Abstract
The present study compared blood oxygen level dependent (BOLD) response in behaviorally inhibited and behaviorally non-inhibited adolescents to positive and negative feedback following their choice in a reward task. Previous data in these same subjects showed enhanced activation in striatal areas in behaviorally inhibited subjects to cues predicting gain or a loss. However, no analyses had examined responses following actual gains or losses. Relative to non-inhibited subjects, behaviorally inhibited subjects in the current study showed enhanced caudate response to negative but not positive feedback, indicating that striatal sensitivity to feedback may be specific to aversive information. In addition, compared to non-inhibited subjects, behaviorally inhibited subjects exhibited reduced differentiation between positive and negative feedback in ventromedial prefrontal cortex (vmPFC). This suggests a perturbed ability to encode reward value.
Keywords: fMRI, striatum, vmPFC, reward, motivation, temperament
Introduction
Behavioral inhibition is a temperament type identified in early childhood that is characterized by heightened sensitivity to novelty, enhanced responses to threat, and a tendency to withdraw from social situations (Fox et al., 2001; Fox et al., 2005). Behaviorally inhibited children are more socially reticent than their peers, more reluctant to play with objects that indicate potential risk (Kagan, et al., 1988; 1989), and are at greater risk for developing anxiety disorders in childhood and adolescence (Chronis-Tuscano, et al., 2009). These children also differ physiologically from non-inhibited peers, displaying higher baseline cortisol levels, greater right frontal electroencephalogram (EEG) asymmetry, and higher heart rates (Fox et al, 2008). Two recent neuroimaging studies (Guyer, et al., 2006; Bar-Haim, et al., 2009) examining adolescents with a history of inhibited and non-inhibited temperament found functional differences in the striatum, a brain region involved in the control of behaviors related to reward and punishment (Seymour, et al., 2007). In the first of these studies (Guyer et al., 2006), adolescents with a childhood history of behavioral inhibition were administered the Monetary Incentive Delay (MID) task, which is known to elicit striatal activation to events that signal the potential future delivery of incentives (Knutson et al., 2000). Relative to peers with no history of inhibition, the behaviorally inhibited adolescents showed heightened blood-oxygen-level-dependent (BOLD) response throughout the striatum to cues indicating that their behavior could lead to a monetary gain or loss. The second study (Bar-Haim et al., 2009) demonstrated that this heightened striatal response was specific to events that signaled that potential outcomes were contingent on the subjects’ actions. Specifically, inhibited adolescents, relative to non-inhibited adolescents, showed enhanced nucleus accumbens response only to cues indicating that the upcoming reward would be contingent upon their response. On non-contingent trials, when reward was expected regardless of choice behavior, no group differences were observed.
Numerous studies have shown that, in addition to its response to incentive cues, the striatum also displays functional activation to rewarding outcomes, such as monetary gain (Knutson, et al., 2000; Pagnoni, et al., 2002; Knutson, et al., 2003; Hardin, et al., 2009), and to punishing outcomes, including pain (Seymour, et al., 2004), aversive sounds (Levita, et al., 2009), or monetary loss (Seymour, et al., 2007, Hardin, et al., 2009). Furthermore, individual differences in striatal activation to particular stimulus types have been linked to related differences in personality traits. Heightened striatal response to monetary reward has been correlated with higher self-ratings on the traits of thrill- and adventure-seeking, and exploratory excitability (Abler, et al., 2006), while enhanced striatal response to novel stimuli correlates with the trait of novelty-seeking (Wittman, et al., 2008). The results of these studies raise the possibility that behaviorally inhibited individuals, who are characterized by an avoidant coping style and a tendency to withdraw from novelty, may show heightened striatal activation specific to negative outcomes. While two prior studies (Bar-Haim et al. 2009; Guyer et al. 2006) examine reward prediction in behavioral inhibition, no prior study has examined neural response following delivery of rewards or punishments.
To examine whether behaviorally inhibited individuals show a valence-specific enhanced striatal response to positive or negative outcomes, we assessed striatal responses to the trial-by-trial feedback presented to participants in the Bar-Haim et al. study (2009), a prior report that focused on striatal response to cue anticipation. In this study, after making a button press, subjects received feedback indicating whether or not their choice had been correct, and thus whether or not they received a monetary reward. For the current study, we hypothesized that behaviorally inhibited individuals, relative to non-inhibited individuals, would show greater striatal activation to negative feedback, but that these groups would not differ in their striatal response to positive feedback.
