Abstract
Background/Aims
Infliximab (IFX), an antibody to tumor necrosis factor, (TNF)-α has efficacy in treating Crohn's disease (CD). However, knowledge of the potential effects of IFX on patients' immune profiles is lacking. The purpose of this study was to reveal the immunological effects of IFX.
Methods
Twenty-two patients with a CD activity index (CDAI) of 194.2±92.9 and an average duration of disease of 3.26 months and 21 healthy controls were included. Patients were to have their first IFX remission induction therapy with 3 infusions (5 mg/kg) at weeks 0, 2, and 6. Oral 5-aminosalicylic acid was the only ongoing medication in the patient population. Blood samples at baseline, 12 hours after the first infusion and at week 14 were labeled with anti-CD4/CD25 antibodies for immunohistochemical measurement of regulatory T-cells (Treg). Serum cytokines and chemokines were measured by suspension array and ELISA.
Results
CDAI significantly decreased prior to the second IFX infusion (p<0.001). Clinical remission rates were 77.3% and 91% by the second and third infusions, respectively. At baseline, interleukin (IL)-6 (p<0.03), IL-8 (p<0.03), IL-10 (p=0.050), IL-13 (p<0.01), transforming growth factor-β1 (p<0.01), and 'regulated on activation, normal T cell expressed and secreted' (RANTES) (p<0.01) were elevated in patients. After the initial IFX infusion, TNF-α (p<0.04), IL-6 (p<0.03), interferon (IFN)-γ (p<0.04), IFN-γ-inducible protein-10 (p<0.01), monocyte chemoattractant protein-1 (p<0.01), macrophage inflammatory protein-1β (p<0.01), and RANTES (p<0.01) were decreased. IFX infusion was associated with an increase in Treg (p<0.01) and a decrease in the Th1 (IFN-γ)/Th2 (IL-4) ratio (p<0.03).
Conclusions
IFX use was associated with restoration of the Th1/Th2 balance after a single infusion and seemed to promote induction of naïve Th0 lymphocytes to Treg. This knowledge should have clinical relevance.
Keywords: Crohn's disease, Infliximab, Transforming growth factor-β1, RANTES, Regulatory T-cell
INTRODUCTION
Crohn's disease (CD) together with ulcerative colitis (UC) are the major phenotypes of the idiopathic inflammatory bowel disease (IBD), which afflicts millions of individuals throughout the world with symptoms that impair quality of life (QoL) and ability to function.1 Currently, the etiology of CD is not understood well. However, tissue edema, increased gut epithelial cell permeability, and extensive infiltration of the colonic mucosa by leukocytes of the myeloid lineage 2011-03-17are often pathologic features of this immune disorder.
Environmental and genetic factors in combination with the microbial flora or specific microorganisms are suspected to trigger the activation of intestinal immune response which, represent the immune pathology in CD. Immune and non-immune cells create a so-called cross talk via secretion of soluble mediators and expression of cell adhesion molecules that can aggravate the inflammation. Cytokines and chemokines are now known to have a major role in the initiation and the perpetuation of immune pathology in CD.2 In line of this thinking, the medical therapy of IBD has changed dramatically over the past decade by the introduction of 'biologics' notably anti-bodies to tumor necrosis factor (TNF)-α like infliximab (IFX).
IFX (Remicade®; Centocor, Malvern, PA, USA), a chimeric monoclonal IgG1 antibody to TNF-α, has shown efficacy in patients with moderate-to-severe CD who show an inadequate response to conventional therapy.2 Targan et al.1 reported that a single intravenous (iv) injection of IFX induced a response at four weeks in 50% to 81% of CD patients with refractory luminal disease and clinical remission in 25% to 48%. Further, the response could be maintained by repeated infusions at appropriate intervals.3 In deed, by the introducti on of IFX, the therapeutic strategies for both remission induction and maintenance in CD has changed with significant improvement in patients' QoL. Therefore, it is logical to assume that potentially, IFX might influence the patients' immune profile and such knowledge should have clinical relevance.
