Abstract
Objective
To examine patterns of executive and oculomotor control in a group of both boys and girls with attention-deficit/hyperactivity disorder (ADHD).
Method
Cross-sectional study of 120 children aged 8 to 12 years, including 60 with ADHD (24 girls) and 60 typically developing controls (29 girls). Oculomotor paradigms included visually guided saccades (VGS), antisaccades, memory-guided saccades, and a go/no-go test, with variables of interest emphasizing response preparation, response inhibition, and working memory.
Results
As a group, children with ADHD demonstrated significant deficits in oculomotor response preparation (VGS latency and variability) and response inhibition but not working memory. Girls, but not boys with ADHD, had significantly longer VGS latencies, even after controlling for differences in ADHD symptom severity. The ADHD subtypes did not differ on response preparation or inhibition measures; however, children with the Inattentive subtype were less accurate on the working memory task than those with the Combined subtype.
Conclusions
Sex differences in children with ADHD extend beyond symptom presentation to the development of oculomotor control. Saccade latency may represent a specific deficit among girls with ADHD.
Keywords: eye movement, inhibitory control, executive function, visual attention, frontal
MOTOR AND EXECUTIVE CONTROL IN ATTENTION-DEFICIT/HYPERACTIVITY DISORDER
Attention-deficit/hyperactivity disorder (ADHD) is associated with dysfunction in motor intentional systems mediated by frontostriatal circuitry.1 The term intention includes four components: initiation, sustaining, inhibition, and shifting2 and is thought to occur between sensation/perception and action, involving a preparedness to respond.3 Together, intention and working memory are subsumed under the construct of executive function. Children with ADHD commonly exhibit deficits in executive control of behavior, including difficulties with inhibition,4 working memory,5 and response preparation.6,7 Executive and motor control systems develop in parallel,8 suggesting shared neural circuitry including frontostriatal systems and the cerebellum.9,10 Models of frontal lobe structure and function describe at least five parallel frontal-subcortical circuits.11 The most posterior are related to motor and oculomotor function (originating in skeletomotor and oculomotor regions of the cortex); the more anterior are thought to be crucial in control of higher-order behavior (e.g., cognitive “executive” and socioemotional control). Children with ADHD often present with motor dysfunction, including inefficient motor speed and coordination,12,13 excessive overflow movements,14,15 slowed processing speed,7,16 and variability of motor response,17 which has also been linked to anomalous function of the frontal-subcortical circuits.18,19
Oculomotor Motor Paradigms in ADHD
Oculomotor paradigms provide unique opportunities to examine executive control skills that exist at the interface between movement and cognition20 and can complement other diagnostic and exploratory studies because they afford a degree of quantification about information processing and timing not found in methods such as magnetic resonance imaging or neuropsychological testing.21 Studies using oculomotor testing have contributed to the understanding of the neurobiological basis of ADHD and have generally supported motor intentional hypotheses, with ADHD groups demonstrating abnormalities related to response inhibition22,23 and (less consistently) response preparation.24–26 The ADHD groups (especially those with predominantly or exclusively male samples) have shown inconsistent deficits in the accuracy of memory-guided saccades (MGS), thought to assess spatial working memory.23,27 However, in a study of only girls, there was a strong trend (p = .07) for reduced MGS accuracy,22 highlighting the importance of studying boys and girls with ADHD simultaneously. To date, however, there have been no studies with large enough samples of contemporaneously recruited boys and girls with ADHD to elucidate sex-specific deficits in oculomotor intention and executive control.