In addition to our focus on the striatum, we also examined activation in ventromedial prefrontal cortex (vmPFC). Previous studies examining neural activation to the receipt of monetary rewards consistently show activation of the vmPFC (Knutson, et al., 2001; Knutson et al., 2003; Ramnani, et al., 2004), a prefrontal region thought to update and encode the reward value of stimuli (Kringelbach, 2005). Failure to receive a reward often leads to deactivation in this region (Knutson, et al., 2003; O’Doherty, et al., 2001). The vmPFC projects to the striatum and is thought to shape striatal response to reward (Haber, et al., 1995). Prior data suggest that behaviorally inhibited individuals are more sensitive than non-inhibited individuals to negative outcomes. Therefore, we hypothesized that these two groups would show comparable activation to positive outcomes in the vmPFC but that inhibited individuals would show greater deactivation to negative outcomes than non-inhibited individuals.
Methods
Participants
Thirty-two adolescents (aged 14 to 18 years; mean age, 16.2 years) completed the study (see, Bar Haim et al., 2009). Subjects were selected from a population participating in a longitudinal study of temperament and affect regulation. At four months of age, 433 subjects were screened for motor and emotional reactivity to novel visual and auditory stimuli (Calkins et al., 1996; Fox et al., 2001). A total of 153 of the screened infants who displayed either low or heightened levels of reactivity were selected for inclusion in the longitudinal study. Behavioral inhibition was coded at 14 and 24 months. Social reticence was coded at 48 months of age. At each age questionnaire measures of temperamental shyness were collected from parents of the children. Behavioral and questionnaire scores were standardized at each age and were used to create a behavioral inhibition composite score across all three ages. Subjects with scores in the upper half of the distribution were identified as behaviorally inhibited, and those with scores in the lower half of the distribution were categorized as non-inhibited. A total of 35 subjects from this population were recruited to participate in the present study. Of these subjects, 32 (15 female; 16 behaviorally inhibited) provided usable data.
In accordance with the selection criteria, the behaviorally inhibited group had significantly higher scores on the behavioral inhibition composite (M=0.57, SD=0.63) than the non-inhibited group (M=−0.72, SD=0.31), t=7.41, df=30, p<.001. The two groups did not differ on age, IQ, or percentage of males and females (see Table 1). Furthermore, the groups did not differ on measures of current anxiety or depression (State-Trait Anxiety Inventory, Screen for Child Anxiety Related Emotional Disorders (SCARED)-Child, SCARED-Parent, and Child Depression Inventory) or psychiatric status, as assessed by the Schedule for Affective Disorders and Schizophrenia for School Aged Children (KSADS) (Kaufman et al., 1997). Hence, any observed between-group differences in neural response are expected to reflect differences specifically in developmental manifestations of temperament as opposed to ongoing psychopathology.
Table 1.
Subject Demographic Characteristics and Questionnaire Measures at Scan
| Behaviorally Inhibited, Mean (SD) |
Behaviorally Non- Inhibited, Mean (SD) |
Statistics, t-value (d.f.) |
p-value | |
|---|---|---|---|---|
| Behavioral Inhibition Composite |
0.57 (0.63) | −0.72 (0.31) | 7.41 (30) | 0.001 |
| Age | 16.50 years (1.37 years) |
15.94 years (1.18 years) |
1.24 (30) | 0.22 |
| IQ | 106.92 (20.52) | 107.56 (10.25) | 0.85 (19) | 0.93 |
| Gender | 68.8% male | 37.5% male | Fisher’s exact test | 0.16 |
| State-Trait Anxiety Inventory— State pre-scan |
28.50 (3.18) | 28.38 (3.28) | 0.11 (30) | 0.92 |
| State-Trait Anxiety Inventory— Trait |
26.19 (4.92) | 25.81 (5.26) | 0.21 (30) | 0.84 |
| Screen for Child Anxiety Related Emotional Disorders - Child |
7.15 (8.30) | 10.12 (10.41) | 0.79 (22) | 0.44 |
| Screen for Child Anxiety Related Emotional Disorders - Parent |
4.40 (4.78) | 5.80 (5.66) | 0.73 (28) | 0.47 |
| Child Depression Inventory |
30.75 (3.30) | 31.20 (3.32) | 0.38 (29) | 0.71 |
| Schedule for Affective Disorders and Schizophrenia for School Aged Children |
20% | 28.6% | Fisher’s exact test | p = .682 |
Experimental Paradigm
