0.4 of photoresist are required to pattern a 20 cm × 20 cm sheet of Whatman Chromatography paper No. 1). The photoresist is composed of: EPON SU-8 resin (52% by mass), triarylsulfonium hexafluorophosphate salts (photoacid) (5% by mass), and PGMEA (43% by mass).9,10
Source of UV light
Any source of UV light could be used to polymerize photoresist in paper so long as the photon flux is high enough to penetrate through the thickness of the paper. We chose a 600 W metal halide lamp (UVitron Intelliray 600) for this purpose. The lamp delivers high intensity (~100 mW cm−2), long wave (365 nm) ultraviolet light that requires exposure times of only 8–14 s in the applications shown here. Alternatively, sunlight can be used to pattern paper, but with less control of intensity day-to-day than a fixed-intensity UV lamp.
Experimental details
Preparation of FLASH paper
We poured photoresist onto a piece of paper and spread the photoresist evenly around the paper using a wooden rolling pin. The photoresist-impregnated paper was baked on a hotplate set at 130 °C for 5–10 min (the baking time depends on the type of paper) to evaporate the PGMEA from the photoresist.
The paper was allowed to cool to room temperature. We covered it with an adhesive transparency film (Computer Grafix clear adhesive backed ink-jet film; one side of this plastic film is sticky), and placed this label-coated paper on an 8.5 in × 11 in piece of black construction paper; the black paper serves as an optical filter to minimize reflected UV light when the paper is exposed (Fig. 1A). The three components (transparency film/photoresist-impregnated paper/black construction paper) were held together by sealing the adhesive border of the transparency to the construction paper (the photoresist-impregnated paper is ~0.5 cm smaller on all sides than the transparency and construction paper).
Fig. 1.
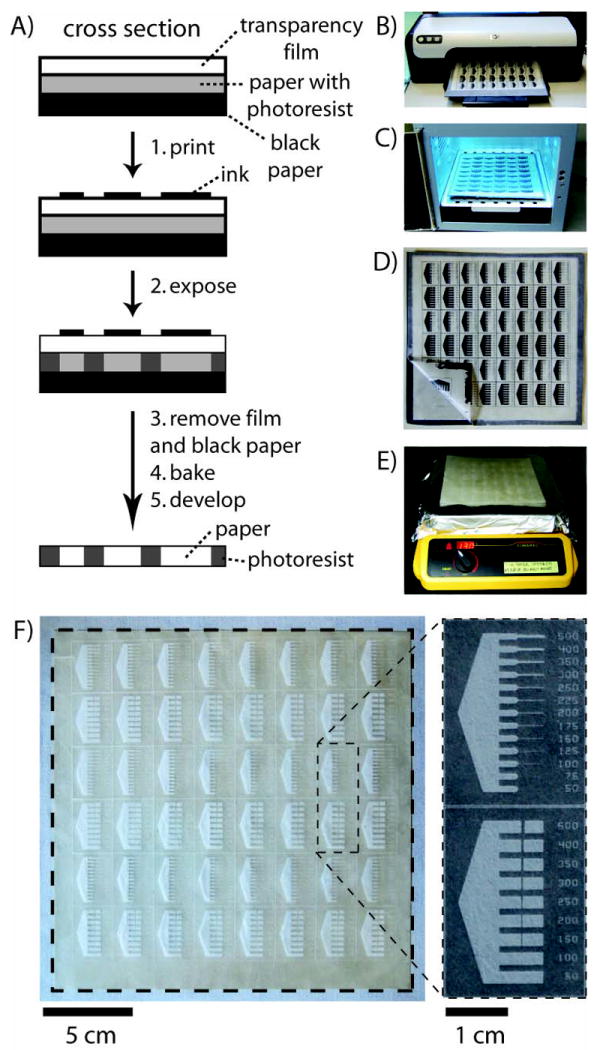
Procedure for FLASH fabrication of microfluidic devices in paper. (A) Schematic of the method. (B) Designs for microfluidic channels were printed directly onto FLASH paper (the paper is Whatman Chromatography paper No. 1). (C) FLASH paper was exposed to UV light. (D) The photoresist-impregnated paper was removed from the transparency film and black construction paper. (E) The paper was baked on a hotplate. (F) After developing the paper in acetone and 70% isopropyl alcohol, the microfluidic devices are ready for use. The dotted lines indicate the edges of the paper.