Sex Differences in Children With ADHD
Boys are more commonly diagnosed with ADHD than girls and more often treated28; nevertheless, functional impairment in girls with ADHD is well established.29,30 The available research has yet to specify a clear behavioral or neurobiological profile unique to girls with ADHD31; however, among typically developing children, there is evidence that the brains of boys and girls develop and mature at different rates,32 with girls maturing anatomically earlier than boys in most regions, including the frontal lobes and the basal ganglia.33 Motor development in typically developing children follows a similar trajectory, with girls maturing earlier than boys.34 The findings in studies assessing sex differences in motor skills among children with ADHD have been mixed. Although parent ratings of motor skill were not different between boys and girls with ADHD,35 studies involving direct assessment of motor function suggested that boys may retain abnormal subtle motor signs (e.g., motor overflow) longer than girls.15
Anatomic and functional magnetic resonance imaging studies have identified differences in brain development between children with ADHD and controls, including abnormalities in regions important for motor control, such as the frontal cortex,36 the premotor and motor regions,37 and the interconnected subcortical structures.38,39 Unfortunately, most of these studies reported findings on samples that were predominantly (or exclusively) male, for which conclusions about female-specific anomalies could not be drawn. The few that do highlight female samples reveal fewer frontal anomalies among girls with ADHD.40 For example, in a recent study of basal ganglia structure, Qiu et al.41 found multifocal abnormalities in children with ADHD, including regions in circuits with motor/premotor as well as pre-frontal cortex. Although this study included both boys and girls, the findings were seen predominantly in boys. Electrophysiological studies also suggest that the anomalous development within ADHD may be sex specific. For example, girls and boys with ADHD manifest different patterns of cerebral organization, in which boys with ADHD have a less right-lateralized frontal alpha asymmetry (compared with control boys), whereas girls with ADHD have a more right-lateralized asymmetry pattern than control girls.42 When sex-specific norms are used, behavioral dysfunction reported by parents of girls with ADHD can be more deviant, relative to age- and sex-matched peers, than that reported for boys with ADHD.43 Taken together, these findings suggest that, when studying neurobehavioral development in children with ADHD, data from girls and boys should be analyzed separately and compared with sex-matched controls/norms, in addition to comparing boys and girls with ADHD with each other, to elucidate the developmental anomalies unique to each sex, especially among the earlier maturing girls. Thus, the purpose of this study was to examine executive and motor intentional control in girls and boys with ADHD using oculomotor paradigms designed to assess response preparation, response inhibition, and working memory.
METHOD
Participants
Approval was granted for this study from the Johns Hopkins Medicine institutional review board. Parents of participants signed written consent, and the participants provided written assent. A total of 120 children, aged 8 to 12 years, were included. Two groups were formed: typically developing controls (n = 60) and children with ADHD (n = 60). All children met the following criteria: Full Scale IQ (FSIQ) of 80 or higher on the WISC-IV44 (n = 117) or WISC-III45 (n = 3); and no history of speech/language disorder, reading disability, visual or hearing impairment, or neurological disorder.
The children with ADHD were recruited from outpatient clinics and from local area pediatricians, local chapters of Children and Adults with ADHD, schools, social/service organizations, and advertisements in the community. The diagnosis of ADHD was determined by a structured parent interview that uses DSM-IV criteria (Diagnostic Interview for Children and Adolescents, Fourth Edition [DICA-IV])46 and administration of ADHD-specific and broad behavior rating scales (Conners’ Parent Rating Scale [CPRS]-Revised).47 The children with DSM-IV diagnoses other than oppositional defiant disorder (ODD) and specific phobias were excluded. Those with ADHD were excluded if they were taking longer-acting psychoactive medications other than stimulants. The parents of children with ADHD taking stimulants were asked not to administer the medication on the day of and day before testing.
Classification of ADHD subtype was made based on DICA-IV interview and rating scales. The children were classified as Inattentive subtype if they met criteria for inattentiveness but not hyperactivity/impulsivity on the DICA-IV, a T score of 65 or greater on the CPRS Scale L (DSM-IV Inattentive), and a T score of 60 or less on the CPRS Scale M (DSM-IV Hyperactive-Impulsive). The children were classified as Hyperactive-Impulsive subtype if they met criteria for hyperactivity/impulsivity but not inattention on the DICA-IV and a T score of 65 or greater on the CPRS Scale M and a T score of 60 or less on the CPRS Scale L. All other children who met criteria for ADHD were classified as Combined subtype. Only two children met criteria for Hyperactive-Impulsive subtype; consequently, their data were collapsed into the Combined subtype group.
The controls were recruited through the local school districts and community flyers and were required to have no history of mental health services for behavior or emotional problems, no diagnoses on the DICA-IV, and no clinically significant elevation on the CPRS. Oppositional defiant disorder and specific phobias were not specifically excluded among the controls; however, none met criteria for these diagnoses.
Oculomotor Testing
The general procedures used in the present study for eye movement recording have been described previously.21,23 Participants were seated in front of a tangent screen on which an array of light-emitting diodes (LEDs) was located centrally at 0° of visual angle and 10°, 20°, and 30° to the right and left of center. Head movements were restricted with use of a chin rest and bite bar. Four paradigms were used for the current study and were administered in a fixed order (listed below).
Visually Guided Saccade Paradigm
Each trial began with the illumination of a central LED. At a random time (1,400–2,400 milliseconds), direction (right or left), and amplitude (10°, 20°, or 30°), one of six peripheral LEDs was illuminated, and the central light turned off. One hundred trials were presented, with a minimum of 15 at each target position. All children were given the same instruction: “When the target light comes on, immediately, with your eyes only, look at the target light. When it goes off, look back to the center.” This paradigm tests the child’s ability to initiate saccades to a suddenly appearing, unpredictable visual stimulus, as measured by latency, and represents a measure of oculomotor motor response preparation. In addition, coefficient of variation was used as a measure of intraindividual variability of ocular motor response and was calculated as follows: (100 × [SD of latency/mean latency]).