The experiment was divided into 3 runs of 40 trials each. Each trial began with the presentation of a circle cue for 1000 ms, to which the participant was instructed to respond by pressing one of two buttons on a hand-held button response box. Circles were one of three colors, with each color corresponding to a particular type of trial: Non-contingent, contingent, or motor (color-to-condition mapping was counterbalanced across participants). On non-contingent trials, the number 1 or the number 2 was displayed in the center of the circle, and subjects were instructed to press the button that corresponded to the number in the circle to receive a monetary gain. On contingent trials, a question mark was displayed in the center of the circle, and subjects were instructed to choose which button to press. Subjects were told that they would receive a monetary gain if they chose the correct button. In reality, participants received positive feedback on a randomly selected 50% of the trials. After button press on both contingent and non-contingent trials, subjects received feedback that was presented for 1000 ms after a variable interval of 0 to 4000 ms. The feedback indicated whether their selection resulted in a gain or no gain for that trial and displayed their cumulative gain for the session. Potential rewards were of two magnitudes, either 3 or 6 points, signaled by the size of the circle cue. On the third trial type, motor trials, participants were instructed to press either of the two buttons in response to the circle. They were told that there was no opportunity for monetary reward on these trials, and no feedback was provided. Each of the three runs consisted of eight trials of each of the five types (small contingent, large contingent, small non-contingent, large non-contingent, motor) randomized for a total of 40 trials per run. A prior report presents analysis of neural activation to the contingent, non-contingent, and motor cues (Bar-Haim et al., 2009). Immediately following completion of the task in the scanner, participants completed a 29-item post-scan questionnaire. Six items assessed subjects’ understanding of the six cues presented during the task, and 23 items probed the subjects’ experience of the task.
Image acquisition
Data acquisition was performed on a 3T General Electric (Waukesha, WI) Signa Magnet. Task stimuli were displayed via back-projection from a head-coil mounted mirror to a screen at the foot of the scanner bed. Foam padding was used to constrain head movement. Behavioral data were recorded using a Cedrus (San Pedro, CA) Lumina hand-held two-button response box.
Thirty sagittal slices (4 mm thickness) per volume were obtained using a T2*-weighted echo-planar sequence (repetition time, 2500 ms; echo time, 23 ms; flip angle, 90°; 64 × 64 matrix; field of view, 240 mm; in-plane resolution, 3.75 × 3.75 mm2). A total of 165 volumes were collected in each run. To improve the localization of activations, a high-resolution structural image was also recorded from each participant during the same scanning session using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence with the following parameters: 124 1.2-mm sagittal slices; repetition time, 8100 ms; echo time, 32 ms; flip angle, 15°; 256 × 256 matrix; field of view, 240 mm; in-plane resolution, 0.86 × 0.86 mm2; NEX, 1; bandwidth, 31.2 kHz.
Image processing and analysis
Analysis of fMRI data was performed using Analysis of Functional and Neural Images (AFNI) software version 2.56b (Cox, 1996). Echo-planar images (EPI) were visually inspected to confirm image quality and minimal movement. Three subjects (all behaviorally inhibited) were excluded due to movement greater than 3 mm, in keeping with the exclusion criteria used in Bar-Haim, et al. (2009). Standard preprocessing of EPI data included slice-time correction, motion correction, and spatial smoothing with a 4 mm full-width half-maximum Gaussian smoothing kernel. Each subject’s data were transformed to a percent signal change using each subject’s voxel-wise time series mean blood oxygen level dependent (BOLD) activity as a baseline. Images were analyzed using an event-related design.
Time series data for each individual were analyzed using multiple regression (Netter et al., 1996). The entire trial was modeled using a gamma-variate basis function, including the five cue and eight feedback events. The model also included six nuisance variables modeling the effects of residual translational and rotational motion (motion in the x, y, and z planes, as well as roll, pitch, and yaw), and regressors for both baseline and linear trend. To increase statistical power, analyses were collapsed across trials of small and large incentive size. However, given our hypotheses, analyses in the present study were restricted to contingent trials, when participants received unpredictable feedback as to whether their button-press selection resulted in a gain or no gain. All non-contingent trials resulted in a sure gain, so long as subjects followed the task instructions. Therefore, these non-contingent trials were not included in the feedback analyses since there was no negative feedback.
