Abstract
Hepatitis C virus (HCV) infection usually progresses to chronic hepatitis, with rare cases of spontaneous viral eradication. We present herein four cases involving patients that were initially declared to have failed to respond to treatments, based on the presence of HCV RNA that was still detectable after completion of the standard treatment for chronic hepatitis C with genotype 2. However, the HCV RNA became undetectable, with a delayed response, after discontinuation of therapy. Two of the four patients were diagnosed as treatment failures after extended treatment, and the other two received no further treatment after the standard treatment. All four patients maintained a sustained virological response during the periodic follow-up after delayed viral clearance.
Keywords: Delayed viral clearance, Chronic hepatitis C, Sustained virological response
INTRODUCTION
Chronic hepatitis C, one of the main causes of chronic liver disease and liver cirrhosis, is highly responsive to combination therapy with pegylated interferon and ribavirin; active treatment is recommended if a patient shows treatment tolerance and a good treatment response. For genotype 1, if early virological response (EVR) occurs, 75% of the patients achieve sustained virological response (SVR), and if it does not occur, 97% of the patients do not achieve SVR. Therefore, for patients with genotype 1, EVR can be used as a predictor of SVR.1 For patients with genotype 2, however, EVR occurs in 97% of patients, with about 80% achieving SVR; therefore, EVR cannot be used as a predictor of SVR.2 With genotype 2, some patients that have an EVR after receiving standard treatment will be diagnosed with treatment failure without achieving a SVR. For patients that have received standard treatment, retreatment is not recommended. This is because using the same regimen results in a less than 5% response rate.3 A spontaneous viral eradication, after infection with chronic hepatitis C, is rare. The loss of hepatitis C virus (HCV) RNA due to a delayed virological response with SVR maintenance is also rarely reported.4,5 Presented herein are two cases that were initially diagnosed as treatment failures after three months of extended treatment after their standard treatment for chronic hepatitis C. In addition, another two cases were initially diagnosed to have had treatment failure after the standard treatment. All four patients later achieved SVR by delayed viral clearance during follow-up.
CASE REPORT
All four patients presented herein, had an EVR after standard treatment (pegylated interferon alfa 2a and ribavirin) for chronic hepatitis C, but did not achieve end-of-treatment response (ETR). Among them, two patients received 12-week extended treatments after the standard treatment; the other two were followed as outpatients after treatment failure.
1. Patient 1
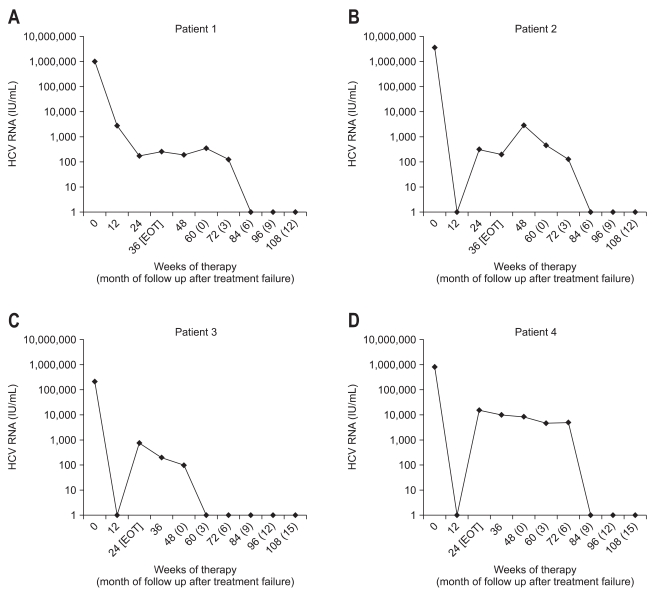
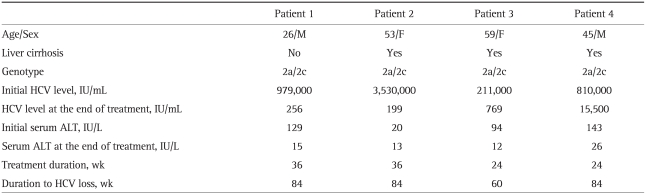
A 26-year-old male had an ALT 129 IU/L, HCV genotype 2a/2c, and serum HCV RNA 979,000 IU/mL. The abdominal ultrasound showed chronic liver disease; however, a liver biopsy was not performed. At 12-weeks of treatment, HCV-PCR testing showed a partial EVR (2,800 IU/mL). HCV RNA was detectable on qualitative HCV-PCR testing that was conducted at the treatment end point (24 weeks). It was still detectable even after 12-weeks of extended treatment with the same drugs as used during the initial standard treatment (HCV RNA level, 256 IU/mL). The patient did not achieve a SVR at 24 weeks after termination of the extended-treatment; HCV RNA was still detectable. Since then, a quantitative HCV RNA assay was carried out every three months. Six months after treatment failure was diagnosed, HCV RNA negativity was found; HCV RNA was undetectable by quantitative HCV RNA testing. Follow-up testing over more than two years showed SVR maintenance with no HCV RNA detected (Fig. 1A).
