Abstract
Collecting duct (CD) renin is stimulated by angiotensin (Ang) II providing a pathway for Ang I generation and further conversion to Ang II. Ang II stimulates epithelial sodium channel (ENaC) via Ang II type 1 receptor (AT1R) and increases mineralocorticoid receptor (MR) activity due to increased aldosterone release. Our objective was to determine if CD renin augmentation is mediated directly by AT1R or via ENaC and MR. In vivo studies examined the effects of ENaC blockade (amiloride; 5 mg/kg/day) on CD renin expression and urinary renin content (URC) in Ang II-infused rats (80 ng/min, 2 weeks). Ang II infusion increased systolic blood pressure (SBP), medullary renin mRNA, URC and intrarenal Ang II levels. Amiloride co-treatment did not alter these responses despite reduction in the rate of progression of SBP. In primary cultures of inner medullary CD (IMCD) cells, renin mRNA and (pro)renin protein levels increased with Ang II (100 nmol/L), and candesartan (AT1R antagonist) prevented this effect. Aldosterone (10−10 to 10−7 mol/L) with or without amiloride did not modify the upregulation of renin mRNA in Ang II treated cells. However, inhibition of protein kinase C (PKC) with calphostin C prevented the Ang II-mediated increases in renin mRNA and (pro)renin protein levels. Furthermore, PKC activation with phorbol 12-myristate 13-acetate (PMA) increased renin expression to the same extent as Ang II. These data indicate that AT1R-mediated increase in CD renin is induced directly by Ang II via PKC pathway and that this regulation is independent of MR activation or ENaC activity.
Keywords: Angiotensin II-dependent hypertension, collecting duct renin, renin gene expression, protein kinase C, cell signaling pathway
Introduction
Regulation of renin expression in kidney juxtaglomerular (JG) apparatus has been widely studied; however, the mechanisms regulating expression of renin in collecting ducts (CD) and its augmentation in animal models of hypertension remain unclear.1, 2 Increased renin synthesis is mediated by the angiotensin II (Ang II) type 1 receptor (AT1R) independent of changes in blood pressure.3, 4 In vitro studies also suggest that CD renin synthesis and secretion can be directly increased by Ang II;5 however the mechanisms involved are poorly understood.
Ang II is present at high levels in the kidneys of hypertensive animals,6 and an important fraction of this Ang II is generated by de novo synthesis7 as a consequence of augmented angiotensinogen (AGT) expression in proximal tubule cells, leading to augmented AGT excretion in urine.8, 9 Along with the expression of angiotensin converting enzyme (ACE) in the distal nephron,10 increases in CD renin expression and activity may have a key role in the intrarenal Ang II content, contributing to additional Ang II generation in distal nephron segments.
AT1R activation suppresses renin synthesis in JG cells via protein kinase C (PKC) and Ca+2 ;11, 12 however, in CD cells the signaling pathways responsible for Ang II-mediated upregulation of CD renin are unclear. In vivo and in vitro evidence suggest that the possible mechanisms could be different from the mechanisms described in JG cells. AT1R are expressed in CD principal cells and activate the epithelial sodium channel (ENaC), stimulating sodium reabsorption.13 In Ang II-dependent hypertension, high Ang II plasma levels lead to increased levels of plasma aldosterone and activation of minealocorticoid receptor (MR) with corresponding increase in Na+ reabsorption through ENaC in principal CD cells.14–17 Thus, increased CD renin in the Ang II infusion model could be due to enhanced MR activity or ENaC stimulation.
To address these possibilities, we compared CD renin mRNA levels primarily in renal inner medullary tissues and the urinary renin content (URC) in Ang II-infused rats with or without co-treatment with the ENaC blocker, amiloride. To further evaluate the effects of Ang II, aldosterone and ENaC blockade on renin mRNA and protein levels, we used rat primary cultures of inner medullary collecting duct (IMCD) cells. Because PKC mediates some of the effects of AT1R activation,11 we further evaluated the effects of PKC inhibition in Ang II treated IMCD cells and the effect of direct activation of PKC on renin expression.