Contrasts of BOLD activity were created for each subject for positive vs. negative feedback on contingent trials. Based on our a priori hypotheses, we examined group differences in the following regions of interest (ROIs): nucleus accumbens, caudate, putamen, and vmPFC. For completeness, we also examined two additional ROIs that are involved in the processing of positive and negative outcomes: the amygdala, and the anterior cingulate cortex. Talairach anatomical boundaries provided by AFNI were used to define voxels that fell within each ROI after spatial normalization (Tailarach and Tournoux, 1988). The statistical threshold for individual voxels was set to p < 0.005, uncorrected. Correction for multiple comparisons was applied following Monte Carlo simulations to determine appropriate contiguity thresholds to achieve a corrected alpha level of p < 0.01. In addition, a whole-brain analysis was performed, with an uncorrected threshold of p < 0.005, and a whole-brain cluster size correction threshold of p < 0.05. This analysis was performed separately for behaviorally inhibited subjects and behaviorally non-inhibited subjects so that general patterns of activation could be examined for each group. A final whole-brain analysis including all subjects was conducted, as well.
Results
Behavioral Data
Participant responses on post-scan questionnaires were examined to determine if group differences emerged in task strategy or understanding of the task. No significant group differences (all t’s < 1.45, p’s > 0.16) were detected in responses to the following items: “I could tell when I was going to win;” “I had a hard time deciding which button to press;” “I had a strategy to decide which button to choose.” In addition, participants indicated clear understanding of the meaning of each cue type. As feedback was passively received, no behavioral response to the feedback could be analyzed.
Imaging Data
The whole-brain analysis across all subjects is presented in Table 2. This analysis yielded twelve significant clusters in the main effect analysis (positive feedback vs. negative feedback), including left caudate, right caudate, and right mPFC. In behaviorally non-inhibited subjects alone, two clusters reached statistical significance: one in the right caudate, and one in left vmPFC. When the analysis was restricted to behaviorally inhibited subjects, no clusters reached statistical significance in the striatum or mPFC. Instead, clusters emerged bilaterally in the temporal gyrus, and in the right middle temporal gyrus. Significant clusters for each group are presented in fig. 1 and Table 3.
Table 2.
Contingent Positive Feedback vs. Contingent Negative Feedback Whole-Brain Analysis across all Subjects
| Region | Talairach Coordinates | Z | No. of voxels |
|---|---|---|---|
| R Cerebellum | [11 −71 −16] | 5.60 | 17599 |
| L Cerebellum | [−43 −42 −18] | 4.90 | 5981 |
| R Caudate | [10 14 −5] | 5.14 | 1922 |
| L Caudate | [−9 16 −4] | 4.82 | 1752 |
| L vlPFC | [−43 53 13] | 4.67 | 1383 |
| R dlPFC | [50 12 37] | 4.73 | 1351 |
| L dlPFC | [−46 3 36] | 4.69 | 1228 |
| L Putamen/Claustrum | [−28 −9 8] | 4.49 | 886 |
| R Precentral | [33 −25 63] | 4.43 | 818 |
| R mPFC | [18 67 23] | 4.33 | 591 |
| Brainstem | [−17 −23 −11] | 3.90 | 587 |
| L Precuneus/Inf Parietal | [−31 −66 38] | 3.99 | 557 |
Figure 1.
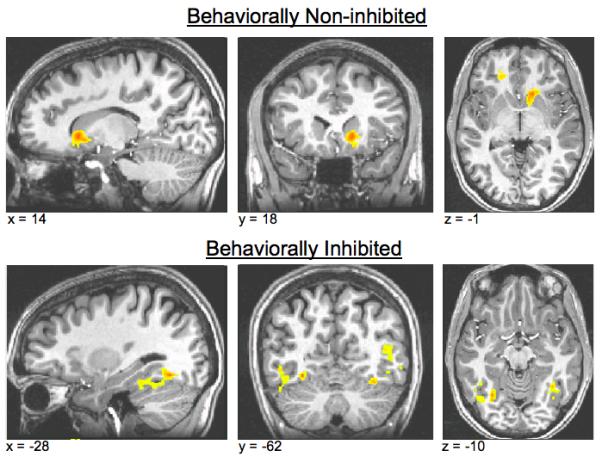
BOLD activation to contingent positive feedback vs. contingent negative feedback within temperament groups. Top row shows significantly active clusters in the behaviorally non-inhibited subjects; bottom row shows significantly active clusters in the behaviorally inhibited subjects. Behaviorally non-inhibited subjects showed greater activation in right caudate and left ventromedial prefrontal cortex to positive feedback than negative feedback. Behaviorally inhibited subjects failed to show this pattern of activation, instead showing greater activation to positive feedback than negative feedback in the fusiform gyrus bilaterally and in the right middle temporal gyrus.