Antisaccade Paradigm
Each trial began with the illumination of a central LED. At a random time (1,400–2,400 milliseconds), direction (left or right), and amplitude (10°, 20°, or 30°), one of the peripheral LEDs illuminated. The child was instructed to make a saccade to the mirror position opposite to that of the illuminated LED or to “quickly move your eyes in the opposite direction of the target light when it comes on.” After 750 milliseconds, that LED was extinguished and an LED located in the mirror position (where the child was supposed to look) was illuminated. The percentage of errors indicates the degree to which the participant was unable to suppress or inhibit reflexive saccades to the inappropriate target and is considered a measure of response inhibition. A minimum of 72 trials was used to determine percent directional errors, with at least 12 trials at each target position.
MGS Paradigm
Each trial began with the illumination of a center fixation LED. After an interval between 1,300 and 1,700 milliseconds, a peripheral target (cue light) also appeared and was illuminated for 100 milliseconds to the right or left of the central fixation LED (10°, 20°, or 30°). After a variable 4,500- to 5,000-ms delay, the central fixation point diminished, and the child was instructed to make a saccade to the remembered location of the target flash. The screen remained blank for 1,000 milliseconds, during which time the child was to look at the remembered location of the peripheral target. The instruction was as follows: “as long as the center light is on, look only at the center light. Do not look at the flash when it occurs. When the center light goes out, then immediately look to the place where you saw the flash; keep looking there and the light will come on in the place where you saw the flash.” A minimum of 72 trials was presented. This paradigm tests the subject’s ability to make volitional saccade to a remembered target location and thus assesses both response inhibition and visuospatial working memory. Variables of interest included the percent anticipatory saccades (i.e., saccades made before the disappearance of the central fixation point—assessing response inhibition) and accuracy—assessing working memory, which was calculated as percent amplitude error (100 × absolute value of (1 − [amplitude of initial saccade/target amplitude]).
Go/No-Go Task
The go/no-go task was modeled after the paradigm described by Castellanos et al.22 Images on monitor included 5 white squares, placed at 10° left, 5° left, 0°, 5° right, and 10° right. Targets included a green (go) or red (no-go) square overlaying one of the white squares. The task was to look at the squares cued by the green target and ignore the targets cued by the red target. This paradigm took advantage of a well-ingrained stimulus-response set (i.e., green = go; red = stop) and thus minimized the demands for working memory. Participants were instructed to look at the center white square to start and to keep their eyes on the center fixation target until they saw one of the squares change color. When a green square appeared over one of the other white squares, they were to quickly look at that target and maintain fixation until they saw a green square appear over another target. If a red cue appeared over one of the white squares, they were to ignore it and continue to look at the current square. Each target presentation was 300 milliseconds, and interstimulus intervals varied between 1,200 and 2,200 milliseconds. Target presentation was set at 75% green and 25% red in a random sequence. There were 100 target presentations. The variable of interest was percent commission errors, considered a measure of response inhibition.
Data Analysis
Eye movements were recorded using bitemporal electrooculography, which involves recording of the electrical potential in the eyes. Two dim overhead red lights (25 W each) illuminated the testing room, with the target lights providing the only other illumination. The position signals were low-pass filtered (90 Hz bandwidth) and digitized at a sample rate of 500 Hz. Data from each individual trial were shown on a video monitor for subsequent offline analysis. Maximum saccadic velocities, amplitudes, and latencies were determined by using an interactive computer analysis program that showed each trial for review by the examiner (A.L.). To verify that the participants were looking at the target, they were required to maintain gaze at the final eye position for 500 milliseconds. Saccade latency was determined from the time the cue appeared to when the saccade began, with saccade onset defined when the eye velocity reached 25° per second. For all paradigms, saccades made before the presentation of the cue to move the eyes were eliminated. Additionally, saccades initiated after the signal to move the eyes, but before 115 milliseconds, were defined as anticipatory and eliminated.
Statistical Analysis
Distributions for the six dependent measures were analyzed, and log transformations were performed for variables that were not normally distributed. Six variables were chosen a priori from among the four oculomotor paradigms to assess the three components of executive control under investigation: response preparation (visually guided saccade [VGS] latency and coefficient of variability), response inhibition (percent antisaccade directional errors; percent memory-guided saccade response suppression errors; percent go/no-go commission errors), and working memory (memory-guided saccade accuracy). First, group and sex differences on the dependent measures were examined using 2 (group) × 2 (sex) factorial analysis of variance. Significant group-by-sex interactions were followed by separate group- and sex-specific analyses. These analyses were followed by a series of planned contrasts, examining oculomotor control differences between the ADHD subtypes and between each subtype and controls.