Patterning hydrophilic and hydrophobic channels in paper
Black designs can be printed onto FLASH paper using an inkjet printer (Fig. 1B). After exposing the paper to UV light for 10 s using a 600 W metal halide lamp (UVitron Intelliray 600 set to 100% intensity), we peeled the transparency and construction paper from the photoresist-impregnated paper, and heated the paper on a hotplate at 130 °C for 5 min (Fig. 1C–E). Unpolymerized photoresist is removed from the paper by soaking the paper in acetone for 1 min, and by rinsing the paper with acetone (1×) and 70% isopropyl rubbing alcohol (2×) using a squirt bottle. The devices were ready for use once dry; we let the devices dry under ambient conditions (Fig. 1F).
For patterning FLASH paper using sunlight instead of a UV lamp, we placed FLASH paper in direct sunlight on a flat surface for 6 min (Cambridge, MA at 12 pm, June 25, 2008). The paper was washed after 6 min to remove unpolymerized photoresist; no baking step was required (the temperature outside was ~27 °C).
Results and discussion
Resolution of the hydrophobic and hydrophilic patterns
We patterned a series of hydrophobic lines and hydrophilic channels of increasing width into Whatman Chromatography paper No. 1, ITW Technicloth, and Scott hard roll paper towel to determine the minimum-sized features that give functional barriers and channels for controlling the position of fluids in paper (Table 1, Fig. 2).
Table 1.
Summary of the experimental details and results for FLASH patterning Whatman Chromatography paper No. 1, ITW Technicloth and Scott hard roll paper towel. All the dimensions are given as the average ± one standard deviation of ten measurements.
| Paper | Costa/$/m−2 | Thickness/μm | Photo-resist/g m−2 | Pre-bake/min | Exposure/s | Post-bake/min | Channel/μm | Barrier/μm |
|---|---|---|---|---|---|---|---|---|
| Chromatography | 6.7 | 175±5 | 155 | 10 | 10 | 5 | 256±20 | 210±30 |
| 360b | 0b | 248±14b | 186±13b | |||||
| Technicloth | 1.2 | 245±10 | 265 | 10 | 14 | 5 | 184±12 | 370±18 |
| Paper towel | 0.15 | 70±16 | 120 | 5 | 8 | 5 | 214±20 | 242±14 |
These values are commercial prices for each type of paper, not bulk costs.
FLASH paper exposed to sunlight.
Fig. 2.
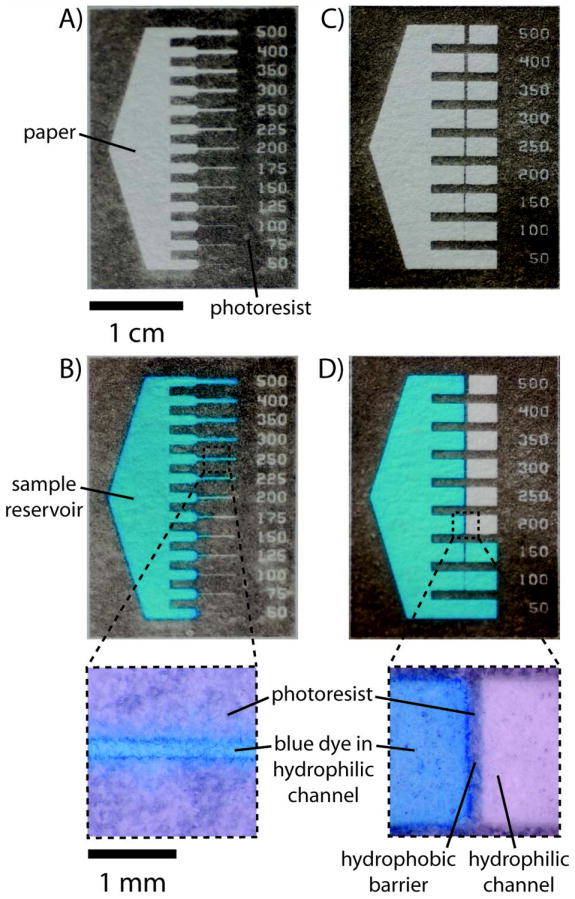
(A) A μPAD made from Whatman Chromatography paper No. 1 designed to measure the smallest functional hydrophilic channel. A large sample reservoir leads into a series of channels of decreasing widths (from 500 μm to 50 μm). (B) The device shown in (A) after adding 40 μL of 1 mM Erioglaucine in water to the sample reservoir. The aqueous dye filled the channels as small as 250 μm in width for chromatography paper. (C) A μPAD designed to measure the smallest functional hydrophobic barrier. A large sample reservoir leads into a series of channels that contain hydrophobic barriers of decreasing widths (from 500 μm to 50 μm). (D) The device shown in (C) after adding 40 μL of 1 mM Erioglaucine. The aqueous dye crossed the barriers that were less than 200 μm in width.