Materials and Methods
Experimental animals and sample collections
All experimental protocols were in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (150 to 175 g) were cage-housed and maintained in a temperature-controlled room with 12-hours light/dark cycle, with free access to tap water and standard rat chow. Ang II was infused at a rate of 80 ng/min via osmotic minipump for 14-days as previously described;2, 3 amiloride (Sigma Chemical Co, St. Louis, MO) was administered daily by oral gavage at a dose of 5 mg/kg/day 17, 16 according to the described half-life of this compound.18 Sham-operated rats were used as controls. Following a training period of one week before treatments, the systolic blood pressures (SBP) were monitored by tail-cuff plethysmography (Visitech, BP-2000, Apex, NC) on days 1, 3, 5, 7, 9 and 12. At the end of the study, rats were placed in metabolic cages for a 24-h urine collection to determine renin content, sodium concentration and creatinine levels. Rats were sacrificed by conscious decapitation and trunk blood samples were collected for determination of serum creatinine, plasma renin activity (PRA) and plasma Ang II concentration as previously described.7
Urinary renin content (URC)
Active renin content in the urine was measured in the absence of trypsin using a modified protocol of PRA (GammaCoat PRA125I RIA kit; Diasorin Inc; St Louis, MO) according to instructions of the manufacturer. Briefly, 500 ml of urine from 24 h collection were spiked with 1 nmol synthetic renin tetradecapeptide for 30 min in a 37° C water bath to measure the amount of Ang I generation by RIA. To exclude the effect of peptidases, replicates were spiked with the specific renin inhibitor WFML peptide (AnaSpec, Fremont, CA) as a control. Values are expressed as International Enzyme Units/day, and each enzyme unit is defined as micromoles of substrate converted into product per hour.
Quantitative real-time RT-PCR
Total RNA was isolated from micro-dissected inner medullary rat tissues. 20 ng of total RNA was used to amplify renin gene (Ren1c) by qRT-PCR using primers and probes sequences previously described.4 Primers for α-ENaC amplification were 5′-ACTCCCTTGGGCTTAGGGTA-3′ (sense); 5′-TGTGCATTCACTCCTGCTTC-3′ (antisense) and 5′-6-FAM- TGATCAAGAAGTGTGGCTGTGCC-BHQ1-3′ (fluorogenic probe). Data were normalized against β-actin mRNA levels using the following primers: 5′-ATCATGAAGTGTGACGTTGA-3′ (sense) 5′-GATCTTCATGGTGCTAGGAGC-3′ (antisense) and 5′-6-HEX-TCTATGCCAACACAGTGCTGTCTGGT-BHQ2-3′ (fluorogenic probe).
Western blot analysis
A rabbit polyclonal anti-renin IgG (sc-H-105; Santa Cruz Biotechnology, Inc; Santa Cruz, CA) mapping to an internal region of (pro)renin/renin (amino acids 116-220) of human origin was used at a 1:200 dilution followed by a Donkey anti-rabbit IgG IRDye® 800 CW (Li-cor Biosciences, Lincoln, NE) at a 1:30,000 dilution. Densitometric analyses were done by normalization against β-actin (Santa Cruz Biotechnology; Santa Cruz, CA). Recombinant human renin and (pro)renin (Lee BioSolutions, St Louis, MO) were used as positive standards.
Primary cultures of rat inner medullary collecting duct (IMCD) cells
To assess CD (pro)renin/renin expression, IMCD cell cultures from 6–8 independent experiments were prepared as described previously19 using inner medullary tissues to avoid contribution from JG renin component. After kidney excision, inner medullary tissues were digested in 10 ml of DMEM-Ham’s F-12, 20 mg of collagenase B, 7 mg of hyaluronidase, 80 mM urea, and 130 mM NaCl, and incubated at 37°C under continuous agitation for 90 min. After centrifugation, the pellet was washed in pre-warmed culture media without enzymes [DMEM-Ham’s F-12, 80 mM urea, 130 mM NaCl, 10 mM HEPES, 2 mM L-glutamine, penicillin-streptomycin (10,000 U/ml), 50 nM hydrocortisone, 5 pM 3,3,5-triiodothyronine, 1 nM sodium selenate, 5 mg/l transferrin, without FBS (pH 7.4, 640 mosmol/kgH2O)]. The resulting IMCD cell suspension was seeded in 3 mm petri-dishes and incubated with treatments described in the results section. Ang II, aldosterone, candesartan, amiloride, calphostin C, and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma (Sigma Chemical Co, St. Louis, MO).
