Abstract
OBJECTIVE:
To generate and report standardized growth curves for weight, length, head circumference, weight/length, and BMI for non–growth hormone–treated white infants (boys and girls) with Prader-Willi syndrome (PWS) between 0 and 36 months of age. The goal was to monitor growth and compare data with other infants with PWS.
METHODS:
Anthropometric measures (N = 758) were obtained according to standard methods and analyzed from 186 non–growth hormone–treated white infants (108 boys and 78 girls) with PWS between 0 and 36 months of age. Standardized growth curves were developed and the 3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentiles were calculated by using the LMS (refers to λ, μ, and σ) smoothing procedure method for weight, length, head circumference, weight/length, and BMI along with the normative 50th percentile using Centers for Disease Control and Prevention national growth data from 2003. The data were plotted for comparison purposes.
RESULTS:
Five separate standardized growth curves (weight, length, head circumference, weight/length, and BMI) representing 7 percentile ranges were developed from 186 non–growth hormone–treated white male and female infants with PWS aged 0 to 36 months, and the normative 50th percentile was plotted on each standardized infant growth curve.
CONCLUSIONS:
We encourage the use of these growth standards when examining infants with PWS and evaluating growth for comparison purposes, monitoring for growth patterns, nutritional assessment, and recording responses to growth hormone therapy, commonly used in infants and children with PWS.
Keywords: Prader-Willi syndrome, standardized growth curves, obesity, growth hormone therapy
WHAT'S KNOWN ON THIS SUBJECT:
Standardized growth curves do not exist for infants with Prader-Willi syndrome. Syndrome-specific growth charts are required for monitoring growth and nutritional status, particularly during growth hormone treatment and follow-up for this disorder during infancy.
WHAT THIS STUDY ADDS:
Standardized growth curves are reported to be used in monitoring growth and development of infants with Prader-Willi syndrome of both genders from 0 to 36 months of age.
Prader-Willi syndrome (PWS), first reported in 1956,1 is a complex genomic imprinting disorder characterized in infancy by central hypotonia, a poor suck and feeding difficulties with failure to thrive, growth hormone deficiency, and hypogonadism. However, hyperphagia occurs in early childhood, leading to obesity if left uncontrolled.2–5 Mental deficiency is present with an average IQ of 65 along with behavioral problems (eg, obsessive-compulsive disorder, temper tantrums, skin-picking), hypopigmentation, small hands and feet, short stature, hypogonadism, hypogenitalism, and a characteristic facial appearance.2,3,5–7
PWS is estimated to occur at 1 in 10 000 to 20 000 live births and is considered to be the most common syndromic cause of life-threatening obesity.2 The syndrome is caused by loss of paternally expressed genes known to be imprinted. PWS is usually a result of a paternal chromosome 15q11-q13 deletion in ∼70% of people, whereas 25% have maternal disomy 15 (both 15s from the mother), and the remaining people with PWS have imprinting center defects controlling the activity of imprinted genes in the chromosome 15q11-q13 region. It is estimated that 350 000 to 400 000 people are affected worldwide, and the syndrome presents in all ethnic groups.8,9 Most cases are sporadic, and the chance of recurrence is <1%, although those with imprinting center defects may have a much higher risk (eg, 50%).
Two distinct stages of clinical course development have been reported in PWS, with the first stage recognized in neonates and infants noted by a delay in reaching developmental milestones, a poor suck and feeding difficulties, decreased muscle mass, and profound hypotonia. The second stage is characterized by the onset of hyperphagia and subsequent obesity in early childhood (ie, 2–3 years of age). Food foraging and behavioral problems (temper tantrums and stubbornness) are often initiated by withholding food. Because of better awareness and early diagnosis, nutritional intervention and growth hormone therapy are frequently prescribed during infancy and early childhood. The impact on growth is illustrated by a study by Eiholzer et al10 in 12 children (age: 0.6–14.6 years) with documented PWS treated with human growth hormone and followed up for 1 year. The children were divided into 3 groups: group 1, overweight and prepubertal; group 2, underweight and prepubertal; and group 3, pubertal. Growth hormone treatment led to dramatic changes in body composition and physical activity, including a distinct increase in growth velocity, height and muscle mass, and improvement in physical performance. Fat mass and weight for height decreased in the overweight group, and weight for height increased in the underweight group.