Table 3.
Contingent Positive Feedback vs. Contingent Negative Feedback Whole-Brain Analysis Separately in Behaviorally Non-inhibited Subjects and Behaviorally Inhibited Subjects
| Behaviorally Non-inhibited | |||
|---|---|---|---|
| Region | Talairach Coordinates | Z | No. of voxels |
| R Caudate | [14 18 −1] | 5.73 | 1454 |
| L vmPFC | [−23 33 3] | 6.96 | 591 |
| Behaviorally Inhibited | |||
|---|---|---|---|
| Region | Talairach Coordinates | Z | No. of voxels |
| L Fusiform Gyrus | [−28 −62 −10] | 6.36 | 2979 |
| R Fusiform Gyrus | [33 −69 −7] | 6.74 | 1769 |
| R Mid Temporal Gyrus | [46 −56 3] | 7.85 | 1689 |
Clusters with a significant group (inhibited vs. non-inhibited) × valence (positive feedback vs. negative feedback) interaction were seen in the dorsal area of the left caudate nucleus (Talairach coordinates: −14, 9, 19; cluster size = 194) and right vmPFC (BA10; Talairach coordinates: 2, 51, −5; cluster size = 225).
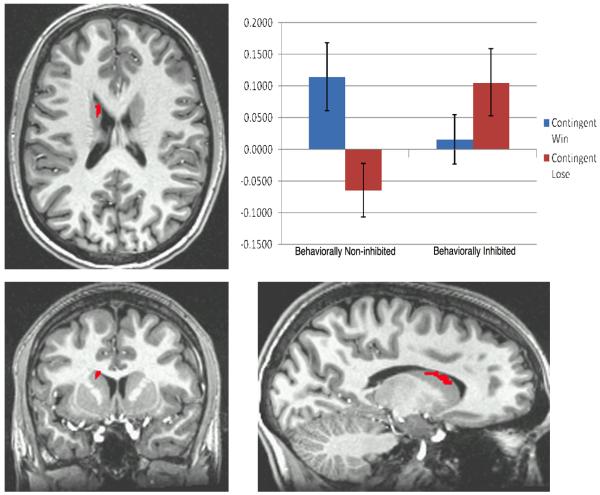
The interaction in the left caudate was in the expected direction, with behaviorally inhibited adolescents showing relatively more caudate activation to no-gain feedback, and non-inhibited adolescents showing relatively more caudate activation to gain feedback (see fig. 2).
Figure 2.
Talairach coordinates: x=−14, y=9, z=19. Bars represent average percent signal change of BOLD activation in voxels in the left caudate that surpassed an alpha threshold of p < 0.005 in the Valence (contingent positive feedback vs. contingent negative feedback) × Group (inhibited vs. non-inhibited) analysis.
The striatal finding was lateralized, with a significant effect found in the left caudate, but not the right. To investigate whether this finding was truly lateralized or simply an artifact of the chosen thresholding criteria, we gradually lowered the thresholding criteria. When the statistical threshold for individual voxels was changed from p<.005 to p<.008, a cluster appeared in the right dorsal caudate in a location roughly symmetrical to the left caudate cluster. The interaction effect in this cluster was similar to that in the left cluster, with behaviorally inhibited individuals showing greater activation to negative feedback and suppressed activation to positive feedback. Thus, there does not appear to be strong evidence for a truly lateralized effect.
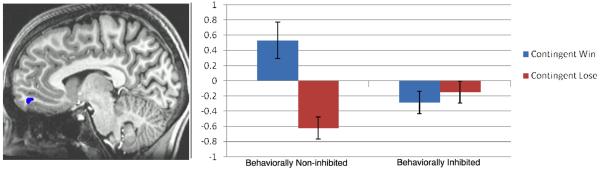
In the right vmPFC, behaviorally inhibited adolescents showed no activation to either gain or no-gain feedback, while non-inhibited adolescents showed a clear differentiation of response to these distinct feedback categories, displaying a significant BOLD activation to gain feedback and deactivation to no-gain feedback (see fig. 3). This cluster extended into the left vmPFC, as well.