Immunofluorescence
For immunofluorescence experiments, 50–60 % subconfluent IMCD cells cultured in chamber slides (Nalge Nunc, Rochester, NY) were fixed in cold methanol for 20 minutes, blocked with phosphate buffered saline (PBS)-tween (0.1 %) plus bovine serum albumin (3%) for 1 hour, stained with rabbit anti-aquaporin-2 (AQP2; Calbiochem, San Diego, CA) or anti-renin (sc H-105, Santa Cruz Biotechnology Inc, Santa Cruz, CA) at 1:200 dilutions and detected with Alexa Fluor 488-conjugated to anti-rabbit IgG (Invitrogen Life Science, Co; Carlsbad, CA). Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen). Negative controls were obtained by omission of the specific primary antibody.
Calcium measurements in primary cultures of rat IMCD cells
Changes in free intracellular Ca+2 were measured in subconfluent IMCD cells plated on glass coverslips incubated with the membrane-permeant acetoxymethyl (AM) ester form of Fura-2 (2 μM; Molecular probes) following the protocol described previously.20 Ratio of 340:380 nm was recorded at a rate of 1/s and analyzed with the Photon Technology International software at basal levels and after incubation with Ang II (10−8 and 10−7 mol/L).
Statistical Analyses
Results are expressed as mean ± SEM. Data were evaluated by Grubb’s test followed when appropriate by paired and unpaired Student’s t test or by one-way ANOVA with Tukey post-test. For mRNA and protein data, control levels were defined as 100 %. Significance was defined as P<0.05.
RESULTS
In vivo studies
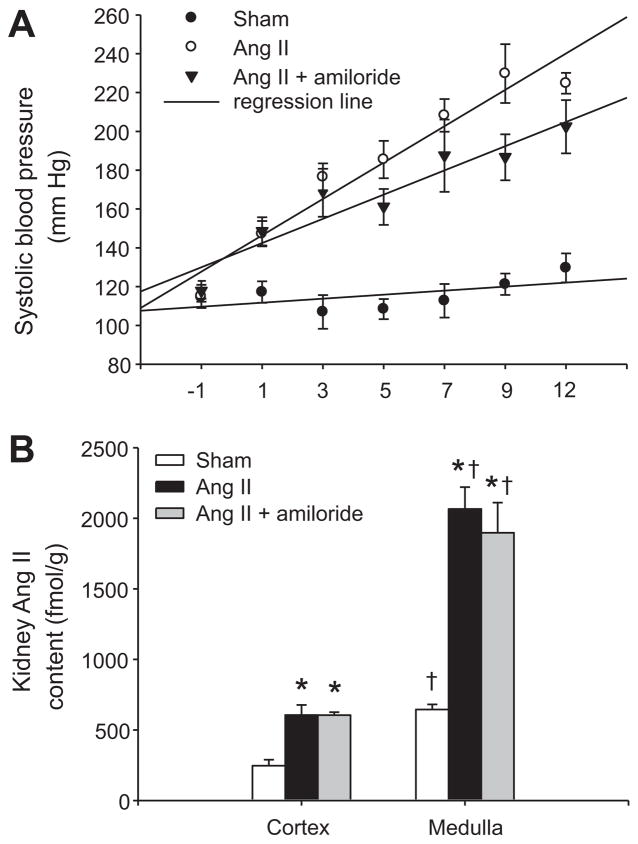
As shown in Table 1, Ang II treated rats with or without amiloride had reduced body weight (P<0.001), higher 24 hr-urine flow (P<0.001 in Ang II + amiloride and P<0.05 in Ang II infused rats), and higher plasma Ang II levels (P<0.05 in Ang II + amiloride and P<0.001 in Ang II infused rats) as compared to sham-operated rats. Ang II-infused rats treated with amiloride showed higher sodium excretion rates compared to sham-operated rats. No changes in plasma sodium, creatinine clearance and fractional sodium excretion were detected among the groups. Amiloride treatment slightly but significantly reduced the rate of SBP progression with Ang II infusion (R2: 0.65 ± 0.06 for Ang II infused rats plus amiloride vs. 0.83 ± 0.02 for Ang II infused rats; P<0.05, Figure 1A). As expected, Ang II infusion suppressed PRA, and amiloride treatment did not alter this effect (Table 1). To evaluate if ENaC blockade influences the response to chronic Ang II infusions, we measured Ang II levels in cortex and medulla. Ang II contents were augmented in both groups compared to sham-operated rats in cortex (606 ± 71 and 605 ± 21 vs. 247 ± 43 fmol/g, P<0.05) and medulla (2,066 ± 155 and 1,897 ± 214 vs. 645 ± 36 fmol/g, P<0.05, Figure 1B).