With growth hormone treatment at an early age leading to improvement in stature, body composition, energy level, and metabolism,3 access to growth standards for PWS are needed during infancy. Previously, Butler and Meaney11 reported selected standardized growth charts for people with PWS aged between 2 and 22 years, but a paucity of syndrome-specific growth standards exists for this disorder, particularly in infants of either gender. Standardized growth curves would be useful for monitoring growth and nutritional status, especially during the time of initiation and treatment with growth hormone and for follow-up. Therefore, we developed standardized curves for weight, length, head circumference, weight/length, and BMI for non–growth hormone–treated white infants (boys and girls) with PWS aged between 0 and 36 months to be used in the clinical setting for evaluation, management, and care of infants with PWS.
METHODS
Subjects
Growth data were available from 224 infants with genetic confirmation of PWS. Thirty-eight infants were excluded because of prematurity (gestation < 37 weeks). Therefore, our selected subject cohort consisted of 186 non–growth hormone–treated white infant boys (n = 108) and girls (n = 78) with PWS and an age range of 0 to 36 months. Sixty-eight percent of the infants with PWS were reported to have the typical 15q11-q13 deletion consistent with the distribution of genetic subtypes reported in the literature.2,3,5 The majority of subjects (n = 102) had repeated measurements (median, 5; range, 2–26) for weight, length, and/or head circumference with a total of 758 data points distributed from 0 to 36 months. Approximately 25% of the data were available from 0 to 3 months; 35% from 3 to 12 months; 25% from 12 to 24 months; and 15% from 24 to 36 months. For comparison purposes, the 50th percentile was calculated and plotted from normal infants using growth data collected from year 2003 by the Centers for Disease Control and Prevention (CDC) (www.cdc.gov/growthcharts/percentile_data_files.htm)12 and plotted on each standardized growth curve.
Methods and Measurements
Nearly all subjects were followed up in our study for caloric intake with enhanced nutrition when necessary with the use of nasogastric or gastrostomy tubes and/or by extended oral feedings, as failure to thrive is typical during infancy with PWS.2 The anthropometric measurements obtained on our infants were made by the authors according to standard techniques.11–13 The measurements consisted of weight, length, and head circumference. The majority of length measurements were taken in the supine position using a horizontal calibrated board. Head circumference was measured to the nearest millimeter with a steel tape. Weight was measured with a balanced beam or electronic digital scale recorded to the nearest 0.01 kg.
The standardized growth curves were derived for each gender using the LMS (refers to λ, μ, and σ) method developed by Cole in 1990 and later by Cole and Green in 1992.14,15 This method summarizes the distribution of an anthropometric variable at each covariate (eg, age) value, using 3 parameters: power of the Box-Cox transformation λ (L), median μ (M), and coefficient of variation σ (S). Because percentiles based on a normal distribution are more precise, the LMS method determines an age-dependent (Box-Cox) transformation such that the transformed anthropometric data are approximately normally distributed. The optimal power value of the Box-Cox transformation is estimated for each of a series of age groups and its trend is summarized by an L curve (eg, L = 0 for log transformation, L = 1 for using raw data). Similarly, trends in the estimated medians and coefficients of variation over the age groups are summarized by M and S curves, respectively. Maximum penalized likelihood estimation technique16 is used to determine the L, M, and S curves as cubic smoothing splines by nonlinear regression, and the extent of smoothing is expressed in terms of equivalent degree of freedom (edf). The percentiles were calculated by using the L, M, and S curves applying the method for constructing normalized growth standards.14,15
In this study, the standardized growth curves were constructed for weight, length, head circumference, and BMI (kg/m2) according to gender by using the LMS Chartmaker.17 For each curve, deviance test and Q test were conducted conjointly to identify the best model and evaluate its goodness of fit. Change in deviance statistic between 2 competing models approximately follows χ2 distribution with corresponding change in edf. For example, a 4-unit change in deviance statistic per 1-edf change was considered as a significant improvement (or deterioration) in the model fit at a .05 α level. In addition, the Q test determines the adequacy of the edf by testing whether the SD scores or z scores calculated from the L, M, and S curves are normally distributed independent of age.18,19 If the z scores fell within the 95% confidence intervals (CIs) across the edf continuum, the model was considered to provide adequate fit. The standardized growth curve for weight/length was generated using the z scores from the curves for weight and length.