Fig. 1.
Serum hepatitis C virus (HCV) RNA levels during therapy and follow-up of patients with posttreatment delayed viral clearance. (A) Patient 1. (B) Patient 2. (C) Patient 3. (D) Patient 4.
EOT, end of treatment.
2. Patient 2
A 53-year-old female had an ALT 20 IU/L, HCV genotype 2a/2c, serum HCV RNA 3,530,000 IU/mL and platelet count 138,000/uL. The abdominal ultrasonography showed liver cirrhosis, but a liver biopsy was not performed. Clinically, the patient had compensated liver cirrhosis; the HCV RNA became undetectable (complete EVR was achieved) after the 12 week standard treatment. HCV RNA was detectable at the treatment end point, indicating a virological breakthrough. HCV RNA was still detectable even after the 12-week extended treatment using the same regimen as used in the standard treatment (HCV RNA level, 199 IU/mL). SVR was not achieved as HCV RNA was still detectable at 24 weeks after extended-treatment was discontinued. Since then, the quantitative HCV RNA assay was performed every three months. The follow-up test after six months showed that the HCV RNA was negative. SVR was maintained as the HCV RNA was not detected in the follow-up test for up to two years (Fig. 1B).
3. Patient 3
A 59-year-old female had an ALT level of 143 IU/L, HCV genotype 2a/2c, serum HCV-PCR 211,000 IU/mL and platelet count 74,000/uL. The abdominal ultrasonography showed liver cirrhosis; however, a liver biopsy was not performed. Clinically, the patient was diagnosed with compensated liver cirrhosis. The quantitative HCV-PCR after 12 weeks of standard treatment showed that the HCV RNA was undetectable (complete EVR was achieved). However, the testing performed at 24 weeks showed detectable HCV RNA, indicating a virological breakthrough and ETR failure (HCV RNA level, 769 IU/mL). The HCV RNA testing at 24 weeks after treatment termination showed that it was still detectable (SVR was not achieved). The quantitative HCV RNA assay performed three months later showed negative HCV RNA, and this was confirmed by the qualitative HCV RNA testing. SVR was maintained based on further follow-up tests with no HCV RNA detected (Fig. 1C).
4. Patient 4
A 45-year-old male had an ALT level of 94 IU/L, HCV genotype 2a/2c, serum HCV PCR 810,000 IU/mL and platelet count 131,000/uL. The abdominal ultrasonography showed liver cirrhosis; however, a liver biopsy was not carried out. Clinically, the patient was diagnosed with compensated cirrhosis. After the 12-week standard treatment, HCV RNA became undetectable on the HCV-PCR testing (complete EVR was achieved). The peripheral blood test at 10 weeks of treatment showed a decrease in the absolute neutrophil count and a 25% dose reduction of the pegylated interferon was provided. As a decrease in the absolute neutrophil count progressed, the pegylated interferon was discontinued at 11 weeks of treatment. By 15 weeks of treatment, the absolute neutrophil count recovered; the administration of pegylated interferon started again. At 24 weeks of treatment, the HCV-PCR showed detectable HCV RNA, indicating a virological breakthrough (HCV RNA level, 15,500 IU/mL). Testing at 24 weeks after treatment termination, showed detectable HCV RNA (SVR was not achieved). Since then, the quantitative HCV RNA assay has been performed every three months. At nine months the HCV RNA was negative, and the qualitative HCV RNA test confirmed that the HCV RNA was undetectable. Since then, the SVR has been maintained as HCV RNA has not been detected in follow-up testing for up to 1.5 years (Fig. 1D).