Table 1.
Physiological parameters at day 14.
| Variable | Sham | Ang II | Ang II + amiloride |
|---|---|---|---|
| Body weight (g) | 318 ± 2 | 269 ± 4† | 275 ± 7† |
| Plasma Na+ (mEq/L) | 142 ± 1 | 142 ± 1 | 144 ± 2 |
| Na+ excretion (mEq/24 h) | 2.6 ± 0.1 | 2.8 ± 0.2 | 3.1 ± 0.1* |
| Fractional Na+ excretion (%) | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.2 |
| Creatinine clearance (mL/min/100g body weight) | 0.52 ± 0.12 | 0.59 ± 0.10 | 0.60 ± 0.12 |
| Urinary volume (24 h) | 12 ± 2 | 37 ± 4* | 40 ± 2† |
| Plasma renin activity (ng Ang I/ml/hr) | 9.60 ± 1.21 | 0.19 ± 0.06* | 0.05 ± 0.03* |
| Plasma Ang II (fmol/mL) | 34.6 ± 5.9 | 169.8 ± 52.9† | 98.0 ± 17.8* |
Values are mean ± SEM. No differences were observed between Ang II and Ang II plus amiloride groups for the variables analyzed.
P<0.05,
P<0.001 versus sham operated rats, n=10
Figure 1.
A. Progression of systolic blood pressure in control rats (Sham operated), Ang II-infused and Ang II-infused treated with amiloride. Regression line curve (R2) is shown for each group (n=10). B. Ang II content in kidney cortex and medulla. *P<0.05 versus Sham operated rats, †P<0.05 versus corresponding group in kidney cortex (n=5).
Effect of ENaC blockade on Ang II-mediated stimulation of renin mRNA levels and URC
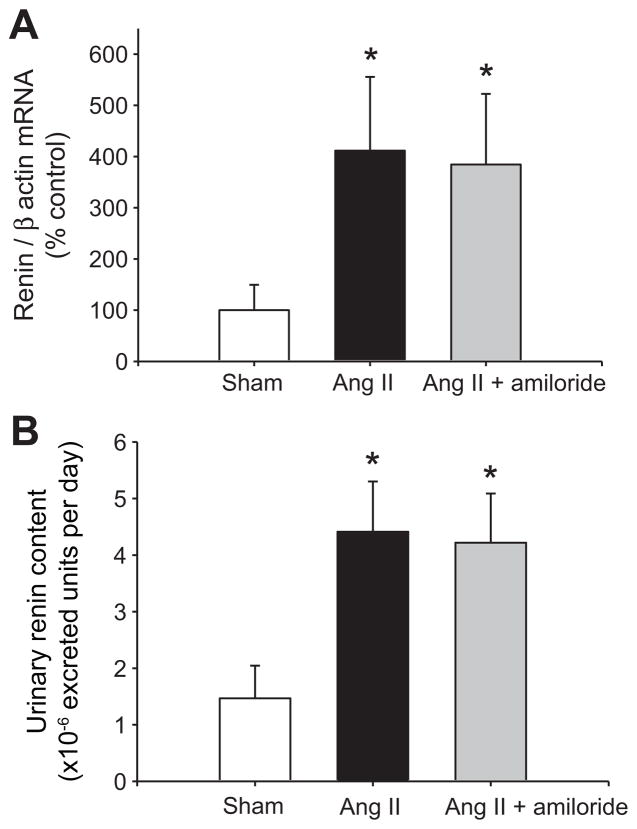
Medullary renin mRNA levels were significantly augmented in Ang II-infused rats compared to sham operated rats (411 ± 144 vs. 100 ± 48 %, P<0.05), and this augmentation was not affected by amiloride treatment (384 ± 136 vs. 100 ± 48 %, P<0.05, Figure 2A). As shown in Figure 2B, URC was augmented both, in Ang II-infused and Ang II-infused rats treated with amiloride compared to sham (4.4 ± 0.9 and 4.2 ± 0.8 vs. 1.5 ± 0.6 × 10−6 enzymatic units per day respectively, P<0.05). Thus, ENaC blockade did not alter the augmentation of CD renin in Ang II-infused rats.
