Abstract
OBJECTIVE:
The purpose of this study was to evaluate the angle for performing lumbar punctures in children aged 0 to 12 years. We hypothesized that the angle changes for different stages of development.
METHODS:
Children aged 0 to 12 years who presented to the Yale–New Haven Children's Hospital at a low-acuity triage level, in need of a lumbar puncture, their accompanying siblings and authors' children were eligible for the study. Subjects in 3 age groups were recruited and grouped as follows: group 1, 0 to 12 months; group 2, 12 to 36 months; and group 3, 3 to 12 years. Ultrasound images of the L3-L4 and L4-L5 lumbar space were taken with subjects in the lateral recumbent and sitting positions. The angle from the interspinous space to the skin was measured.
RESULTS:
Thirty-six subjects were included. The mean angles in the lateral recumbent and sitting positions were group 1, 47.8° (SD: 8.2) and 51.1° (SD: 8.5), respectively; group 2, 58.8° (SD: 6.8) and 59.6° (SD: 5.5); and group 3, 60.5° (SD: 6.6) and 61.9° (SD: 4.0). The results of group 1 were significantly different from those of groups 2 or 3 in both positions (lateral recumbent P = .00526 and 0.00160; sitting P = .0499 and .00282).
CONCLUSIONS:
The angle for lumbar puncture was more acute for infants than for older children in this study. Future studies should assess the difference in success rates of lumbar punctures when clinicians have knowledge of these angles.
Keywords: lumbar puncture, ultrasound, angle, interspinous space
WHAT'S KNOWN ON THIS SUBJECT:
Ultrasound has been used to characterize the depth and width of the interspinous space. A study by Abo et al found that the maximum width of the interspinous space is in the sitting position with flexed hips.
WHAT THIS STUDY ADDS:
This study further characterizes the interspinous space by describing the optimal angle at which to point the needle. We found that the optimal angle for performing a lumbar puncture is ∼50° in infants and 60° in children aged 1 to 12 years.
Lumbar puncture (LP) for cerebrospinal fluid evaluation is a common procedure used in pediatrics for the assessment of patients with suspected sepsis and meningitis. It involves inserting a needle into the interspinous space of the lower lumbar vertebrae into the subarachnoid space to collect a sample of cerebrospinal fluid. If the needle is not inserted along the interspinous space, it will encounter bone and have to be withdrawn and redirected. An LP is considered successful when a sufficient amount of nonbloody cerebrospinal fluid is collected. Failed attempts include those that contain peripheral blood or no spinal fluid at all. Success rates of LPs vary, with reported rates ranging from 45% to 54.2%.1,2 This procedure is especially challenging in infants because the target space is small.3 Furthermore, respiratory status can be compromised with positioning.4,5 It is also difficult in obese patients, as the bony landmarks are often obscured by subcutaneous fat. Repeated attempts of the potentially painful procedure are often required and have been shown to increase the incidence of postdural puncture headache in adult patients.6 Failed LPs can lead to unnecessary hospitalizations and prolonged use of antibiotics.7 Although there have been prospective studies concerning the variables associated with successful LPs (eg, resident experience, patient position, draping), knowledge of the angle of the LP needle in relation to the skin may improve the success rates of this difficult procedure.8,9
Ultrasound has been used to guide LPs. Specifically, it has been used to measure the depth to the epidural space, with high correlation to actual values.10,11 In addition, it has been shown to be useful in pediatrics in guiding LPs.12 However, musculoskeletal applications using ultrasound in a clinical setting is a relatively new field in pediatric emergency medicine. In a recently published study in Pediatrics, Abo et al3 found, using ultrasound, that the width of the interspinous space was the largest in the sitting position with the hips flexed. Using ultrasound to place and identify epidural catheters has been addressed in pediatric anesthesia literature.13,14 A study by Ferre et al15 used static ultrasound images in adults to identify landmarks but did not describe the angles obtained in their images. However, to the best of our knowledge no studies have determined the relationship of the angle of needle entry in children to patient factors such as age. This knowledge could provide clinicians with further guidance in deciding how to orient the needle. The goal of our study was to determine and explore the effect of position and age on the angle between the interspinous spaces and the skin. We hypothesized that the angle changes in different stages of development.