We thought that circulating CD4+ T cell phenotype expressing CD25High, which are known as the functional regulatory T cell (Treg) should be an appropriate lead to look at the likely effect of IFX on immune function. The Treg constitutes 5-10% of peripheral T cells in normal naïve mice and in humans, but is compromised in patients with IBD.4,5 The Treg is known to down-regulate immune responses to both foreign and selfantigens. Recently, we found that the number of circulating Treg was inversely correlated with the level of inflammation in patients with UC,4 while the number of these lymphocyte subset in the colonic mucosa, the site of UC expression increased in parallel with UC activity.5
Therefore, as the objective of the present study, we investigated the Treg expression profile in CD patients during remission induction therapy with IFX. Moreover, we looked at a broad spectrum of cytokines and chemokines, since we have hypothesized that IFX has validated efficacy in patients with CD in addition to blocking TNF-α. This biologic might influence one or more arms of the immune system. CD patients were to be steroid as well as immunomodulator (thiopurine) naïve so that we could exclude the contribution of other therapeutic interventions to maximize of chance of identifying an immune arm that was significantly impacted by IFX in the absence of another major therapeutic intervention. Our view is that monitoring the peripheral immune arms is essential for not only to understand the mode of action of biologics, including IFX, but also the underlying immune characteristics of CD.
MATERIALS AND METHODS
1. Subjects
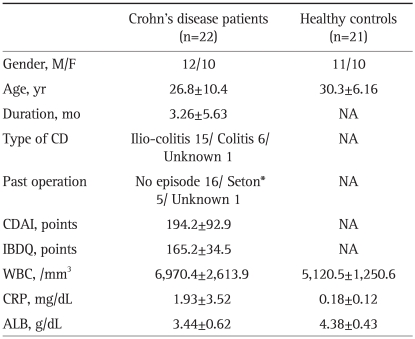
A single center prospective study was conducted in our institute from May 2007 to April 2010. Diagnosis of CD was based on the official criteria established by the research committee of the Japan Ministry of Health and in Western countries.6,7 We have requited CD patients admitted for remission induction therapy with three iv doses of IFX, and receiving IFX for the first time. Also, we have selected the potential candidate that they have had to treat with peroral 5-aminosalicylic acid (5-ASA; Pentasa®; Ferring Pharmaceuticals, Lausanne, Switzerland) alone for over 4 weeks prior to entry, with no change in dosage during this study. Patients were excluded if they had other acute or chronic diseases with known effects on the immune system or were taking corticosteroids, nutritional therapy or immunomodulators within 8 weeks prior to this study. Among the study period, total twenty-two Japanese patients with active CD, 12 men and 10 women, mean age 36.9±12.7 years together with 21 age and gender matched healthy volunteers as a control group (HC) were satisfied these conditions and included. The subjects' demography is prese nted in Table 1. The mean CD activity index (CDAI)8 was 194.2±92.9, range 153.4 to 337.0, and the mean duration of CD since diagnosis was 3.26±5.63 months, range 0.5 to 19 months. The protocol was reviewed and approved by the Institutional Ethics Committee and all subjects provided informed consent to participate in this study.
Table 1.
The Demographics of the Study Subjects upon Entry into This Study
Data are presented as mean±SD.
NA, not applicable; CD, Crohn's disease; CDAI, Crohn's disease activity index; IBDQ, inflammatory bowel disease questionnaire; WBC, white blood cell count; CRP, C-reactive protein; ALB, serum albumin.
*A rubber band seton for the management of abscess-anal fistulae.
2. IFX administration
Prior to entry, patients were investigated to be free from tuberculosis by chest X-ray, tuberculin test and pelvic MRI as well as being free from other chronic infections. The IFX remission induction therapy consisted of three iv IFX (5 mg/kg) infusions at weeks 0, 2, and 6 according to the ACCENT1 trial.3
3. Collection of the samples
From the CD patients, peripheral blood samples were obtained before and 12 hours after each IFX infusion and again 14 weeks after starting the IFX therapy. HC subjects provided blood samples at entry to this study. Obtained peripheral whole blood was immediately submitted for the Treg measurement, and the remainder was immediately submitted for centrifugal separation to obtain serum. The serum was then stored under -80℃ condition until the cytokine and chemokine measurements.