RESULTS
Demographic Information
Demographic information for the sample is provided in Table 1. The sample was drawn from largely middle class socioeconomic status and was 74% white, 16% African American, 7% biracial, 1% Asian, 1% Native American, and 1% Hispanic. For the entire sample, there were no significant differences between ADHD and control groups in age (F1,118 = 0.002, p = .97), sex ratio (χ2 = 0.36, p = .46), racial distribution (χ2 = 4.00, p = .55), handedness (χ2 = 2.64, p = .27), or socioeconomic status (F1,107 = 0.01, p = .92). The ADHD group included 37 children with Combined subtype (16 girls) and 23 with Inattentive subtype (8 girls), with no differences in sex ratio between the two subtype groups (χ2 = 0.52, p = .35). Ten (28%) of the 36 boys and 6 (25%) of the 24 girls with ADHD had comorbid ODD (χ2 = 0.06, p = .81), whereas 10 (28%) of the 36 boys and 4 (17%) of the 24 girls had specific phobias (χ2 = 0.99, p = .32).
TABLE 1.
Demographic and Screening Information
| Controls |
ADHD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls (n = 29) |
Boys (n = 31) |
Total (n = 60) |
Girls (n = 24) |
Boys (n = 36) |
Total (n = 60) |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, y | 10.4 | 1.3 | 10.3 | 1.3 | 10.3 | 1.3 | 10.0 | 1.3 | 10.5 | 1.3 | 10.3 | 1.3 |
| Hollingshead index | 52.9 | 9.1 | 50.5 | 11.6 | 51.7 | 10.4 | 52.2 | 7.9 | 51.7 | 9.6 | 51.9 | 9.0 |
| Full Scale IQ | 113.4 | 11.5 | 114.0 | 11.9 | 113.7 | 11.6 | 108.1 | 14.9 | 109.8 | 13.1 | 109.3 | 13.7 |
| CPRS-R DSM-IV Totala,b,c | 45.6 | 4.6 | 43.3 | 3.2 | 44.4 | 4.1 | 78.6 | 13.6 | 68.1 | 8.0 | 72.3 | 11.7 |
Note: CPRS-R = Conners’ Parent Rating Scale-Revised.
ADHD > controls, p < .001.
Girls > boys (ADHD only), p < .001.
Girls > boys (controls only), p < .05.
The controls had marginally higher FSIQ than the ADHD group (F1,118 = 3.87, p = .06); however, this difference was driven by ADHD-related reduction in processing speed index (F1,118 = 18.08, p < .001), which depends highly on response preparation and motor speed. The ADHD and the control groups did not differ on IQ indices less dependent on response preparation and motor speed, that is, verbal comprehension (F1,118 = 0.16, p = .69), perceptual reasoning (F1,118 = 0.52, p = .47), or working memory (F1,115 = 2.55, p = .11). As such, it was felt that covarying for FSIQ was not appropriate when measuring group differences on oculomotor skills, given the significant overlap with motor speed demands of the processing speed index. The girls with ADHD were rated by their parents as having more severe symptoms (CPRS Scale N: DSM-IV Total) than the boys with ADHD (F1,58 = 14.17, p < .001), whereas the control girls had higher rating of symptoms than the control boys (F1,58 = 5.14, p = .03). As such, ADHD symptom severity (CPRS Scale N) was used as a covariate in subsequent analyses between the boys and the girls.
Group and Sex Differences in Oculomotor Control
Means and SDs for the six oculomotor control variables are listed in Table 2. There were no significant main effects for sex on any of the six variables. In contrast, there were significant main effects for group (ADHD worse than controls) on both response preparation variables: VGS latency (F1,116 = 5.17, p = .025, eta-square [η2]p = 0.04) and VGS variability (F1,116 = 5.10, p = .026, η2p = 0.04), and all three response inhibition variables: AS directional errors (F1,116 = 12.16, p = .001, η2p = 0.09), MGS response suppression errors (F1,116 = 23.7, p < .0001, η2p = 0.17), go/no-go commission errors (F1,116 = 7.72, p = .006, η2p = 0.06) but not on the working memory variable: MGS accuracy (F1,116 = 0.21, p = .65, η2p = 0.002).
TABLE 2.