METHODS
This was a prospective observational study of a convenience sample of children aged 0 to 12 years. Informed consent was obtained through a written form in English and verbal explanation of the risks and benefits of the study. The study protocol was approved by the Yale Human Investigation Committee. The goal of the study was to enroll 10 to 20 subjects in each of the following age groups: 0 to 12 months; 12 to 36 months; and 3 to 12 years. An LP was not required as part of the subject's treatment plan.
Inclusion criteria were all patients who were categorized as triage level III or IV at the Pediatric Emergency Department of Yale–New Haven Children's Hospital and children who required an LP as part of their treatment plan. There are no specific criteria for triage level III or IV, but patients' conditions are considered to be nonurgent. In addition, siblings of patients and children of authors were also eligible. Exclusion criteria included the inability of the parents to speak or read English, any wounds or obvious trauma to the lumbar spine, skin breakdown, rash in the region of the L2 to S1 vertebrae, or if the subject's clinical condition did not allow for the patient to assume the lateral recumbent or sitting position.
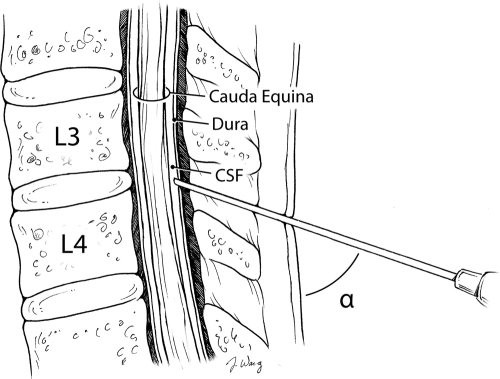
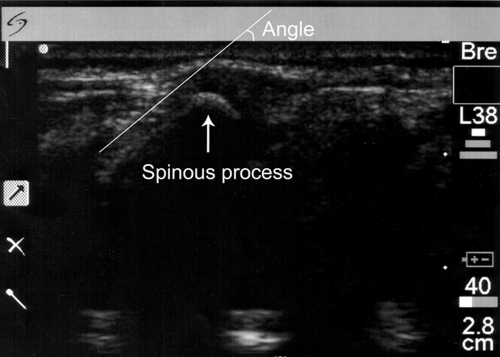
After written signed consent was obtained, the subject's birth date, date of study, weight, and gender were recorded. Ultrasound images were obtained using a SonoSite 180 (Sonosite, Inc, Bothell, WA) ultrasound with an L38 high-frequency linear probe using frequencies of 8 to 12 MHz. Static images were taken in both the lateral recumbent and sitting positions (Figs 1 and 2). Staff or parents assisted in maintaining the subject's position as if the patient were actually to receive an LP. The legs were positioned with the hips flexed. The probe was covered with ultrasound gel and placed between the posterior superior iliac crests along the vertebrae that correlate to the L3-L4 and L4-L5 interspinous spaces. The patient was then positioned in the alternate position, and images were taken again. Each static image was printed and a line with a ruler was drawn from the skin to the interspinous space along the spinous process. Given that the skin was flush with the probe, the outer edge of the image was considered the skin and the horizontal line of the protractor was aligned with this edge. The angle between the spinous process and the skin was then measured (Figs 3 and 4).
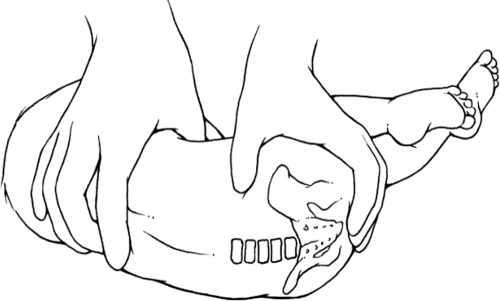
FIGURE 1.