4. Measurement of Treg expression
Similar to our previous study on Treg,4,5 tests were immediately double stained with fluorochrome-conjugated monoclonal antibodies for 20 minutes at room temperature (IOTest; Immunotech, Marseille, France). Antibody combination used for double-colour fluorescence, fluorescein isothiocyanate (FITC), phycoerythrin (PE) was anti CD4CD25. Isotype-matched irrelevant FITC-, PE-labeled monoclonal antibodies served as control. Labeled tests were then washed in buffer 3 times and fixed in 2% paraformaldehyde just prior to flow cytometry. Measurements were done immediately after test preparations by using a Beckman Coulter Epics XL flow cytometer and System II software (Coulter Corp., Hialeah, FL, USA). The analysis and sort gates were restricted to the population of lymphocytes according to their specific forward and side scatter dimensions. The regulatory CD25High+CD4+ phenotype was sorted from CD4+CD25+ T-lymphocyte subpopulation.4,5 The functional Treg cells expressing high levels of the interleukin (IL)-2 receptor, and characterized by the expression of the transcription factor FOXP3 are shown as CD4+CD25High+. The flow cytometer was set to count and measure percentages and fluorescent intensities of the immune-stained lymphocytes of aspirated samples, which contained 100 µL/sample of patients' peripheral whole blood. Each measurement was continued for either 300 seconds or until 20×104 cells were counted.
5. Measurements of cytokines and chemokines
Peripheral blood samples were obtained from both the CD and the HC groups at the same time points for Treg measurement. Samples were immediately centrifuged, and the sera were stored at -80℃ until assay. Cytokines including interleukin (IL)-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, interferon (IFN)-γ, TNF-α, and chemokines including CCL2/MCP-1, CCL3/macrophage inflammatory protein (MIP)-1α, CCL4/MIP-1β, CCL5/regulated on activation, normal T-cell expressed and secreted (RANTES), CCL11/eotaxin, CXCL10/IFN-γ-inducible protein (IP)-10 were assayed by using a suspension-array method (Bio-Plex Human Cytokine Assay System; Bio-Rad Laboratories, Tokyo, Japan) according to the manufacture's protocol. In brief, a 50 µL of an antibody-immobilized beads suspension and a 50 µL of each sample were mixed in wells of a 96-well plate, incubated for 30 minutes at room temperature (RT) and aspiration-filtered three times. A 25 µL per well of detection antibody was added, and incubated for 30 minutes at RT, and aspiration-filtered three times. The beads were reacted with 50 µL of streptavidin-PE solution, incubated for 10 minutes at RT and aspiration-filtered three times. Then, the beads were resuspended in 125 µL/well of assay buffer prior to measuring the cytokine concentrations based on the intensity of the fluorescence at wavelengths specific to each bound cytokine.
6. Assessments of Th1/Th2, Th1/Th17 and TGF-β1/IL-6 ratios
We were interested to see if IFX impacts the T-helper (Th) cell system. According to previous report,9 we have defined the IFN-γ/IL-4 ratio reflecting the Th1/Th2 balance. Also, the Th1/Th17 balance was evaluated by monitoring the IFN-γ/IL-17 ratio. These were determined at baseline, post 1st IFX infusion or at week 14. Similarly, we measured serum transforming growth factor (TGF)-β1, a key cytokine for Th17 or Treg differentiation together with IL-6 level by using a commercially available ELISA kit (R&D Systems, Tokyo, Japan). Assays were according to the manufacture's protocol which involves measurement of the fluorescence intensity at a specific wavelength for TGF-β1.
7. Statistics
When appropriate, data are presented as the mean±SD values or as the median (interquartile range [IQR]) (if specified). Comparisons of the fluorescent intensities of T-cell surface markers and serum cytokine levels between groups were done by using the Mann-Whitney U test. For comparisons within the same subjects, the Wilcoxon signed-rank test was applied. Statistical analysis was performed by using an SPSS version 11.0J software (SPSS Inc., Tokyo, Japan). p<0.05 was considered statistically significant.
RESULTS
1. Clinical efficacy of IFX therapy
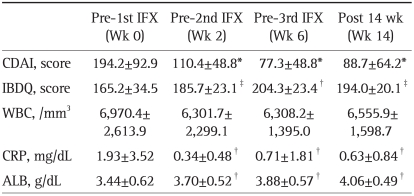
The clinical efficacy of IFX was assessed by determining the CDAI as well as the IBD questionnaire (IBDQ). The latter is reported to be a reliable indicator of QoL.7 Table 2 shows the clinical efficacy outcomes of the remission induction therapy with 3 infusions of IFX at weeks 0, 2, and 6. Both, the overall CDAI (p<0.001) and the IBDQ (p=0.037) scores in the 22 patients with active CD improved significantly prior to 2nd IFX infusion (2 weeks post 1st infusion). The clinical remission (CDAI≤150) rates at the 2nd and the 3rd IFX infusions were 77% (17 of 22) and 91% (20 of 22) of the patients, respectively. The improved CDAI and IBDQ were maintained up to 14 weeks post 1st IFX infusion. Biochemical markers, C-reactive protein (CRP) and serum albumin (ALB) also significantly improved after the 1st IFX infusion and up to week 14. No significant change in total white blood cell count was observed during this study.