RESULTS
No significant differences in growth measurements were seen when comparing the data among infants (boys or girls) with PWS having the 15q11-q13 deletion or other genetic defects including maternal disomy 15. Therefore, the boys and girls that represented each genetic subtype were grouped in the production of individual standardized growth curves for each gender.
Figures 1 through 5 show standardized growth curves for weight, length, head circumference, BMI, and weight/length with 7 percentiles (3rd, 10th, 25th, 50th, 75th, 90th, and 97th) for both male and female infants with PWS aged 0 to 36 months. For female weight (edf = 1,4,2), length (edf = 1,6,2), and head circumference (edf = 1,5,4), normality of data holds and thus no transformation was necessary (ie, L = 1). For male weight (edf = 2,6,3), length (edf = 0,7,2), head circumference (edf = 2,6,3), and BMI (edf = 0,8,4) and female BMI (edf = 0,5,1), Box-Cox transformation preceded the LMS smoothing procedure for curve development.
FIGURE 1.
Standardized curves for weight of male (upper) and female (lower) infants with PWS (solid lines) and normative 50th percentile (broken line).
FIGURE 5.
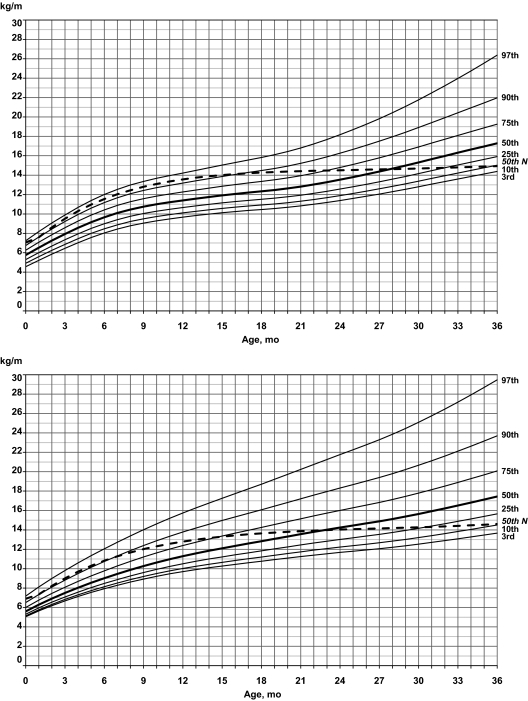
Standardized curves for weight/length of male (upper) and female (lower) infants with PWS (solid line) and normative 50th percentile (broken line).
FIGURE 2.
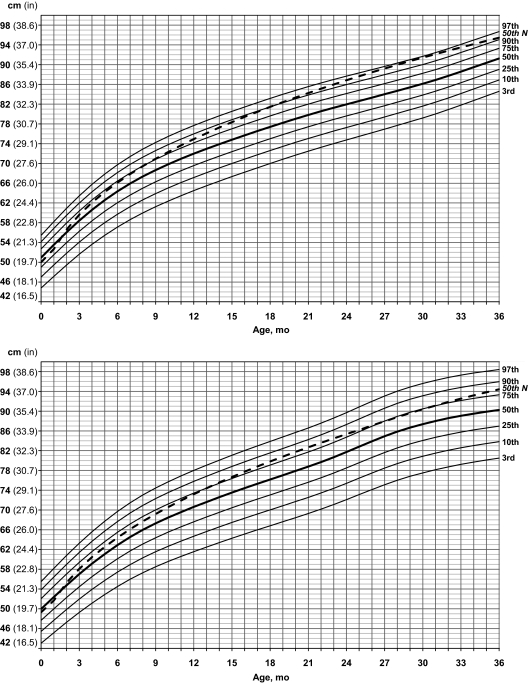
Standardized curves for length of male (upper) and female (lower) infants with PWS (solid lines) and normative 50th percentile (broken line).