Figure 3.
Talairach coordinate: x=2. Bars represent average percent signal change of BOLD activation in voxels in the right vmPFC that surpassed an alpha threshold of p < 0.005 in the Valence (contingent positive feedback vs. contingent negative feedback) × Group (inhibited vs. non-inhibited) analysis.
Regardless of group, a main effect for valence (gain vs. no gain) was found in the rostro-ventral areas of the left and right caudate nuclei (Talairach coordinates: −9, 16, −4; 10, 14, −5), a location distinct from the dorsal caudate region where the previously noted interaction emerged, and in the right vmPFC (Talairach coordinates: 9, 48, −7), with all three regions showing greater activation to gain feedback than no-gain feedback. No significant main or interaction effects emerged in the amygdala or the anterior cingulate cortex.
Discussion
In the current study, adolescents with and without a history of behavioral inhibition were presented with feedback signifying that their actions had positive (receipt of monetary gain) or negative (omission of monetary gain) consequences. Whole-brain analyses conducted separately for behaviorally inhibited and behaviorally non-inhibited participants indicated that behaviorally inhibited adolescents show atypical processing of positive and negative feedback. While non-inhibited adolescents showed greater activation in both the caudate and the vmPFC to positive monetary feedback than to negative monetary feedback, behaviorally inhibited adolescents did not show sufficiently large differences in activation in either region for statistically significant clusters to emerge. Additional region of interest analyses examining group by valence effects in the striatum and vmPFC allowed us to more precisely characterize these group differences, indicating that behaviorally inhibited participants differed from their non-inhibited counterparts in their neural responses to the feedback in two ways. First, they showed heightened responses in the striatum to negative feedback, relative to positive feedback. Non-inhibited adolescents showed the opposite pattern. Second, behaviorally inhibited adolescents showed less differentiation than non-inhibited adolescents between positive and negative feedback in the vmPFC, a region that encodes the reward value of stimuli (Knutson et al., 2003); non-inhibited participants displayed the expected pattern of activation in the vmPFC.
Our first main finding concerns the striatum. In this region, based on prior research, non-inhibited individuals showed the typical, expected pattern of response: relative activation to receipt of reward and deactivation to omission of reward. Consistent with our hypothesis, inhibited individuals, in contrast, showed an opposite pattern: relative activation to omission of reward and minimal activation to the receipt of reward, suggesting a greater striatal response to negative events than to positive ones. This pattern of findings is especially unusual as striatal activation is rarely seen to the omission of reward. Typically, a more overtly negative event than failure to gain a reward, such as the loss of money (Delgado, et al., 2000; Hardin, et al., 2009) or administration of a mild shock (Seymour, et al., 2004) is necessary to elicit striatal activation. Thus, the present finding suggests that behaviorally inhibited individuals encode mildly negative events as aversive to a greater extent than their non-inhibited peers. This is consistent with findings in other domains indicating that a heightened sensitivity to mildly aversive events is related to both behavioral inhibition and anxiety (Perez-Edgar, et al., 2010; Reeb-Sutherland, et al., 2009).
Of note, the location of the interaction effect in the striatum is more caudal and dorsal than the striatal regions that respond most robustly to rewarding cues. This is consistent with the data of Seymour and colleagues (2007), which indicate that maximal striatal activation to aversive events is located more caudally and dorsally in the striatum than maximal activation to rewarding events. This relative functional segregation is consistent with animal research, as well. Although it is difficult to make inferences due to the limited spatial resolution of fMRI, rodent studies have also shown an appetitive-aversive gradient in the ventral striatum, where injections of GABA agonists or glutamate antagonists more rostrally elicits appetitive feeding behavior, and equivalent injections more caudally elicits withdrawal behavior and defensive treading (Reynolds & Berridge, 2001, 2002; Faure, et al., 2008). Moreover, environmental contexts can shift the edges of the gradient, so that regions of the striatum that would elicit approach behaviors in a safe environment will elicit defensive behaviors in a stressful environment (Reynolds & Berridge, 2008). Thus, our results could reflect a shifting of this appetitive-aversive gradient in behaviorally inhibited individuals, such that the region in which the interaction arises responds principally to appetitive stimuli in non-inhibited individuals and to aversive stimuli in inhibited individuals.