Figure 2.
A. Renin mRNA levels in medullary tissues in each group. B. Urinary renin content was increased in Ang II infused rats and Ang II infused rats treated with amiloride. *P<0.05 versus sham operated rats, n=5.
In vitro studies
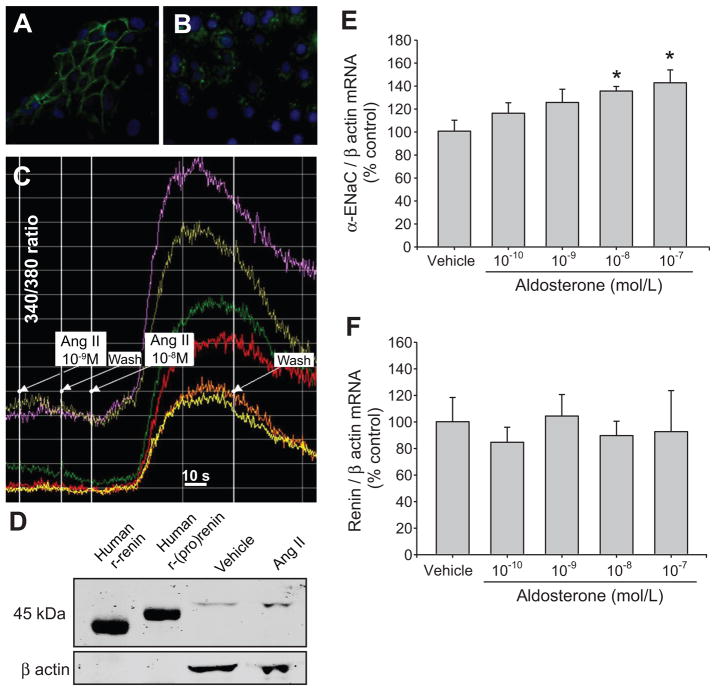
Methanol-fixed IMCD cells from primary cultures showed specific AQP-2 immunolabeling in the plasma membrane of principal cells (Figure 3A). Likewise, these principal cells immunoexpressed renin/(pro)renin1, 2 in a granular pattern (Figure 3B). Functional expression of AT1R in the plasma membrane was evaluated by intracellular Ca+2 measurements in response to Ang II (10−9 and 10−8 mol/L). As shown in Figure 3C, Ang II at 10−8 mol/L increased the ratio 340:380 nm, indicating increases in intracellular Ca+2. By immunoblotting and using recombinant human renin and (pro)renin as positive controls, it was apparent that most of the protein levels detected in IMCD cell extracts was (pro)renin (47 kDa). Post-transcriptional processing such as glycosylation of endogenous (pro)renin in the rat may explain differences in molecular weight compared to recombinant human (pro)renin (45 kDa). Ang II treatment (10−7 mol/L) increased (pro)renin protein levels (Figure 3D and 4C).
Figure 3.
Characterization of inner medullary collecting duct (IMCD) cells. A. Immunofluorescence localization of aquaporin-2 (green) in the plasma membrane. B. Immunofluorescence localization of renin/(pro)renin (green). Nuclei were counterstained with DAPI (blue). C. 340:380 ratio in IMCD cells exposed to Ang II (10−9 and 10−8 mol/L, n=6). D. Effect of Ang II incubation (10−7 mol/L, 8 hours) on protein expression, recombinant human renin (Human r-renin) and (pro)renin (Human r-(pro)renin) demonstrated that (pro)renin is the main source in IMCD cells. Dose response curve for α-ENaC (E) and renin (F) mRNA levels in IMCD cells incubated for 6 hours with aldosterone (10−10 to 10−7 mol/L, n=8). *P<0.05 versus vehicle.