Lateral recumbent position.
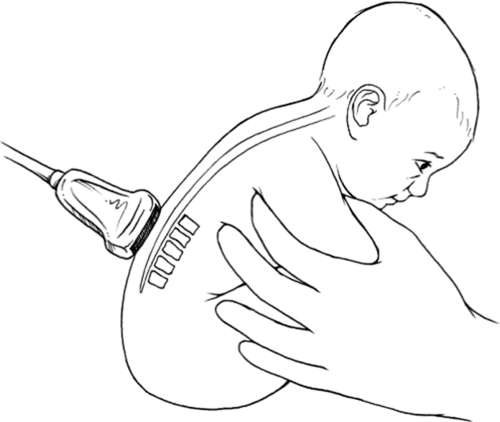
FIGURE 2.
Sitting position.
FIGURE 3.
Angle measured during the study.
FIGURE 4.
Sample image showing spinous processes.
The mean, median, and SD statistics were computed by using Excel 2007 (Microsoft, Redmond, WA) and R 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria).16 Because the sample sizes were small, the nonparametric tests of the Kruskal-Wallis test and Wilcoxon rank tests were used. These tests were performed by using the statistical program R 2.8.1. Linear and power regressions were generated by using Excel 2003 and 2007 editions.
RESULTS
Thirty-seven subjects were enrolled in the study from June 16, 2006, until December 29, 2007. Of these patients, 36 had sufficient data that could be included in the study. One subject's data were lost because of lack of disk space in the ultrasound machine. Table 1 presents the characteristics of the study subjects. Because of a lack of patient cooperation, 4 patients in the 0- to 12-month age group could only be imaged in the lateral recumbent position; the remaining 9 were imaged in both positions. The ligamentum flavum was identified in all subjects except for 1. Regardless, the slope of the spinous process in relation to the skin was used to guide the lines drawn to measure the angle.
TABLE 1.
Characteristics of Subjects Enrolled in Study
| Group | Age | n | Gender |
Lateral Recumbent Position |
Sitting Position |
|||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Age, Mean (SD), d | Weight, Mean (SD), kg | Age, Mean (SD), d | Weight, Mean (SD), kg | |||
| 1 | 0–12 mo | 13 | 9 | 4 | 117 (107) | 5.7 (2.3) | 122 (110) | 6.1 (2.5) |
| 2 | 12–36 mo | 10 | 8 | 2 | 722 (233) | 12.4 (1.6) | 722 (233) | 12.4 (1.6) |
| 3 | 3–12 y | 13 | 6 | 7 | 2210 (778) | 22.5 (6.9) | 2281 (861) | 24.3 (9.1) |
| Overall | 0–12 y | 36 | 23 | 13 | — | — | — | — |
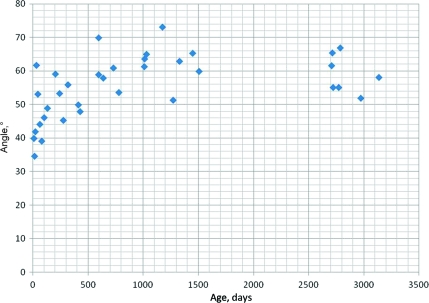
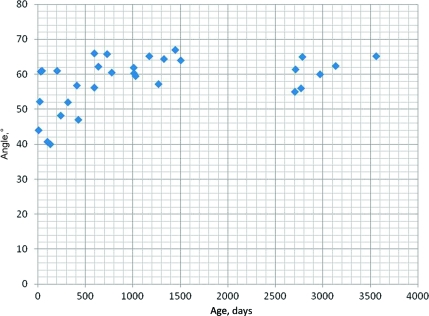
For all subjects, the mean angle was 55.3° (SD: 9.2) for the lateral recumbent position and 58.0° (SD: 7.5) for the sitting position. The mean angles in the lateral recumbent position were as follows: group 1, 47.8° (SD: 8.2); group 2, 58.8° (SD: 6.8); and group 3, 60.5° (SD: 6.6) (Fig 5). The mean angles for the sitting position were as follows: group 1, 51.1° (SD: 8.5); group 2, 59.6° (SD: 5.5); and group 3, 61.9° (SD: 4.0) (Fig 6).