FIGURE 3.
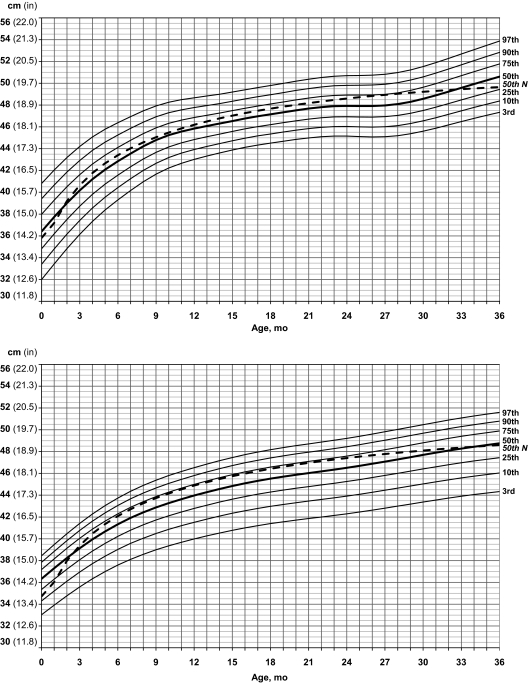
Standardized curves for head circumference of male (upper) and female (lower) infants with PWS (solid lines) and normative 50th percentile (broken line).
FIGURE 4.
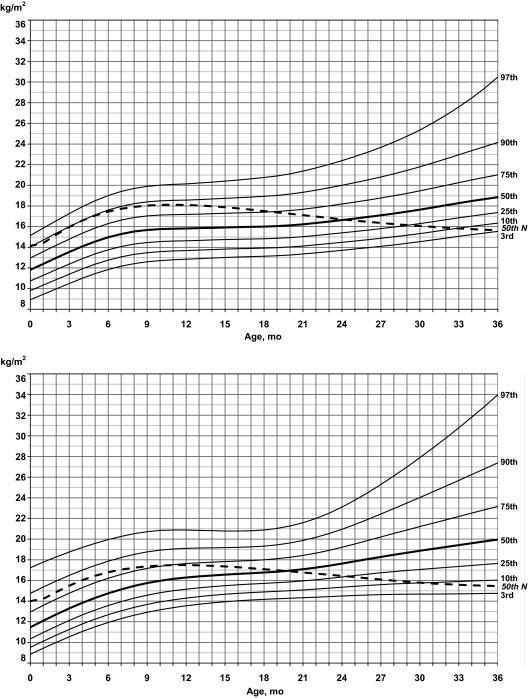
Standardized curves for BMI of male (upper) and female (lower) infants with PWS (solid lines) and normative 50th percentile (broken line).
For weight in infant boys with PWS, the normative 50th percentile generated from the 2003 CDC national infant growth data set followed the 90th percentile for PWS beginning at 2 months of age and continued to 18 months. At this point, weight increased rapidly with the normative 50th percentile following the 25th percentile for PWS by 36 months of age. For weight in infant girls with PWS, the normative 50th percentiles followed between the 75th and 90th percentiles until ∼15 months of age; weight increased rapidly with the normative 50th percentile following the 25th percentile for PWS by 36 months of age.
For length in boys with PWS, the normative 50th percentile was greater than the 50th percentile for PWS by 2 months of age, then crossed the 75th percentile in PWS by 9 months and the 90th percentile by 21 months then remained between the 90th and 97th percentiles until 36 months of age. A similar pattern was seen early for girls with PWS, and the normative 50th percentile was equal to the 50th percentile for PWS at 2 months of age; by 14 months, the normative 50th percentile followed the 75th percentile in PWS until ∼36 months of age.