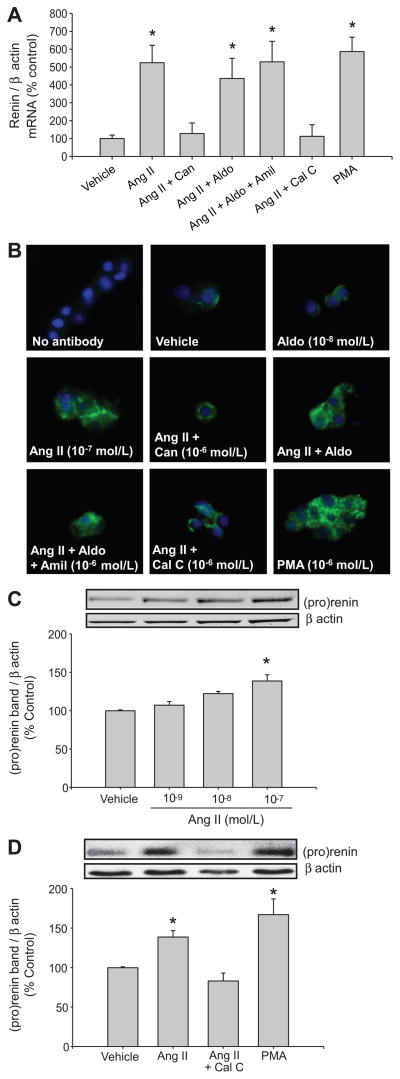
Figure 4.
Renin mRNA and protein levels in IMCD cells. A. Renin mRNA levels were analyzed after 6 hours of incubation (n=8). B. Renin immunofluorescence in subconfluent primary cultures of IMCD cells after 8 hours of treatment. Negative control was performed by ommision of primary antibody. Aldo: aldosterone; Can: candesartan; Amil: amiloride; Cal C: calphostin C; PMA: phorbol 12-myristate 13-acetate. C. Representative Western blot showing Ang II dose response expression of (pro)renin (n=6). D. Representative Western blot showing the effect of PKC inhibition by calphostin C (10−6 mol/L) in IMCD cells incubated with Ang II (10−7 mol/L) and the effect of PMA alone after 8 hours. Densitometric analyses were done from 6 independent experiments according to the ratio between (pro)renin and β actin bands. Control levels were defined as 100 %. *P<0.05.
Effects of MR activation and ENaC blockade on renin mRNA expression in IMCD cells treated with Ang II
The efficacy of aldosterone treatment in IMCD cells was assessed from α-ENaC mRNA expression at different concentrations (10−10 to 10−7 mol/L). Compared to vehicle treated cells, aldosterone treatment (6 hours) significantly increased α-ENaC expression in IMCD cells at concentrations of 10−7 mol/L (143 ± 11 vs. 100 ± 9 %, P<0.05) and 10−8 mol/L (136 ± 4 vs. 100 ± 9 %, P<0.05) (Figure 3E); however, aldosterone (10−10 to 10−7 mol/L) did not stimulate renin mRNA levels (Figure 3F). Nevertheless, Ang II treatment (10−7 mol/L) increased renin mRNA expression compared to vehicle treated cells (524 ± 96 vs. 100 ± 19 %, P<0.05), and this effect was blocked by candesartan (126 ± 36 vs. 100 ± 19 %, P=NS) showing that this effect is mediated by AT1R activation. In Ang II-dependent hypertension, high circulating levels of aldosterone and Ang II stimulate α-ENaC expression and ENaC activity respectively; thus, we evaluated the effects of Ang II plus aldosterone in IMCD cells with or without amiloride. A dose of amiloride 10 times higher than IC 50 (~10−7 mol/L) was used. This concentration also avoids nonspecific inhibition of other transporters, observed in the range of 10−3 mol/L.21 Both groups showed a significant increase in renin mRNA as compared to vehicle treated cells (Ang II plus aldosterone: 436 ± 102 %, P<0.05; Ang II plus aldosterone plus amiloride: 529 ± 115 %, P<0.05), but no differences between both groups or versus the Ang II group were detected. Additionally, no effects were observed with amiloride alone (108 ± 26 vs. 100 ± 19 %, P=NS).