FIGURE 5.
Angle versus age in the lateral recumbent position.
FIGURE 6.
Angle versus age in the sitting position.
There were significant difference between groups 1 and 2 and groups 1 and 3 and no significant difference between groups 2 and 3 in both positions (Table 2.) We also compared the angles between the lateral recumbent position and the sitting position to determine if there was a significant difference between the angles in each position. Using the Wilcoxon rank tests, the comparison between the positions for groups 1, 2, and 3 showed no significant differences (group 1: P = 1; group 2: P = .610; and group 3: P = .657). Overall, there was no significant difference between all subjects in either position (P = .565). These comparisons found that the angle did not significantly change regardless of whether the patient was in the lateral recumbent or the sitting position. The regression analyses for angle versus weight resulted in power equations for the best fit curves with R2 = 0.418 and 0.258 for the lateral recumbent and sitting positions, respectively. In terms of gender and angle, there were no significant differences between girls and boys in either position in all individual groups (Table 3).
TABLE 2.
Statistical Significance Between Angles Found in Each Group
| Group | Statistically Significant? | P |
|---|---|---|
| Lateral recumbent 1 and 2 | Yes | .00526 |
| Lateral recumbent 1 and 3 | Yes | .00160 |
| Lateral recumbent 2 and 3 | No | .510 |
| Sitting 1 and 2 | Yes | .0499 |
| Sitting 1 and 3 | Yes | .00282 |
| Sitting 2 and 3 | No | .391 |
TABLE 3.
P Values of Gender Comparisons Between Groups
| Group | Lateral Recumbent Position | Sitting Position |
|---|---|---|
| 1 | 1.00 | .106 |
| 2 | 1.00 | .296 |
| 3 | .277 | .222 |
DISCUSSION
In this pilot study, we reported statistically significant differences in the angle of needle placement in infants compared with older children. Furthermore, this difference applied to both common positions used for performing LPs. This study adds to the current literature in studying factors that could improve success rates of LPs. Considering that there are numerous pediatric LPs performed annually, it is conceivable that the results of this study could lead to increased success rates of LPs in children. Other factors that have been researched in the success rates of LPs and entering the epidural space (in the case of anesthesia) include the size and design of the needle, advancing the needle with the stylet in place, use of local anesthetic, and patient movement.8,17,18 In 1996, Kokki and Hendolin17 found higher success rates in spinal anesthesia when using a 25-gauge needle instead of a 29-gauge needle as a successful spinal blockade. Furthermore, although Kokki et al18 found no difference in the success rates of a spinal block using cutting point or pencil point needles, there was a significant difference (P = .002) with the need for repeated skin puncture. They found that the cutting point needle required less repeated punctures. Nigrovic et al9 found that young age (defined as <3 months), less physician experience with LPs, lack of anesthetic, patient movement, and advancement of the needle with a stylet in place were risk factors for unsuccessful or traumatic LPs (red blood cell count > 500 cells per μL). Of those, anesthetic use, patient movement, and advancement of the needle without the stylet in place are the most modifiable.