Our second finding concerns the vmPFC. The vmPFC encodes the reward value of items present in the environment (Rolls, 2000). In humans, this region shows greater BOLD activation to receipt of pleasant than unpleasant feedback (O’Doherty et al., 2001; Knutson et al., 2003), and the amount of BOLD activation is correlated with the reported pleasantness of the stimulus (Kringelbach et al., 2003). Additionally, the vmPFC has been proposed to play a key role, in conjunction with the striatum, in both learning the reward value of stimuli and selecting actions that will lead to reward (O’Doherty et al., 2004). In the present study, individuals with no history of inhibition displayed the expected pattern of vmPFC response: activation to receipt of reward and deactivation to the omission of reward. We hypothesized that behaviorally inhibited subjects, relative to non-inhibited subjects, would display a similar vmPFC response to receipt of reward and a heightened deactivation response to the omission of reward. In reality, behaviorally inhibited adolescents showed little vmPFC response to either stimulus class and did not differentiate between them.
The muted response to positive and negative outcomes in the vmPFC in inhibited individuals could indicate a diminished ability to discriminate the reward value of a stimulus. Because the vmPFC densely innervates the striatum and modulates striatal responses (Haber et al., 1995), the vmPFC pattern seen here could contribute to the diminished striatal responses to positive feedback seen in inhibited individuals. Further studies are needed to gain a more precise understanding of the respective contributions of each of these regions to reward processing in behavioral inhibition.
The current study has several limitations. The heightened striatal activation in behaviorally inhibited individuals to negative feedback appears to reflect greater striatal sensitivity in behaviorally inhibited individuals to aversive events. However, in this paradigm, negative feedback reflected the omission of reward, which typically elicits striatal deactivation, rather than the receipt of punishment, which typically elicits striatal activation. To extend the current results, future studies might examine responding among inhibited individuals to punishment, in addition to omission of reward. Additionally, the present study does not permit assessment of the development of this neural pattern with age and its contribution to the developmental trajectory of behavioral inhibition. Longitudinal research is critical to begin to address this question.
Together, the results of the present study demonstrate that temperamentally inhibited adolescents process positive and negative information differently from non-inhibited adolescents, who show a typical activation pattern. Behaviorally inhibited adolescents exhibit enhanced striatal activation to negative information, and a reduced differentiation between positive and negative feedback in the vmPFC. The enhanced striatal response to negative information may underlie the temperamental bias associated with behavioral inhibition towards avoidance at the expense of approach behavior. Avoidance orientation is theorized to be a factor underlying multiple forms of psychopathology (Hayes, et al., 1996), suggesting a possible connection between enhanced striatal response to negative information and the increased rates of social anxiety symptoms seen in behaviorally inhibited teens (Chronis-Tuscano, et al., 2009). Further research must be done to directly test the links among enhanced striatal response to aversive events, avoidance behavior, and psychopathology.
Acknowledgements
This work was supported in part by the National Institute of Mental Health Grant R01 MH 074454 (N.A.F.), the National Institute of Child Health and Development Grant 5R37 HD 017899-20 (N.A.F.), the Intramural Research Program of the National Institutes of Health—National Institute of Mental Health, and by a National Science Foundation Graduate Research Fellowship (S.M.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:1218–1223. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JFW. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: parametric functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. Journal of Neuroscience. 2008;28:7184–92. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Perez-Edgar K, White LK. The biology of temperament: An integrative approach. In: Nelson CA, Luciana M, editors. The handbook of developmental cognitive neuroscience. MIT Press; Cambridge, MA: 2008. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. Journal of Neuroscience. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Pine DS, Ernst M. The influence of context valence in the neural coding of monetary outcomes. NeuroImage. 2009;48:249–257. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experiental avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. Journal of Consulting and Clinical Psychology. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick J, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick J, Gibbons J. Inhibited and uninhibited types of children. Child Development. 1989;60:838–845. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule of Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper J. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. NeuroImage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4th ed Irwin; Chicago: 1996. [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink C, Montague P, Berns G. Activity in human ventral striatum locked to errors of reward prediction. Nature Neuroscience. 2002;5:97–8. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Elliot R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. NeuroImage. 2004;23:777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland B, Helfinstein S, Degnan K, Perez-Edgar K, Henderson H, Lissek S, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Berridge K. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. The Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Berridge K. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. The Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Berridge K. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11:423. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Fackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann B, Daw N, Seymour B, Dolan R. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58:967–73. doi: 10.1016/j.neuron.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]