We quantified the dimensions of the features by imaging the patterns using a digital camera (Nikon DXM1200) attached to a stereomicroscope (Leica MZ12), and analyzing the images using Adobe®Photoshop®. The smallest functional channel (defined as a channel that could wick a 1 mM aqueous solution of Erioglaucine) was designed to be 200 μm in width and was measured to be 184 ± 12 μm (based on 10 measurements) (Table 1). Smaller channels did not wick the dye. The smallest functional barrier (defined as a barrier that prevented aqueous solutions from crossing it for at least 10 min) was designed to be 200 μm in width and was measured to be 186 ± 13 μm (based on 10 measurements) (Table 1).
FLASH patterning using a photocopying machine
FLASH paper (prepared using adhesive transparency film designed for photocopying machines instead of inkjet printers) can be loaded directly into a photocopying machine, and a design (black features on white paper) can be photocopied onto it. This method works well for features ~0.5–1 mm wide (Fig. 3). For smaller or larger features, the toner from the photocopying machine does not apply evenly or thick enough on the FLASH paper to block transmission of UV light. It is possible, however, to print patterns on FLASH paper at the larger (0.5–1 mm) dimensions with throughput of ~1 sheet per second. This method of patterning is ideal for situations where large numbers of devices of the same design are required.
Fig. 3.
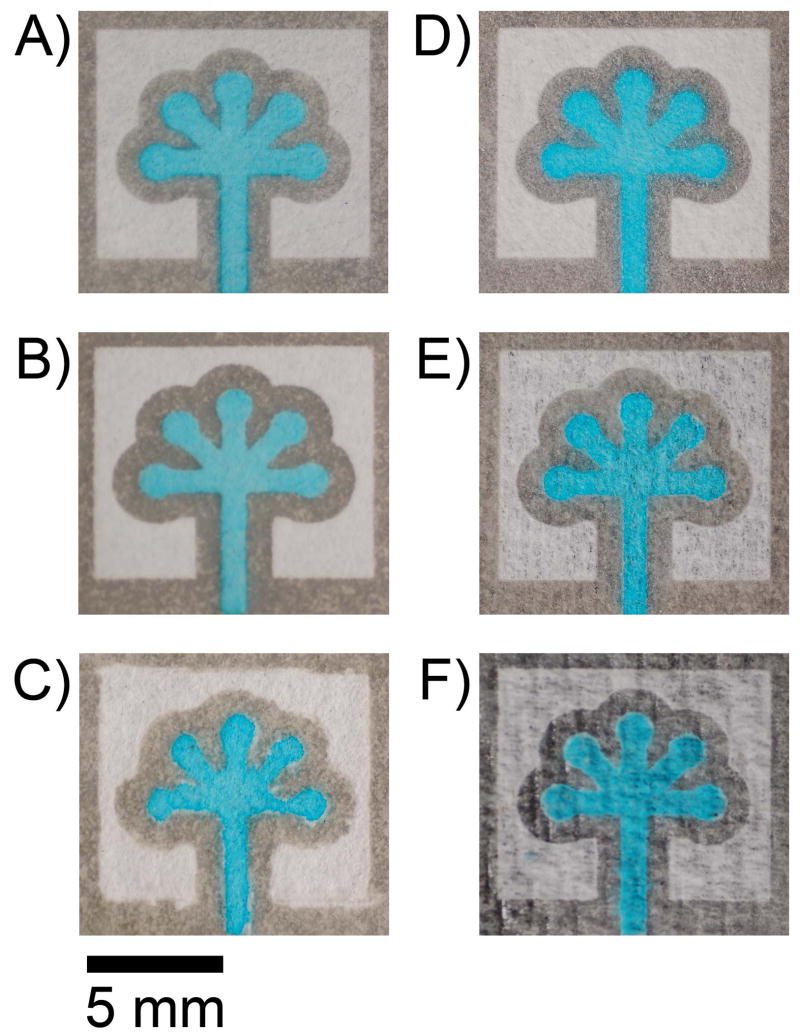
Micro-PADs produced using chromatography paper by (A) printing the pattern with an ink-jet printer, (B) printing the pattern with a photocopy machine, and (C) drawing the pattern through a stencil using a waterproof black pen. A μPAD patterned using sunlight (D). A μPAD from Technicloth (E), or a paper towel (F). The devices were filled with an aqueous blue dye (1 mM Erioglaucine).