For head circumference in boys with PWS, the normative 50th percentile was equal to the 50th percentile in PWS at 2 months of age and then plotted between the 50th and 75th percentiles until 32 months of age; the normative 50th percentile then fell below the 50th percentile in PWS and leveled off at the 25th percentile for PWS by 36 months of age. A similar pattern was observed for the girls with PWS for head circumference, with the normative 50th percentile equal to the 50th percentile for PWS at 2 months of age and followed the 75th percentile from 6 to 24 months before leveling off at the 50th percentile in PWS by 36 months of age.
Growth charts from the CDC are not available for BMI for infants younger than 2 years of age because of rapid changes in growth in both body weight and length during this time.20 However, standardized curves were generated for both BMI and weight/length using normative data from the CDC for comparison purposes with PWS infants and other similarly affected infants. For BMI in boys with PWS, the normative 50th percentile followed the 90th percentile for PWS until ∼6 months of age when it gradually dropped to the 75th percentile at 18 months, then to the 50th percentile at 24 months, and to the 3rd percentile by 36 months. For BMI in girls with PWS, the normative 50th percentile followed between the 75th and 90th percentiles until 10 months of age, when it dropped to the 75th percentile in PWS, then the 50th percentile by 20 months, and to less than the 10th percentile by 36 months of age.
For weight/length in boys with PWS, the normative 50th percentile followed between the 90th and 97th percentiles for PWS until 15 months, when it dropped to the 90th percentile; it then dropped to the 75th percentile at 23 months and to the 10th percentile by 36 months of age. For weight/length in girls with PWS, the normative 50th percentile followed the 90th percentile until 8 months; it then dropped to the 75th percentile by 15 months, to the 50th percentile by 23 months, and to the 10th percentile by 36 months of age.
DISCUSSION
Anthropometric standards have been developed for several syndromes and have been used successfully in the medical management of patients.21,22 The use of growth standards has become routine in most clinical genetics centers for monitoring growth and development of patients with certain syndromes (eg, Down syndrome). Medical care of people who have PWS is complex, particularly the monitoring of abnormal growth parameters (eg, weight, height) before and during growth hormone therapy often prescribed in infancy because of growth hormone deficiency and early recognition and diagnosis. Growth hormone deficiency in people with PWS has responded favorably, with increased length, decreased fat mass, and increased physical activity and strength.3,4,23,24
CONCLUSIONS
The use of our anthropometric growth standards should be helpful for those with PWS and being treated with growth hormone. Therefore, the use of PWS standards in infancy should assist the clinician and dietitian in monitoring nutrition, growth, and development. The standards reported herein may also be useful for assisting in identifying those with PWS, for increasing awareness of the syndrome, and for natural history studies. We would encourage the use of these standards in the examination of infants with PWS and for comparison of others similarly affected and in relationship with normative data.
ACKNOWLEDGMENTS
This consortium on the natural history study of Prader-Willi, Angleman, and Rett syndromes is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network. Funding and/or programmatic support for this project has been provided by NIH U54 grants HD061222 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and RR019478 (NCRR) from the NIH Office of Rare Diseases Research.
We thank the families and infants with PWS who participated in this study, the Prader-Willi Syndrome Association (USA), and several research assistants and students. We thank Carla Meister for expert preparation of the manuscript and Cindy Yoo for help in data retrieval. We acknowledge the gracious participation and provision of information by families in the Rare Disease Natural History Study, for which Dr Mary Lou Oster-Granite (Health Scientist Administrator, Eunice Kennedy Shriver National Institute of Child Health and Human Development) provided invaluable guidance, support, and encouragement.