DISCUSSION
For the treatment of chronic hepatitis C, a combination of pegylated interferon and ribavirin is used as standard treatment, with about an 80% treatment response rate, particularly for patients with genotype 2.6 In some patients with HCV genotype 2, SVR is not achieved without reaching ETR, even though an EVR is achieved. As such, if the patient becomes a non-responder after treatment for chronic hepatitis C, the response rate will remain only at 8-10% even if the patient is retreated with pegylated interferon alfa 2a or alfa 2b and ribavirin is again provided for treatment. Some studies have shown that combination therapy with pegylated interferon and ribavirin, with the addition of thymosin alpha 1, as an immunomodulator for non responders that were previously treated with pegylated interferon alfa 2a and ribavirin, was effective.7,8 The current AASLD guidelines do not recommend that retreatment be used for the purpose of HCV eradication in patients previously treated with pegylated interferon and ribavirin.9
However, some studies showed that for patients with genotype 1, the extension of the treatment period from 48 to 72 weeks for slow responders resulted in an elevated SVR without increased side effects.10 However, confirmation of this finding is needed; there have been no studies on the effectiveness of extended therapy for slow responders or non-responders in patients with HCV genotypes 2 or 3. For patient 1 and 2 reported here that received extended therapy its effect on the delayed virological response or SVR was not determined. Although the standard guidelines do not recommend the measurement of EVR in patients with genotype 2, it was nonetheless performed to evaluate the intermediate response of the treatment. Of the four patients who showed delayed viral clearance, three showed complete EVR and the other showed partial EVR. Based on this result, it was postulated that delayed viral clearance is related with the significant reduction in the HCV RNA level in early treatment.
In two cases the serum HCV RNA became undetectable; either as a very rare spontaneous event or as the result of antiviral treatment. On the basis of the scant data available, HCV clearance occurs in about 0.5% of non-treated, chronically infected subjects every year. However, there is no definite evidence of spontaneous viral clearance.11 There have been reports that spontaneous serum HCV RNA clearance occurred in clinically changed environments accompanied by large liver cancer tumors,12 acute hepatitis B virus (HBV) superinfection,13 immune reconstitution after antiretroviral therapy for human immunodeficiency virus (HIV) infection,14 withdrawal of immunosuppressive therapy after solid-organ transplantation,15 pregnancy,16 and total gastrectomy procedures for gastric cancer.17 Delayed HCV RNA clearance rarely occurs; however, in nonresponders, patients with viral breakthrough, and patients with relapses after treatment for chronic hepatitis C. In the two studies that reported delayed clearance, one patient had genotype 1b, two had 2a/2c, and four had 3a.4,5 Of the 340 patients who received standard treatment for chronic hepatitis C, 86 were nonresponders. Among them, four patients, all with genotype 2a/2c, showed delayed viral clearance (Table 1). In the cases reported here, there were no co-morbid diseases or conditions, such as viral superinfection, liver cancer, surgery, pregnancy, and immunotherapy. Up to now, there has been no single case reporting more than four patients with genotype 2 who showed delayed viral clearance. Further clinical studies are thus required to obtain clinical data showing that a delayed response is more apt to occur in patients with genotype 2 than in patients with other genotypes.
Table 1.
Treatment Course in Patients with Delayed Viral Clearance
HCV, hepatitis C virus.
The mechanism involved in the HCV RNA conversion to a negative status by late post-treatment viral clearance is unknown. Given that many patients in the cases reported so far and that were directly experienced by these authors were over 40 years of age and either with obesity, a high HCV RNA level, or liver cirrhosis, the factors that show resistance to the treatment are postulated to be related with the mechanism. As in the case of patient 4, the ineffective therapy based on the side effects of the treatment drug is also seen as related to the delayed response, although the ineffective therapy and the delayed response have nothing in common that perfectly accords with each other. One possible explanation might be due to changes in the immune response.5 Reduction of viral replication or enhancement of anti-HCV host defenses has been suggested to precede viral clearance: e.g., the presence of very large cancer tumors, HBV superinfection, the administration of immunotherapy, pregnancy, or a malignancy. One study showed a delayed biochemical response after chronic hepatitis C therapy was associated with baseline hepatic fibrosis and liver cirrhosis.18 Three of the four patients reported here had liver cirrhosis by abdominal ultrasonography, and the other patient had chronic liver disease; although liver biopsies were not performed to confirm these findings. Advanced liver disease has been associated with delayed virological response.
A common feature among the cases was delayed viral clearance with a significant reduction in the HCV RNA levels during therapy. In all four delayed responders the serum HCV RNA at the end of treatment was decreased by more than 2 log units within 12 weeks. Annicchiarico et al.5 also reported that in cases with delayed HCV RNA clearance, in a patient with a relapse, breakthroughs and non-responders were associated with a significant decrease in the HCV RNA titer at the end of therapy. The mechanism responsible for delayed viral clearance requires further study.