Increased renin mRNA expression is mediated by AT1R via PKC pathway
Because PKC mediates some of the effects of AT1R, we tested the effect of PMA on renin expression in IMCD cells. After 6 hours, renin mRNA levels were increased by PMA (10−6 mol/L) compared to vehicle treated cells (586 ± 80 vs. 100 ± 19 %, P<0.05). We next tested the ability of the PKC inhibitor, calphostin C, to block the stimulatory effect of Ang II. As shown in Figure 4A, calphostin C (10−6 mol/L) blocked the effect of Ang II (10−7 mol/L) on renin mRNA expression (112 ± 65 %, P=NS; Figure 4A). As showed in Figure 4B, immunofluorescence experiments confirmed the results observed by qRT-PCR. Figure 4C shows a (pro)renin protein dose-response curve after 8 hours of Ang II treatment. To confirm the data observed by renin qRT-PCR, we evaluated the changes in the (pro)renin protein levels in IMCD cells according to treatments. A representative Western blot is displayed in the Figure 4D. Percentage versus control (100 ± 2 %) were: Ang II: 137 ± 8 %, P<0.05; Ang II + calphostin C: 83 ± 10 %, P=NS; PMA: 167 ± 20 %, P<0.05.
Discussion
Previous studies have shown that chronic Ang II infusion stimulates CD renin synthesis independently of blood pressure.3, 4 Novel findings from this study demonstrate that augmentation of renin expression is mediated directly by AT1R via a PKC pathway and that this effect is independent of ENaC and MR activation. In vivo data showing that ENaC blockade did not modify the Ang II-mediated augmentation of renin secretion and renin mRNA levels indicate that ENaC is not responsible for the augmented CD renin.
Because AT1R activation directly inhibits renin synthesis in JG cells, our original hypothesis was that CD renin is upregulated through indirect mechanisms. In searching for candidates responsible for upregulation of CD renin in Ang II-dependent hypertension, ENaC emerged as one of the effectors of the renin-angiotensin-system that could be implicated in CD renin regulation. Furthermore, chronic Ang II infusions augments aldosterone secretion,22 which drives activation of MR to promote synthesis of α-ENaC subunit and the functional assembly of the entire channel in the plasma membrane.14,15 Studies showing that chronic Ang II infusion enhances distal nephron sodium reabsorption16 suggest that enhanced CD renin expression may contribute to enhanced sodium reabsorption. Our in vivo results demonstrate that amiloride is able to reduce the rate of progression of hypertension and to enhance natriuresis in Ang II infused rats, suggesting that amiloride effectively reduces sodium reabsorption in the CD. Despite these effects, renin mRNA levels and URC were similarly increased in both groups (Ang II and Ang II plus amiloride), even though PRA was completely suppressed. Moreover, in vitro activation of MR by aldosterone at concentrations that increase α-ENaC mRNA expression did not alter renin mRNA or protein levels. Rohrwasser et al.23 demonstrated in mice that the high-sodium diet plus amiloride treatment increased renin immunoreactivity in connecting tubule cells although there were decreases in JG renin; however, connecting tubule cell renin immunoreactivity in mice did not exhibit significant variation in response to changes in dietary sodium.23, 24 Our results further support that sodium reabsorption by the CD is not a modulator of renin synthesis or excretion in this model.