The views expressed in this article do not necessarily reflect the official policies of the US Department of Health and Human Services, and mention of trade names, commercial practices, or organizations does not imply endorsement by the US government.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- PWS
- Prader-Willi syndrome
- CDC
- Centers for Disease Control and Prevention
- edf
- equivalent degree(s) of freedom
- CI
- confidence interval
- NIH
- National Institutes of Health
REFERENCES
- 1. Prader A, Labhart A, Willi H. A syndrome characterized by obesity, small stature, Cryptorchidism and oligophrenia following a myotonia-like status in infancy [in German]. Schweiz Med Wochensshr. 1956;86:1260–1261 [Google Scholar]
- 2. Butler MG. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet. 1990;35(3):319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler MG, Lee PDK, Whitman BY. Management of Prader-Willi Syndrome. 3rd ed New York, NY: Springer; 2006 [Google Scholar]
- 4. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(11):4183–4197 [DOI] [PubMed] [Google Scholar]
- 5. Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet. 2009;17(1):3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23(3):793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler MG, Haynes JL, Meaney FJ. Intra-familial and mid-parental child correlations and heritability estimates of anthropometric measurements in Prader-Willi syndrome families. Dysmorph Clin Genet. 1990;4:2–6 [PMC free article] [PubMed] [Google Scholar]
- 8. Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinologist. 2000;10(4):3S–16S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bittel DC, Butler MG. Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med. 2005;7(14):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eiholzer U, Gisin R, Weinmann C, et al. Treatment with human growth hormone in patients with Prader-Labhart-Willi syndrome reduces body fat and increases muscle mass and physical performance. Eur J Pediatr. 1998;157(5):368–377 [DOI] [PubMed] [Google Scholar]
- 11. Butler MG, Meaney JF. Standards for selected anthropometric measurements in Prader-Willi syndrome. Pediatrics. 1991;88(4):853–860 [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention Percentile data files with LMS values. Available at: www.cdc.gov/growthcharts/percentile_data_files.htm Accessed August 4, 2010
- 13. Weiner JS, Lourie JA. Human Biology: A Guide to Field Methods. Oxford, United Kingdom: Blackwell Scientific Publications; 1969. International Biological Programme Handbook No. 9 [Google Scholar]
- 14. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60 [PubMed] [Google Scholar]
- 15. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319 [DOI] [PubMed] [Google Scholar]
- 16. Green PJ. Penalized likelihood for general semi-parametric regression models. Int Stat Rev. 1987;55:245–259 [Google Scholar]
- 17. Pan H, Cole TJ. LMS Chartmaker, A Program for Calculating Age-Related Reference Centiles Using the LMS Method [computer program]. Version 2.4. London, United Kingdom: Medical Research Council; 2009 [Google Scholar]
- 18. Pan H, Cole TJ. A comparison of goodness of fit tests for age-related reference ranges. Stat Med. 2004;23(11):1749–1765 [DOI] [PubMed] [Google Scholar]
- 19. Royston P, Wright EM. Goodness-of-fit statistic for age-specific reference intervals. Stat Med. 2000;19(21):2943–2962 [DOI] [PubMed] [Google Scholar]
- 20. Davies PS, Lucas A. Quetelet's index as a measure of body fatness in young infants. Early Hum Dev. 1989;20(2):135–141 [DOI] [PubMed] [Google Scholar]
- 21. Meaney FJ, Farrer LA. Clinical anthropometry and medical genetics: a compilation of body measurements in genetic and congenital disorders. Am J Med Genet. 1986;25(2):343–359 [DOI] [PubMed] [Google Scholar]
- 22. Hall JG, Froster-Iskenius UG, Allanson JE. Handbook of Normal Physical Measurements. Oxford, England: Oxford University Press; 1989 [Google Scholar]
- 23. Myers SE, Whitman BY, Carrel AL, Moerchen V, Bekx MT, Allen DB. Two years of growth hormone therapy in young children with Prader-Willi syndrome: physical and neurodevelopmental benefits. Am J Med Genet A. 2007;143(5):443–448 [DOI] [PubMed] [Google Scholar]
- 24. Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2010;95(3):1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]