Group Performance on Oculomotor Tasks
| Controls |
ADHD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls (n = 29 ) |
Boys (n = 31) |
Total (n = 60) |
Girls (n = 24) |
Boys (n = 36) |
Total(n = 60) |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Response preparation | ||||||||||||
| VGS latency, msa,c,d | 219.8 | 31.1 | 226.0 | 31.1 | 223.0 | 31.0 | 251.3 | 49.6 | 230.5 | 38.5 | 238.8 | 44.1 |
| VGS variability, msa | 22.9 | 5.2 | 24.7 | 5.9 | 23.8 | 5.6 | 26.4 | 5.4 | 25.9 | 5.3 | 26.1 | 5.3 |
| Response inhibition | ||||||||||||
| AS % errorsb | 55.7 | 21.3 | 55.6 | 18.5 | 55.7 | 19.8 | 65.3 | 15.7 | 68.5 | 17.1 | 67.3 | 16.5 |
| MGS % errorsb | 26.5 | 12.9 | 31.3 | 12.1 | 29.0 | 12.7 | 40.3 | 16.4 | 42.4 | 15.1 | 41.6 | 15.6 |
| GNG % commissionsb | 52.3 | 19.3 | 57.9 | 20.9 | 55.2 | 20.2 | 64.2 | 19.3 | 65.2 | 16.9 | 64.8 | 17.8 |
| Working memory | ||||||||||||
| MGS accuracy | 11.4 | 5.1 | 12.7 | 6.7 | 12.1 | 6.0 | 11.1 | 5.5 | 11.8 | 7.0 | 11.5 | 6.4 |
Note: AS = antisaccades; GNG = go/no-go; MGS = memory-guided saccades; VGS = visually guided saccades.
ADHD > controls (total group), p < .05.
ADHD > controls (total group), p < .01.
ADHD > controls (girls only), p < .05.
Girls > boys (ADHD only), p < .05 (covarying for ADHD symptom severity).
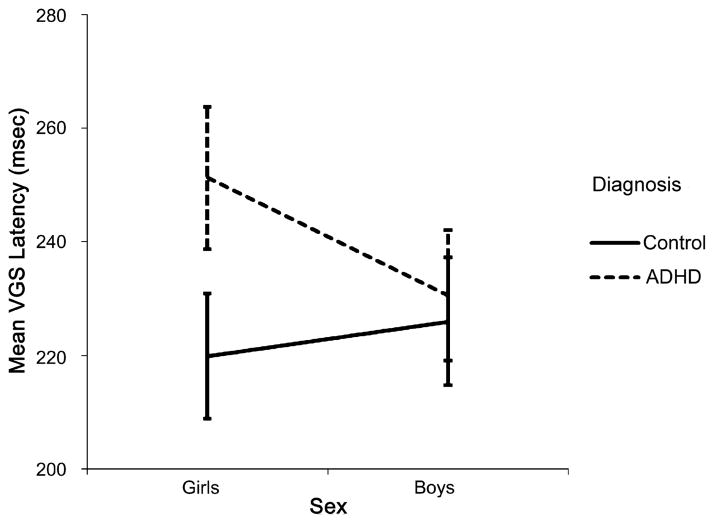
There was also a significant group-by-sex interaction for VGS latency (F1,116 = 3.77, p = .05, η2p = 0.03), which remained after covarying for ADHD symptom severity (F1,115 = 4.59, p = .03, η2p = 0.04). To explore the interaction, group differences (within sex) and sex differences (within group) were examined (Fig. 1). The girls with ADHD had significantly longer VGS latencies than the control girls (F1,51 = 7.94, p = .007, η2p = 0.14); however, the boys with ADHD did not differ from the control boys (F1,65 = 0.27, p = .60, η2p = 0.004). Furthermore, after covarying for ADHD symptom severity, the girls with ADHD had longer VGS latencies than the boys with ADHD (F1,57 = 5.02 p = .029, η2p = 0.081), whereas the control girls did not differ from the control boys (F1,57 = 1.27, p = .27, η2p = 0.02).
Fig. 1.
Group-by-sex interaction for visually guided saccade latency: girls with ADHD > control girls ( p = .007); girls with ADHD > boys with ADHD (p = .029). Error bars = 95% confidence interval.
ADHD Subtype Differences in Oculomotor Control
Planned comparisons revealed significantly longer VGS latency (p = .009) and variability (p = .02) and a higher proportion of errors on AS (p = .003) and MGS (p < .0001) paradigms among children with Combined subtype (compared with the controls). Additionally, compared with the controls, the children with the Inattentive subtype had significantly increased response inhibition errors on AS (p = .018), MGS (p < .0001), and go/no-go commissions (p = .003) and reduced MGS accuracy (p = .05); however, they did not differ from the controls on VGS latency (p = .22) or variability (p = .12). There were no significant differences between ADHD subtypes on any of the response preparation or response inhibition variables. Conversely, the Inattentive subtype had significantly reduced MGS accuracy compared with the Combined subtype (p = .03).