Table 2.
Clinical Efficacy of IFX Therapy
Data are presented as mean±SD.
IFX, infliximab; CDAI, Crohn's disease activity index; IBDQ, inflammatory bowel disease questionnaire; WBC, white blood cell count; CRP, C-reactive protein; ALB, albumin.
*p<0.001, †p<0.03, and ‡p<0.05 vs pre 1st IFX.
2. The impact of IFX on the regulatory T-cells
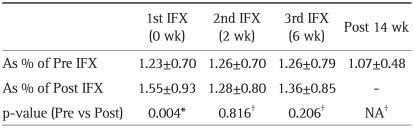
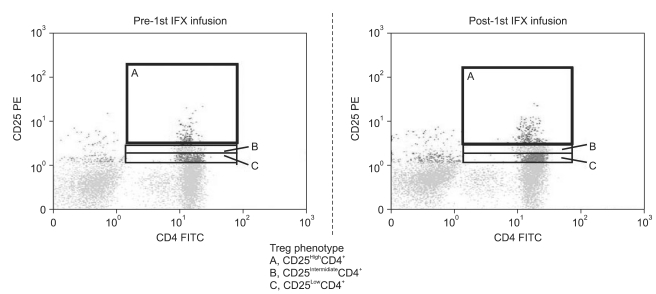
At baseline, the mean CD25HighCD4+ Treg expression (%) tended to be higher in the CD group as compared with the HC group, but without reaching statistical significance, 1.69±1.20 vs 1.06±0.26. Table 3 shows the change in circulating Treg profile during this study. As shown in Fig. 1, relative to baseline, there was a significant increase in Treg post each IFX infusion, but only in tests that were taken approximately 12 hours after the 1st IFX infusion (p=0.004).
Table 3.
Results of the Comparison of Peripheral CD25HighCD4+ Regulatory T-cell Expression
Data are presented as mean±SD.
IFX, infliximab; NA, not applicable.
*p-values were compared between Pre- and Post- each IFX infusion; †p-values were compared between Pre-1st IFX infusion and Pre-2nd, 3rd infusions or Post week 14 after 1st IFX infusion.
Fig. 1.
Flow cytometry profiles showing regulatory T-cells (Treg) (A, CD25High+CD4+; B, CD25Intermidiate+CD4+; C, CD25Low+CD4+ phenotypes within the CD25+CD4+ T-cells). The expression of peripheral Treg increased after a single infliximab (IFX) infusion at a concentration of 5 mg/kg/session. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
3. Results of cytokines, chemokines and related measurements
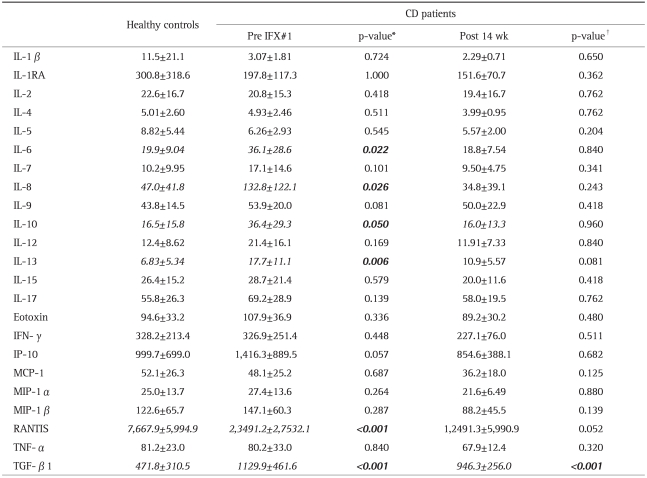
For patients with active CD vs HC, Table 4 shows the results of cytokine and chemokine measurements at entry. The CD group had significantly higher IL-6 (p<0.03), IL-8 (p<0.03), IL-10 (p=0.050), IL-13 (p<0.01), CCL5/RANTES (p<0.001), and TGF-β1 (p<0.001) as compared with the HC group. To obtain a correlation between the CD activity described in the CDAI and demographic/immunological profiles and other measurements, we defined 200 points in CDAI as the boundary between the 2 groups at base-line. Twelve of the 22 patients were categorized as the High group (mean CDAI=267.8±45.12), and the remaining 10 were defined as the Low group (113.9±56.29). Patients of the higher group had significantly lower serum albumin (High vs Low, 3.19±0.45 vs 3.74±0.54; p=0.040) and hemoglobin (High vs Low, 10.2±1.40 vs 13.0±2.12; p=0.021) concentrations (g/dL) among their demographic parameters described in Table 1 at the enrollment to the study. We could not detect any significant difference in cytokines and chemokines secretion at base-line.