References
- 1.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 2.Berg T, Carosi G. Optimizing outcomes in patients with hepatitis C virus genotype 2 or 3. Antivir Ther. 2008;13(Suppl 1):17–22. [PubMed] [Google Scholar]
- 3.Cheruvattath R, Rosati MJ, Gautam M, Vargas HE, Rakela J, Balan V. Pegylated interferon and ribavirin failures: is retreatment an option? Dig Dis Sci. 2007;52:732–736. doi: 10.1007/s10620-006-9457-x. [DOI] [PubMed] [Google Scholar]
- 4.Denis J, Rautou PE, Lambare B, Herbert S, Auray-Cartier V, Asselah T. Chronic hepatitis C: viral clearance several months after discontinuation of therapy in two non-responders. Infection. 2007;35:197–200. doi: 10.1007/s15010-007-6099-7. [DOI] [PubMed] [Google Scholar]
- 5.Annicchiarico BE, Siciliano M, Avolio AW, Grillo RL, Bombardieri G. A 5-year prospective study of the late resolution of chronic hepatitis C after antiviral therapy. Aliment Pharmacol Ther. 2007;25:1039–1046. doi: 10.1111/j.1365-2036.2007.03295.x. [DOI] [PubMed] [Google Scholar]
- 6.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 7.Baek YH, Lee SW, Yoo HS, et al. Thymosin alpha-1 in combination with pegylated interferon and ribavirin in chronic hepatitis C patients who have failed to prior pegylated interferon and ribavirin treatment. Gut Liver. 2007;1:87–89. doi: 10.5009/gnl.2007.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camerini R, Ciancio A, De Rosa A, Rizzetto M. Studies of therapy with thymosin alpha1 in combination with pegylated interferon alpha2a and ribavirin in nonresponder patients with chronic hepatitis C. Ann N Y Acad Sci. 2007;1112:368–374. doi: 10.1196/annals.1415.047. [DOI] [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaglio PJ. Treatment of chronic hepatitis C in a slow responder: a case for extended therapy. Nat Rev Gastroenterol Hepatol. 2009;6:372–375. doi: 10.1038/nrgastro.2009.65. [DOI] [PubMed] [Google Scholar]
- 11.Kondili LA, Chionne P, Costantino A, et al. Infection rate and spontaneous seroreversion of anti-hepatitis C virus during the natural course of hepatitis C virus infection in the general population. Gut. 2002;50:693–696. doi: 10.1136/gut.50.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokosuka O, Kojima H, Imazeki F, et al. Spontaneous negativation of serum hepatitis C virus RNA is a rare event in type C chronic liver diseases: analysis of HCV RNA in 320 patients who were followed for more than 3 years. J Hepatol. 1999;31:394–399. doi: 10.1016/s0168-8278(99)80028-2. [DOI] [PubMed] [Google Scholar]
- 13.Wietzke P, Schott P, Braun F, Mihm S, Ramadori G. Clearance of HCV RNA in a chronic hepatitis C virus-infected patient during acute hepatitis B virus superinfection. Liver. 1999;19:348–353. doi: 10.1111/j.1478-3231.1999.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 14.Fialaire P, Payan C, Vitour D, et al. Sustained disappearance of hepatitis C viremia in patients receiving protease inhibitor treatment for human immunodeficiency virus infection. J Infect Dis. 1999;180:574–575. doi: 10.1086/314910. [DOI] [PubMed] [Google Scholar]
- 15.Somsouk M, Lauer GM, Casson D, et al. Spontaneous resolution of chronic hepatitis C virus disease after withdrawal of immunosuppression. Gastroenterology. 2003;124:1946–1949. doi: 10.1016/s0016-5085(03)00391-3. [DOI] [PubMed] [Google Scholar]
- 16.Zein CO, Abu-Lebdeh H, Zein NN. Spontaneous clearance of chronic hepatitis C during pregnancy. Am J Gastroenterol. 2001;96:3044–3045. doi: 10.1111/j.1572-0241.2001.04697.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa M, Morimoto Y, Shiroi A, Yoshiji H, Kuriyama S, Fukui H. Spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C. Am J Gastroenterol. 2001;96:922–923. doi: 10.1111/j.1572-0241.2001.03650.x. [DOI] [PubMed] [Google Scholar]
- 18.Su WP, Peng CY, Lai HC, et al. Persistent transaminase elevations in chronic hepatitis C patients with virological response during peginterferon and ribavirin therapy. Hepatogastroenterology. 2009;56:798–801. [PubMed] [Google Scholar]