DISCUSSION
As documented in previous studies using oculomotor paradigms, children with ADHD demonstrated robust deficits in oculomotor response inhibition, including increased errors on three different paradigms (antisaccades, MGS, and go/no-go). Additionally, as a group, the finding of increased variability in VGS latencies is consistent with a growing literature showing increased response variability in ADHD.48,49 Increased intraindividual variability has been linked with frontal circuits important for motor response selection and inhibition, particularly those involving the rostral supplementary motor (“pre-SMA”) region.19,50 Oculomotor deficits in spatial working memory have been found less consistently in previous studies, perhaps because of differences in delay periods.51 However, in the present study, the children with ADHD did not differ from the controls on MGS accuracy.
Ignoring diagnosis, the boys and the girls did not differ on any of the oculomotor variables under investigation. Compared with previous oculomotor studies in children, our ADHD group was unique because we oversampled for subtypes less common within each sex (i.e., girls with Combined subtype and boys with Inattentive subtype) to eliminate the disproportionate subtype-by-sex confound that can potentially influence sex-specific patterns of results. Although oculomotor performance among the boys and the girls was highly similar on most parameters, we identified a group-by-sex interaction in which there were more robust deficits among the girls with ADHD in VGS latency, when compared with the control girls and also when compared with the boys with ADHD—a deficit that may impede development of skills dependent on rapid and efficient cognitive control of behavior.52 This isolated weakness among the girls with ADHD is contrasted with patterns observed in skeletomotor control and speed, in which the boys with ADHD have demonstrated equivalent35 and more severe deficits than the girls with ADHD,15 and with previous studies that have shown longer VGS latency in children with ADHD in samples of predominantly boys.26 Increased VGS latency has been linked with anomalous development of frontostriatal circuits53,54; however, patients with lesions in the parietal eye fields55 and the posterior internal capsule56 also demonstrate increased latency on these tasks. As this isolated finding represents one of the few examples in the behavioral and neuropsychological literature in which the girls with ADHD have greater deficits than the concurrently sampled boys with ADHD, it will require replication before conclusions can be drawn with regard to the unique neurobiology of the girls with ADHD. It is possible that the etiology of this increased latency may involve both frontostriatal circuitry and frontoparietal circuits. For example, the role of the caudate nucleus should be explored in future research, given its contribution to oculomotor control via the substantia nigra and the superior colliculus,57,58 and because it has been shown to be larger in female subjects than in male subjects, after relative brain size has been controlled.59,60
Across oculomotor tasks, there were few differences in performance between ADHD subtypes, although the Combined (but not Inattentive) subtype showed relative slowing on response preparation variables. Of note, the Inattentive subtype had poorer MGS accuracy than the children with Combined subtype, which may suggest a link between the behavioral reports of inattention and spatial working memory performance in this group. At the same time, however, those with Inattentive subtype had consistent deficits on all response inhibition measures, highlighting the substantial overlap in symptoms in this age range.61
Although the boys and the girls with ADHD were recruited in an identical manner, parent ratings on the CPRS (which use sex-specific norms) were higher on DSM-IV ADHD Diagnostic Scales for girls than for boys. Despite these differences, deficits in VGS latency among the girls with ADHD (compared with the boys with ADHD) remained after controlling for the severity of ADHD symptoms.
Strengths of the current study include the large sample size and inclusion of contemporaneously sampled groups of girls and boys with ADHD, matched on age, IQ, and subtype. Additionally, the ADHD sample was carefully screened for comorbidities that might confound the interpretation of findings. Nevertheless, although screening for comorbid disorders allowed more direct interpretation of ADHD-specific deficits, results may be less generalizable to clinical samples that typically have multiple comorbidities (beyond ODD and specific phobias). Additionally, the use of a fixed order of presentation of the four paradigms did not allow us to examine the effects of test order on outcome, and we cannot rule out that order effects may have played a role in our findings. Future research should consider sex differences in oculomotor variables not directly examined in the present study (e.g., anticipatory and express saccades for VGS and AS paradigms).
The current study highlights the use of oculomotor paradigms in identifying deficits not routinely found in traditional neuropsychological assessment. In neuropsychology, it is virtually impossible to operationalize “visual attention” without documenting “looking,” which means, “where the eyes are fixed.” Oculomotor paradigms offer a more precise means of assessing components of visual attention that link cognition and neurological development and may be particularly sensitive for detecting these links in school-aged girls. In summary, the finding of longer VGS latency holds promise as a marker for one component of the neurobiological phenotype in the girls with ADHD. Future research is required to replicate this finding and to examine the neural correlates of these behavioral differences.