In terms of the ultrasound and radiographic literature, in 1 study the authors derived an equation for calculating the depth on the basis of the length of the needle inserted, with the angle of the insertion measured with a sterile protractor.19 However, this study did not report the angles measured. Our study adds to this characterization by giving a mean angle to place the needle and further guidance for clinicians performing LPs. Although the use of ultrasound in pediatric emergency departments is increasing and methods for using ultrasound to perform LPs have been described for adults, our study may help the clinician without access to ultrasound for guided procedures by providing the mean angles of needle insertion based on patient ages.20–22
Limitations of our study include the small sample size and the fact that the study did not show how the angles found in static images affected success rates. Furthermore, the study was performed with only 1 investigator (Dr Bruccoleri) measuring the angles; therefore, we were unable to test interrater reliability. Last, we did not evaluate subject height and its relationship to the angle of the interspinous space. There have been studies using height and weight together to predict needle depth to the interspinous space.23,24 Although we found a poor correlation of needle angle to weight, there could be a relationship to height and needle angle or height and weight and needle angle.
Future study designs may include randomized controlled trials to study the success of LPs with providers who are given the knowledge of the angles in this study and providers who are not. Another randomized control trial design would be to study the success rates of LPs between clinicians who are given still ultrasound images of a patient's lumbar space and those who are not. In addition, further studies could explore whether the child's ability to walk upright affects the angle of the interspinous space.
CONCLUSIONS
The angle for LP as determined by ultrasonography in this study was ∼50° in infants and 60° in children aged 1 to 12 years in both the lateral recumbent and sitting positions. The differences in these angles between ages were statistically and clinically significant. Further studies need to be performed to determine if the results of this anatomic study lead to improved outcomes of LPs.
ACKNOWLEDGMENTS
This study was funded by the Department of Pediatrics at Yale School of Medicine. The Office of Student Research funded the Summer Research Fellowship program during 2006. This work was supported in part by grant KL2 RR024138 from the Clinical & Translational Science Awards program of the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
We thank Dr Rajiv Menjoge for help in understanding the statistical analysis, the staff and physicians at the Pediatric Emergency Department of Yale–New Haven Children's Hospital for help in finding subjects and obtaining images, Jenny Wang for illustrations, and the Yale Adult Emergency Department for use of the SonoSite 180 machine.
Dr Bruccoleri acquired data, performed the statistical analysis with the assistance of Dr Menjoge (as noted in the Acknowledgments), and drafted and edited the manuscript, and Dr Chen conceived the idea for the study, obtained institutional review board approval, acquired data, and revised and edited the manuscript. Drs Bruccoleri and Chen both approved the article that was submitted.
FINANCIAL DISCLOSURE: Dr Bruccoleri owns 16 of 394.6 million outstanding shares in Stryker, which is involved in making ultrasound equipment. Dr Chen has indicated he has no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- LP
- lumbar puncture
REFERENCES
- 1. Pinheiro JM, Furdon S, Ochoa LF. Role of local anesthesia during lumbar puncture in neonates. Pediatrics. 1993;91(2):379–382 [PubMed] [Google Scholar]
- 2. Schreiner RL, Kleiman MB. Incidence and effect of traumatic lumbar puncture in the neonate. Dev Med Child Neurol. 1979;21(4):483–487 [DOI] [PubMed] [Google Scholar]