FLASH patterning using a pen
A third option for fabricating μPADs is to draw patterns onto FLASH paper using a black pen or marker (we used a Sarstedt black permanent waterproof pen) (Fig. 3C). The patterns made using this method are not as well defined as those made using a printer, and the size of the pattern depends on the pen and the skill of the technician. This method of patterning is, nonetheless, useful for quick, proof-of-concept experiments in situations where inkjet printers or photocopying machines are unavailable.
One disadvantage of this method is the difficulty in making multiple identical devices by hand. This limitation can be circumvented by using stencils (we generate ours using a VERSA laser cutter and a transparency sheet). Alternatively, if a photocopying machine is available, a hand-drawn pattern can be photocopied directly onto FLASH paper.
Conclusion
This Technical Note describes a lithographic method for prototyping microfluidic devices in paper. The method is rapid, inexpensive, requires no specialized equipment, and can be performed in any laboratory; clean rooms commonly associated with photolithography are not necessary.
We anticipate that microfluidic devices fabricated in paper will be useful in innovative developing countries (IDCs;11 for monitoring the health of large populations that do not have access to centralized medical facilities), and in industrialized countries (for monitoring chronic diseases, for military applications, and for applications in environmental monitoring, agriculture, veterinary medicine, and homeland security).
This method of patterning is a convenient way of producing μPADs, especially when the FLASH paper is prepared in advance. The method requires <30 min to pattern an 8.5 in × 11 in piece of FLASH paper (~10 min to print the pattern using an inkjet printer, ~6 min to expose and bake the paper, and ~10 min to develop and dry the paper). When using a photocopying machine to print the patterns, <20 min is the time required to pattern a single piece of FLASH paper, but additional FLASH paper can be processed in minutes; the photocopying machine allows for much higher throughput than an inkjet printer. The materials used to prepare an 8.5 in × 11 in sheet of FLASH chromatography paper cost less than $1.6 per sheet: ~$0.40 of photoresist, ~$0.27 of paper, ~$0.84 of transparency film, and ~$0.04 of black paper. The cost of the materials per device is ~1–3 cents depending on the size of the device.
The methods we describe for fabricating these devices are straightforward, and should enable others to prototype similar devices that are suitable for local conditions and needs. The goal of this article is to describe a practical method for prototyping μPADs, and to make available a method that is capable of shifting the development stages for diagnostic devices from developed countries to those regions where they are needed most. A prototyping method that is widely available should lead to unexpected uses of μPADs, and should motivate the development of new photoresists that provide additional and exciting function to paper-based microfluidic devices. Extensions of FLASH patterning should be compatible with reel-to-reel processes for manufacturing in bulk.
Supplementary Material
Acknowledgments
This work was supported by the NIH EHS (R01 ES016665) and by the N/MEMS S & T Fundamentals MF3 Center (DARPA). We thank NSF (A.W.M.) and NIH (S.T.P.) for additional support. We also thank Robert Barr (Rohm and Haas Corporation) for his help in formulating the photoresist.
Footnotes
Electronic supplementary information (ESI) available: Further experimental details.
References
- 1.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Angew Chem, Int Ed. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Anal Chem. 2008;80:3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daar AS, Thorsteinsdottir H, Martin DK, Smith AC, Nast S, Singer PA. Nat Genet. 2002;32:229–232. doi: 10.1038/ng1002-229. [DOI] [PubMed] [Google Scholar]
- 4.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 5.Chin CD, Linder V, Sia SK. Lab Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 6.Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 7.von Lode P. Clin Biochem. 2005;38:591–606. doi: 10.1016/j.clinbiochem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Stewart GH. In: Advances in Chromatography. Giddings JC, Keller RA, editors. Vol. 1. Marcel Dekker; New York: 1965. pp. 93–111. [Google Scholar]
- 9.Shaw JM, Gelorme JD, LaBianca NC, Conley WE, Holmes SJ. IBM J Res Dev. 1997;41:81–94. [Google Scholar]
- 10.Hurditch RJ, Nawrocki DJ, Johnson DW. 6 391 523 B1. Mirco Chem Corp, US Pat. 2002
- 11.Morel CM, Acharya T, Broun D, Dangi A, Elias C, Ganguly NK, Gardner CA, Gupta RK, Haycock J, Heher AD, Hotez PJ, Kettler HE, Keusch GT, Krattiger AF, Kreutz FT, Lall S, Lee K, Mahoney R, Martinez-Palomo A, Mashelkar RA, Matlin SA, Mzimba M, Oehler J, Ridley RG, Senanayake P, Singer P, Yun M. Science. 2005;309:401–404. doi: 10.1126/science.1115538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.