Acknowledgments
This study was supported by grant HD-24061 from the National Institutes of Health/National Institute of Child Health and Human Development; grants R01 NS050153, NS 35359, R01 NS043480, R01 NS047781, and K02 NS044850 from the National Institutes of Health/National Institute of Neurological Disorders and Stroke; and grant UL1-RR025005 from the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, a National Institutes of Health/National Center for Research Resources–Clinical and Translational Science Awards program.
Footnotes
Disclosure: Dr. Mostofsky served as a consultant to Bristol-Myers Squibb. The other authors report no conflicts of interest.
Contributor Information
Dr. E. MARK MAHONE, Kennedy Krieger Institute
Dr. STEWART H. MOSTOFSKY, Kennedy Krieger Institute
Dr. ADRIAN G. LASKER, Johns Hopkins University School of Medicine
Dr. DAVID ZEE, Johns Hopkins University School of Medicine
Dr. MARTHA B. DENCKLA, Kennedy Krieger Institute
References
- 1.van der Meere J, Sergeant J. Focused attention in pervasively hyperactive children. J Abnorm Child Psychol. 1988;16:627–639. doi: 10.1007/BF00913474. [DOI] [PubMed] [Google Scholar]
- 2.Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 3. New York: Oxford University Press; 1993. [Google Scholar]
- 3.Denckla MB. Biological correlates of learning and attention: what is relevant to learning disability and attention-deficit/hyperactivity disorder? Dev Behav Pediatr. 1996;17:1–6. [PubMed] [Google Scholar]
- 4.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- 6.Harris EL, Schuerholz LJ, Singer HS, et al. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 1995;1:511–516. doi: 10.1017/s1355617700000631. [DOI] [PubMed] [Google Scholar]
- 7.Willcutt EG, Sonuga-Barke EJ, Nigg JT, Sergeant JA. Recent developments in neuropsychological models of childhood psychiatric disorders. In: Banaschewski T, Rohde LA, editors. Biological Child Psychiatry: Recent Trends and Developments. Basel: Karger; 2008. pp. 195–226. [Google Scholar]
- 8.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 9.Mahone EM, Powell SK, Loftis CW, et al. Motor persistence and inhibition in autism and ADHD. J Int Neuropsychol Soc. 2006;12:622–631. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- 10.Rubia K, Overmeyer S, Taylor E, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 11.Lichter DG, Cummings JL. Introduction and overview. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 1–43. [Google Scholar]
- 12.Denckla MB, Rudel RG, Chapman C, Krieger J. Motor proficiency in dyslexic children with and without attentional disorders. Arch Neurol. 1985;42:228–231. doi: 10.1001/archneur.1985.04060030042008. [DOI] [PubMed] [Google Scholar]
- 13.Watemberg N, Waiserberg N, Zuk L, Lerman-Sagie T. Developmental coordination disorder in children with attention-deficit-hyperactivity disorder and physical therapy intervention. Dev Med Child Neurol. 2007;49:920–925. doi: 10.1111/j.1469-8749.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 14.Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Percept Mot Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- 15.Cole WR, Mostofsky SH, Gidley Larson J, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke AR, Barry RJ, McCarthy R, et al. EEG activity in girls with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2003;114:319–328. doi: 10.1016/s1388-2457(02)00364-4. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos FX, Sonuga-Barke EJ, Scheres A, et al. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suskauer SJ, Simmonds DJ, Caffo BS, et al. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leigh JS, Zee DS. The Neurology of Eye Movements. 4. New York: Oxford University Press; 2006. [Google Scholar]
- 21.Lasker AG, Mazzocco MM, Zee DS. Ocular motor indicators of executive dysfunction in fragile X and Turner syndromes. Brain Cogn. 2007;63:203–220. doi: 10.1016/j.bandc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos FX, Marvasti FF, Ducharme JL, et al. Executive function oculomotor tasks in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Mostofsky SH, Lasker AG, Cutting L, Denckla MB, Zee DS. Oculomotor abnormalities in attention deficit hyperactivity disorder: a preliminary study. Neurology. 2001;57:423–430. doi: 10.1212/wnl.57.3.423. [DOI] [PubMed] [Google Scholar]
- 24.Mostofsky SH, Lasker AG, Singer HS, Denckla MB, Zee DS. Oculomotor abnormalities in children with Tourette syndrome with and without ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:1464–1472. doi: 10.1097/00004583-200112000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Rommelse NN, Van der Stigchel S, Witlox J, et al. Deficits in visuo-spatial working memory, inhibition and oculomotor control in boys with ADHD and their non-affected brothers. J Neural Transm. 2008;115:249–260. doi: 10.1007/s00702-007-0865-7. [DOI] [PubMed] [Google Scholar]