- 3. Abo A, Chen L, Johnston P, Santucci K. Positioning for lumbar puncture in children evaluated by bedside ultrasound. Pediatrics. 2010;125(5). Available at: www.pediatrics.org/cgi/content/full/125/5/e1149 [DOI] [PubMed] [Google Scholar]
- 4. Gleason CA, Martin RJ, Anderson JV, Carlo WA, Sanniti KJ, Fanaroff AA. Optimal position for a spinal tap in preterm infants. Pediatrics. 1983;71(1):31–35 [PubMed] [Google Scholar]
- 5. Weisman LE, Merenstein GB, Steenbarger JR. The effect of lumbar puncture position in sick neonates. Am J Dis Child. 1983;137(11):1077–1079 [DOI] [PubMed] [Google Scholar]
- 6. Seeberger MD, Kaufmann M, Staender S, Schneider M, Scheidegger D. Repeated dural punctures increase the incidence of postdural puncture headache. Anesth Analg. 1996;82(2):302–305 [DOI] [PubMed] [Google Scholar]
- 7. Mazor SS, McNulty JE, Roosevelt GE. Interpretation of traumatic lumbar punctures: who can go home [published correction appears in Pediatrics. 2003;111(5):1127]? Pediatrics. 2003;111(3):525–528 [PubMed] [Google Scholar]
- 8. Baxter AL, Fisher RG, Burke BL, Goldblatt SS, Isaacman DJ, Lawson ML. Local anesthetic and stylet styles: factors associated with resident lumbar puncture success. Pediatrics. 2006;117(3):876–881 [DOI] [PubMed] [Google Scholar]
- 9. Nigrovic LE, Kuppermann N, Neuman MI. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med. 2007;49(6):762–771 [DOI] [PubMed] [Google Scholar]
- 10. Currie JM. Measurement of the depth to the extradural space using ultrasound. Br J Anaesth. 1984;56(4):345–347 [DOI] [PubMed] [Google Scholar]
- 11. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513–516 [DOI] [PubMed] [Google Scholar]
- 12. Coley BD, Shiels WE, II, Hogan MJ. Diagnostic and interventional ultrasonography in neonatal and infant lumbar puncture. Pediatr Radiol. 2001;31(6):399–402 [DOI] [PubMed] [Google Scholar]
- 13. Rapp HJ, Folger A, Grau T. Ultrasound-guided epidural catheter insertion in children. Anesth Analg. 2005;101(2):333–339, table of contents [DOI] [PubMed] [Google Scholar]
- 14. Tsui BC, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology. 2010;112(3):719–728 [DOI] [PubMed] [Google Scholar]
- 15. Ferre RM, Sweeney TW, Strout TD. Ultrasound identification of landmarks preceding lumbar puncture: a pilot study. Emerg Med J. 2009;26(4):276–277 [DOI] [PubMed] [Google Scholar]
- 16. R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009, 2010. Available at: www.r-project.org [Google Scholar]
- 17. Kokki H, Hendolin H. Comparison of 25 G and 29 G Quincke spinal needles in paediatric day case surgery: a prospective randomized study of the puncture characteristics, success rate and postoperative complaints. Paediatr Anaesth. 1996;6(2):115–119 [DOI] [PubMed] [Google Scholar]
- 18. Kokki H, Heikkinen M, Turunen M, Vanamo K, Hendolin H. Needle design does not affect the success rate of spinal anaesthesia or the incidence of postpuncture complications in children. Acta Anaesthesiol Scand. 2000;44(2):210–213 [DOI] [PubMed] [Google Scholar]
- 19. Kil HK, Cho JE, Kim WO, Koo BN, Han SW, Kim JY. Prepuncture ultrasound-measured distance: an accurate reflection of epidural depth in infants and small children. Reg Anesth Pain Med. 2007;32(2):102–106 [DOI] [PubMed] [Google Scholar]
- 20. Chen L, Baker MD. Novel applications of ultrasound in pediatric emergency medicine. Pediatr Emerg Care. 2007;23(2):115–123 [DOI] [PubMed] [Google Scholar]
- 21. Murphy M, Nagdev A. Focus On: Ultrasound Guided Lumbar Puncture. ACEP News. 2007;September:23–25 [Google Scholar]
- 22. Nomura JT, et al. A Randomized Controlled Trial of Ultrasound-Assisted Lumbar Puncture. J Ultrasound Med. 2007;26(10):1341–1348 [DOI] [PubMed] [Google Scholar]
- 23. Chong SY, Chong LA, Ariffin H. Accurate prediction of the needle depth required for successful lumbar puncture. Am J Emerg Med. 2010;28(5):603–606 [DOI] [PubMed] [Google Scholar]
- 24. Abe KK, Yamamoto LG, Itoman EM, Nakasone TAF, Kanayama SK. Lumbar puncture needle length determination. Am J Emerg Med. 2005;23(6):742–746 [DOI] [PubMed] [Google Scholar]