Table 4.
A Comparison of Cytokines and Chemokines between Crohn's Disease (CD) Patients and Healthy Controls (HCs) vs. Measurements Taken before the First IFX* Infusion or after 14 Weeks†
Data are presented as mean±SD (pg/dL). Statistically significant values (p<0.05) are indicated in bold italics.
IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; TGF, transforming growth factor.
Eight weeks after the end of remission induction therapy (14 weeks after the 1st IFX), only TGF-β1 was very markedly elevated in the CD group as compared with the HC group (p<0.001), while RANTES showing a marked difference between the CD and HC, but without reaching statistical significance (p=0.052).
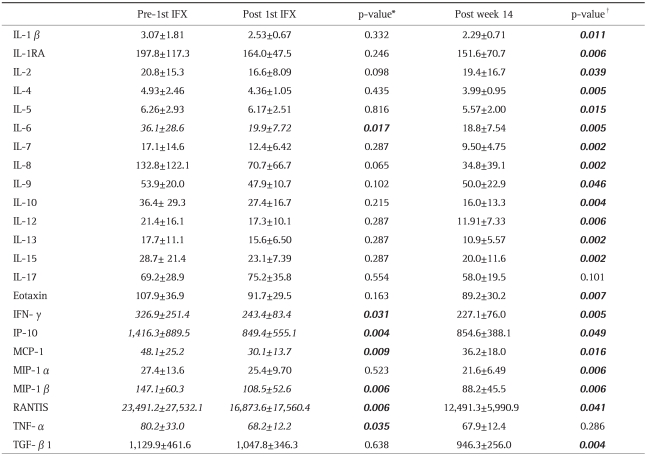
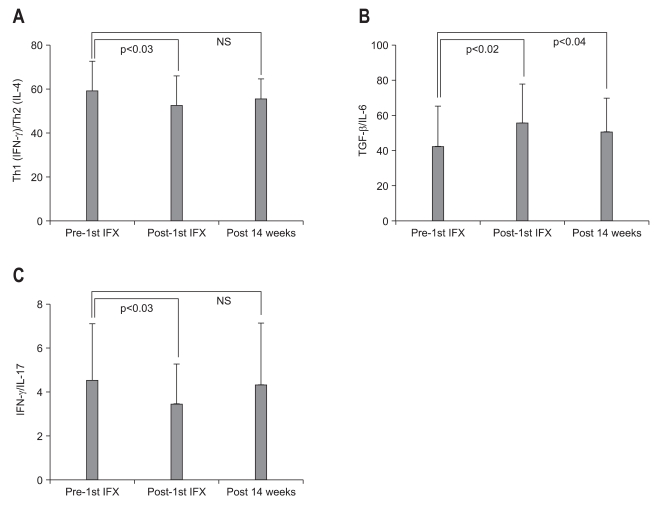
When looking at cytokine levels at baseline vs post 1st IFX infusion (Table 5), we found that serum levels of IL-6 (p<0.02), IFN-γ (p<0.04), TNF-α (p<0.04), together with chemokines, CXCL10/IP-10 (p<0.01), CCL2/MCP-1 (p<0.01), CCL4/MIP-1β (p<0.01), and CCL5/RANTES (p<0.01) were significantly decreased just after one IFX infusion. Similarly, as seen in Fig. 2A, there was a significant decrease in Th1 (IFN-γ)/Th2 (IL-4) ratio between pre and post 1st IFX infusion (p=0.024). The TGF-β1/IL-6 and IFN-γ/IL-17 ratios were evaluated to assess the impact of IFX on the Treg-Th17 pathway (Fig. 2B, C).