- 26.Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- 27.Ross RG, Hommer D, Breiger D, Varley C, Radant A. Eye movement task related to frontal lobe functioning in children with attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1994;33:869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Derks EM, Hudziak JJ, Boomsma DI. Why more boys than girls with ADHD receive treatment: a study of Dutch twins. Twin Res Hum Genet. 2007;10:765–770. doi: 10.1375/twin.10.5.765. [DOI] [PubMed] [Google Scholar]
- 29.Hinshaw SP, Carte ET, Fan C, Jassy JS, Owens EB. Neuropsychological functioning of girls with attention-deficit/hyperactivity disorder followed prospectively into adolescence: evidence for continuing deficits? Neuropsychology. 2007;21:263–273. doi: 10.1037/0894-4105.21.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohan JL, Johnston C. What is the social impact of ADHD in girls? A multi-method assessment. J Abnorm Child Psychol. 2007;35:239–250. doi: 10.1007/s10802-006-9076-1. [DOI] [PubMed] [Google Scholar]
- 31.Biederman J, Kwon A, Aleardi M, et al. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am J Psychiatry. 2005;162:1083–1089. doi: 10.1176/appi.ajp.162.6.1083. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PM, Sowell ER, Gogtay N, et al. Structural MRI and brain development. Int Rev Neurobiol. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- 33.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gidley Larson JC, Mostofsky SH, Goldberg MC, et al. Effects of gender and age on motor exam in typically developing children. Dev Neuropsychol. 2007;32:543–562. doi: 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fliers E, Rommelse N, Vermeulen SH, et al. Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: effects of age and gender. J Neural Transm. 2008;115:211–220. doi: 10.1007/s00702-007-0827-0. [DOI] [PubMed] [Google Scholar]
- 36.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 37.Mostofsky SH, Cooper KL, Kates WR, et al. Smaller prefrontal and pre-motor volumes in boys with ADHD. Biol Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 38.Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- 39.Wellington TM, Semrud-Clikeman M, Gregory AL, et al. Magnetic resonance imaging volumetric analysis of the putamen in children with ADHD: combined type versus control. J Atten Disord. 2006;10:171–180. doi: 10.1177/1087054705284242. [DOI] [PubMed] [Google Scholar]
- 40.Castellanos FX, Giedd JN, Berquin PC, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 41.Qiu A, Crocetti D, Adler M, et al. Basal ganglia volume and shape in children with ADHD. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baving L, Laucht M, Schmidt MH. Atypical frontal brain activation in ADHD: preschool and elementary school boys and girls. J Am Acad Child Adolesc Psychiatry. 1999;38:1363–1371. doi: 10.1097/00004583-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Posner K, Melvin GA, Murray DW, et al. Clinical presentation of attention-deficit/hyperactivity disorder in preschool children: the Pre-schoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) J Child Adolesc Psychopharmacol. 2007;17:547–562. doi: 10.1089/cap.2007.0075. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler DL. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 45.Wechsler D. Wechsler Intelligence Scale for Children-III. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 46.Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 47.Conners CK. Conners’ Rating Scales–Revised. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 48.Di Martino A, Ghaffari M, Curchack J, et al. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmonds DJ, Fotedar SG, Suskauer SJ, et al. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Rommelse NN, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn. 2008;68:391–414. doi: 10.1016/j.bandc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 52.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 53.Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- 55.Pierrot-Deseilligny C, Ploner CJ, Muri RM, et al. Effects of cortical lesions on saccadic: eye movements in humans. Ann N Y Acad Sci. 2002;956:216–229. doi: 10.1111/j.1749-6632.2002.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 56.Gaymard B, Rivaud S, Cassarini JF, et al. Effects of anterior cingulate cortex lesions on ocular saccades in humans. Exp Brain Res. 1998;120:173–183. doi: 10.1007/s002210050391. [DOI] [PubMed] [Google Scholar]
- 57.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 58.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 59.Giedd JN, Castellanos FX, Rajapakse JC, et al. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 60.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 61.Lee SI, Schachar RJ, Chen SX, et al. Predictive validity of DSM-IV and ICD-10 criteria for ADHD and hyperkinetic disorder. J Child Psychol Psychiatry. 2008;49:70–78. doi: 10.1111/j.1469-7610.2007.01784.x. [DOI] [PubMed] [Google Scholar]