To evaluate the possible mechanism involved in the AT1R-dependent renin upregulation, we examined the presence of functional AT1R in IMCD cells. Intracellular Ca+2 measurements in response to Ang II incubations demonstrated increases in intracellular Ca+2 levels, showing that AT1R are functionally expressed in the plasma membrane of IMCD cells. Immunofluorescence studies in vitro also demonstrated the presence of renin/(pro)renin in principal cells of the CD as previously shown.2 Furthermore, AT1R blockade in primary cultures of IMCD cells demonstrated a direct AT1R-dependent mechanism. In view of the evidence that PKC and Ca+2 inhibit JG renin expression,11, 12 in vivo experiments were carried out using medullary tissues to avoid any possible contribution of JG renin. The present study using rat IMCD cells composed mainly of principal CD cells (Figure 3A) suggests a different mechanism for CD renin regulation. In JG cells, Ang II suppresses renin secretion and basal levels of renin mRNA, and these effects can be blunted by PKC inhibition.11 Using a mouse renin 1c promoter luciferase gene constructs expressed in As4.1 cells (JG mouse cell line), Klar et al.12 showed two important regulatory sequences for Ca+2-mediated inhibition of renin gene transcription in the promoter region which were independent of PKC.12 However, in the present study using cultured IMCD cells expressing functional AT1R, PKC activation with PMA increased renin mRNA and (pro)renin protein levels, suggesting that the downstream pathway in the control of CD renin expression is mediated by PKC. Calphostin C inhibits the classical (α), the novels (δ,ε,η) but not the atypical (ζ) PKC isoforms25 described in the rat IMCD cells.26 Treatment with PMA specifically activates classical and novel PKCs,26 suggesting that in this pathway the atypical PKC isoform is not involved.25 The combination of in vivo and in vitro data also demonstrates that AT1R is the main regulator of CD renin/(pro)renin synthesis and secretion, independent of MR activation or ENaC activity.
Perspectives
While the mechanisms for renin regulation in JG cells are well described, the present study provides evidence for a different mechanism in the regulation of renin synthesis in the CD. Our study shows that AT1R activation in CD cells leads to increases in renin mRNA and renin excretion, in vivo, independent of ENaC activity and MR activation. Activation of AT1R increases intracellular Ca+2 levels in IMCD cells suggesting that Ca+2 is not a negative modulator of renin synthesis in CD cells. Furthermore, in vitro studies showed that activation of PKC increases CD renin mRNA and (pro)renin protein levels, while PKC inhibition completely blocked the Ang II-mediated stimulation of CD renin synthesis. Collectively the results provide further evidence for an important role of augmented CD renin to contribute to increased intrarenal Ang II generation in Ang II-dependent hypertension.
Acknowledgments
Sources of Funding
National Institutes of Health (NIH) through the Institutional Developmental Award Program of the National Center for Research Resources (P20RR-017659), HL26371; American Heart Association (AHA; 09BGIA2280440); Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12HD043451). A. A. G. is a recipient of CONICYT postdoctoral fellowship from Chile.
Footnotes
Disclosures
None.
References
- 1.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003;64:2155–2162. doi: 10.1046/j.1523-1755.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting Duct Renin Is Upregulated in Both Kidneys of 2-Kidney, 1-Clip Goldblatt Hypertensive Rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The Collecting Duct Is the Major Source of Prorenin in Diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–662. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 7.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 11.Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflugers Arch. 2002;444:499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 12.Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension. 2005;46:1340–1346. doi: 10.1161/01.HYP.0000192025.86189.46. [DOI] [PubMed] [Google Scholar]
- 13.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 14.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension. 2008;52:137–142. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spahn H, Reuter K, Mutschler E, Gerok W, Knauf H. Pharmacokinetics of amiloride in renal and hepatic disease. Eur J Clin Pharmacol. 1987;33:493–498. doi: 10.1007/BF00544242. [DOI] [PubMed] [Google Scholar]
- 19.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem. 2000;275:36839–36846. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 20.Fuller AJ, Hauschild BC, Gonzalez-Villalobos R, Awayda MS, Imig JD, Inscho EW, Navar LG. Calcium and Chloride Channel Activation by Angiotensin II-AT1 Receptors in Preglomerular Vascular Smooth Muscle Cells. Am J Physiol Renal Physiol. 2005;289:F760–F767. doi: 10.1152/ajprenal.00422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teiwes J, Toto RD. Epithelial sodium channel inhibition in cardiovascular disease. A potential role for amiloride. Am J Hypertens. 2007;20:109–117. doi: 10.1016/j.amjhyper.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz RM, Graciano ML, Seth D, Awayda MS, Navar LG. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am J Physiol Renal Physiol. 2007;293:F139–F147. doi: 10.1152/ajprenal.00504.2006. [DOI] [PubMed] [Google Scholar]
- 23.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 24.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 25.Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 1998;274:F564–F572. doi: 10.1152/ajprenal.1998.274.3.F564. [DOI] [PubMed] [Google Scholar]