Table 5.
Comparisons of Cytokines before and after First IFX* Infusion or after 14 Weeks†
Data are presented as mean±SD (pg/dL). Statistically significant values (p<0.05) are indicated in bold italics.
IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; TGF, transforming growth factor.
Fig. 2.
The transitions of the (A) Th1 (IFN-γ)/Th2 (IL-4), (B) TGF-β 1/IL-6, and (C) IFN-γ/IL-17 ratios during this study are shown in panels A, B, and C, respectively. p<0.05 was considered statistically significant.
IFX, infliximab; IFN, interferon; IL, interleukin; TGF, transforming growth factor; NS, not significant.
To see the sustainability of IFX effects on specific arms of the immune system, we looked at serum cytokine/chemokine levels at week 14 vs entry levels. At week 14, all cytokines and chemokines, except IL-17 and TNF-α had decreased significantly relative to pre IFX baseline levels (Table 5).
DISCUSSION
The development of IFX as a therapeutic agent has changed the clinical course of CD together with a significant improvement in patients' QoL. All the 22 patients with CD in this study showed significant improvements of both clinical and laboratory parameters during the remission induction therapy with 5 mg/kg/session IFX administered at weeks 0, 2, and 6. Over 90% of the patients maintained clinical remission with sustained nutritional status during the 14 weeks follow-up (8 weeks post last IFX infusion). Further, in this study, 77% of the patients responded to a single infusion of IFX within 2 weeks. This seamed to be higher than the response rate (58%) reported by the ACCENT1 trial.3 This might be caused that the patients in our study had a short duration of CD (average 3.26 months) as well as being corticosteroid and thiopurine naïve; these demographic factors might have contributed to higher efficacy outcomes for IFX in this study. Moreover, our patients had relatively mild CD activity (average CDAI, 194.2±92.9).
Our major interest in this study was to see if in addition to blocking TNF-α, the clinical efficacy of IFX could be explained by other specific arms of the immune system that were either activated or otherwise suppressed in association with IFX therapy, given that these patients were not receiving immunomodulators or corticosteroids. This could pave the way for a better explanation of the mode of action of IFX in the treatment of patients with IBD and potentially in other immune related diseases in which this agent has shown efficacy like rheumatoid arthritis.8 With this in mind, we looked at the profiles of a broad set of pro- or anti-inflammatory cytokines, chemokines and Treg lymphocytes which are known to be major players in the expression of CD in an early stage of the disease.
Because CD is regarded as a Th1-shifted immune disorder,9 one might expect that at baseline, patients with active CD show elevated expressions of Th1 cytokines. However, in the present study, although there was no significant deference in Th1 cytokine, including IL-2, IFN-γ and TNF-α, between the HC group and the patients with CD prior to the 1st IFX infusion, Th2 cytokines like IL-6, IL-8, IL-10, and IL-13 were significantly elevated in the CD group at baseline as compared with the HC group. Interestingly, the expressions of both TGF-β1 and RANTES in the HC group showed a marked decrease as compared with either the CD group prior to 1st IFX or post 14 weeks. TGF-β1 is known to have a pivotal role in Th17 differentiation. Recently, TGF-β1 in combination with IL-1β, IL-6 or IL-21 was found to induce the differentiation of human Th17 cells under controlled conditions.10,11 Similarly, TGF-β1 is reported to induce the Treg-specific transcription factor FOXP3 which is a key regulatory gene in the development of Treg,12 while suppressing the T-bet, a transcription factor required for the commitment of Th1 cells.13 In line with this understanding, Bettelli et al.14 have suggested a reciprocal relations hip between Treg cells and Th17 cells. In particular, addition of IL-6 to a system driven by TGF-β1 inhibited the generation of Treg, promoting the transition of naïve CD4+ T cells (Th0) to Th17 cells.13 In this study, our CD patients showed a significant up-regulation of not only TGF-β1 level, but also IL-6 level, although a group in Ankara recently reported that the plasma TGF-β1 level in CD patients was not significantly different from the level in healthy controls or showing correlation with CDAI.15 The marked over expression of TGF-β1 together with increased IL-6 secretion we observed at baseline in this study might reflect patients' background characteristics, like naïve for thiopurines and corticosteroids.
As another line of investigation, we became interested in a recent topic raised by Fantani et al.16 that TGF-β1 signaling in relation to the induction of FOXP3 expressing Treg was interfered with by an over expression of the inhibitory molecule, Sma- and Mad-related protein7 (Smad7) in patients with IBD. Hence, a combined over expression of TGF-β1 and IL-6 observed in our CD patients might suggest that their immune system was geared to selectively induce Th0 cells into the Th17 in response to an appropriate stimulus.
Based on our recent observations in patients with IBD, we hypothesized that a significant elevation of Treg expression following treatment might indicate a restoration of the immuneprofile to a more normal function.4,5 Likewise, in the present study, we were interested in the expression profile of the CD25 intensely expressing CD4+ T-cells (CD25HighCD4+) to see the impact of IFX on Treg lymphocytes which are known to have an essential role in maintaining immune tolerance in the intestinal mucosa.17-20 At baseline, the Treg level in our CD patients was not significantly different from the level in the HC group, in spite of the knowledge that the peripheral Treg level is compromised in patients with active UC4,5 or CD.21 We also looked at various chemokine secretion profiles which mediate leukocyte trafficking.22 Among chemokines, the CC chemokine receptor 5 (CCR5) and the CXC chemokine receptor 3 (CXCR3) are selectively expressed by Th1 cells that mediate cellular immunity and tissue-specific autoimmune disorders.23,24 At baseline, only the secretion of RANTES was significantly increased in CD patients as compared with the HC group, while the secretion of IP-10, CXCL10 (one of the two variants of CXCR3)25 did not show significant difference between the two groups. RANTES is a receptor sharing CCR5 together with MIP-1α/CCL3) and MIP-1β (CCL4).22 Oki et al.26 reported that the noncaseating granulomas cells, which are characteristic of CD, were positive for RANTES/CCL5 protein. Granulomas were often diagnosed early in the course of the disease.27 Therefore, the up-regulation of peripheral RANTES we observed might indicate granulomatous inflammation of the GI-tract, and this could be an initial pathogenic characteristic of CD in a very early stage of the disease.
At 12 hours after an IFX infusion, TNF-α, IL-6, and IFN-γ, representing Th1 cytokines were significantly decreased. Immediate reduction of serum TNF-α level could indicate that the primary action of IFX was blockade of TNF-α, which is expected. Although the mechanism of therapeutic effects of IFX in the treatment of IBD is not fully understood, previous investigations have suggested that the mode of actions of IFX may fall into two categories: blockade of TNF receptor actions and induction of transmembrane TNF-mediated effects.28 In fact, anti-TNF agents have been reported to bind to transmembrane TNF and initiate reverse signaling back into cells leading to apoptosis, cell activation, or cytokine suppression.29,30 Hence, in the present study, other Th1 classified cytokines like IL-6, and IFN-γ might have been suppressed together with CXCR3 or IP-10 by an indirect action of IFX. Further, a significant reduction of IFN-γ achieved after the 1st IFX infusion should contribute to a shift of Th1 (IFN-γ)/Th2 (IL-4) balance towards Th2. Interestingly, the Th1/Th2 balance was not different between pre-1st IFX infusion and week 14. The bottom line could be that IFX seems to suppress more than one pro-inflammatory arm of the over active immune system in patients with CD.
Among various immune function related observations we made in this study, the impact of IFX on the CD4+CD25+ Treg could be very significant as this lymphocyte sub-type is intimately associated with normal immune function in the gut.31 Unequivocal evidence for this perception has been provided by animal models of colitis which showed that depletion of CD4+CD25+ from the circulation led to an inflammatory colitis similar to human IBD.31-33 In the present study, the peripheral Treg level in tests that were taken 12 hours after the 1st IFX infusion showed a significant increase as compared with baseline level. Both IL-10 and TGF-β are reported to be involved in the regulatory activities of the CD4+CD25High+ Treg.18,20,34-36 We found that IFX infusion increased the TGF-β1 (Treg)/IL-6 (Th1) ratio, while decreasing the IFN-γ (Th1)/ IL-17 (Th17) ratio. These results indicated that a single infusion of IFX has the potential to induce transition of peripheral Th0 lymphocytes to Treg and restore the Th1/Th2 balance.
At week 14, after successful remission induction therapy, our patients showed a marked modification in their cytokine expression profiles, most notably, the Treg expression had improved to the HC level. Additionally, our result has demonstrated that the level of IL-17 secretion was not influenced by the IFX therapy. This observation should mean that a homeostatic modulation of the peripheral immune-system of CD patient in very early stage induced a differentiation towards Th17 helped to alleviate the inflammatory drive.
In conclusion, in this study, 77% of the 22 patients responded to a single infusion of IFX within 2 weeks. Our impression is that mild and short duration of CD in the patients of this study and corticosteroid as well as thiopurine naïveness might have been significant factors in this high response rate to IFX. Further, IFX therapy was associated with a significant restoration of the Th1/Th2 balance and seemed to promote maturation of naïve Th0-lymphocytes to Treg via restoration of TGF-β1/ IL-6 balance.
ACKNOWLEDGEMENTS
Conflict of interest: None.
External funding: None.
References
- 1.Targan SR, Hanauer SB, van Deventer SJ, et al. Crohn's Disease cA2 Study Group. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 2.Fiorino G, Rovida S, Correale C, Malesci A, Danese S. Emerging biologics in the treatment of inflammatory bowel disease: what is around the corner? Curr Drug Targets. 2010;11:249–260. doi: 10.2174/138945010790309975. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama Y, Fukunaga K, Fukuda Y, et al. Demonstration of low-regulatory CD25High+CD4+ and high-pro-inflammatory CD28-CD4+ T-Cell subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci. 2007;52:2725–2731. doi: 10.1007/s10620-006-9560-z. [DOI] [PubMed] [Google Scholar]
- 5.Kamikozuru K, Fukunaga K, Hirota S, et al. The expression profile of functional regulatory T cells, CD4+CD25high+/forkhead box protein P3+, in patients with ulcerative colitis during active and quiescent disease. Clin Exp Immunol. 2009;156:320–327. doi: 10.1111/j.1365-2249.2009.03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Caprilli R, Gassull MA, Escher JC, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: special situations. Gut. 2006;55(Suppl 1):i36–i58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winship DH, Summers RW, Singleton JW, et al. National Cooperative Crohn's Disease Study: study design and conduct of the study. Gastroenterology. 1979;77(4 Pt 2):829–842. [PubMed] [Google Scholar]
- 9.Matsumura K, Nakase H, Yamamoto S, et al. Modulation of the Th1/Th2 balance by infliximab improves hyperthyroidism associated with a flare-up of ulcerative colitis. Inflamm Bowel Dis. 2009;15:967–968. doi: 10.1002/ibd.20760. [DOI] [PubMed] [Google Scholar]
- 10.Irvine EJ, Feagan B, Rochon J, et al. Canadian Crohn's Relapse Prevention Trial Study Group. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Gastroenterology. 1994;106:287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PC, Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- 12.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 13.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15.Kiliç ZM, Ayaz S, Ozin Y, Nadir I, Cakal B, Ulker A. Plasma transforming growth factor-beta1 level in inflammatory bowel disease. Turk J Gastroenterol. 2009;20:165–170. doi: 10.4318/tjg.2009.0002. [DOI] [PubMed] [Google Scholar]
- 16.Fantini MC, Rizzo A, Fina D, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–1316. e1–e3. doi: 10.1053/j.gastro.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 21.Chamouard P, Monneaux F, Richert Z, et al. Diminution of Circulating CD4+CD25 high T cells in naïve Crohn's disease. Dig Dis Sci. 2009;54:2084–2093. doi: 10.1007/s10620-008-0590-6. [DOI] [PubMed] [Google Scholar]
- 22.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loetscher P, Uguccioni M, Bordoli L, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 25.Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289–295. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- 26.Oki M, Ohtani H, Kinouchi Y, et al. Accumulation of CCR5+ T cells around RANTES+ granulomas in Crohn's disease: a pivotal site of Th1-shifted immune response? Lab Invest. 2005;85:137–145. doi: 10.1038/labinvest.3700189. [DOI] [PubMed] [Google Scholar]
- 27.Freeman HJ. Granuloma-positive Crohn's disease. Can J Gastroenterol. 2007;21:583–587. doi: 10.1155/2007/917649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Mitoma H, Horiuchi T, Hatta N, et al. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology. 2005;128:376–392. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 31.Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98. [PubMed] [Google Scholar]
- 32.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 33.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 35.Sitohy B, Hammarström S, Danielsson A, Hammarström ML. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin Exp Immunol. 2008;151:326–333. doi: 10.1111/j.1365-2249.2007.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fahlén L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]