Abstract
Background/Aims
Acute gastric injury by alcohol or indomethacin has been reported to be prevented by DA-9601, an extract of the herb Artemisia asiatica. Ghrelin, an endogenously produced gastrointestinal peptide hormone, has also been demonstrated to play a role in gastric mucosal defense. The aim of this study was to investigate the effects of DA-9601 on ghrelin in an acute gastric injury model induced by alcohol or indomethacin.
Methods
A total of 140 Sprague-Dawley rats were divided into two groups, a placebo group and a DA-9601-pretreated group. Thirty minutes later, half of the rats in each group received ethanol injury and the other half received indomethacin injury. Levels of serum ghrelin and gastric mucosal ghrelin mRNA were measured by ELISA and RT-PCR, respectively.
Results
Immediately after ethanol administration, ghrelin increased in both groups pretreated with DA-9601 and placebo. However, the increase occurred more rapidly and was higher in the DA-9601-pretreated rats than in the controls that did not receive DA-9601-pretreatment. Similarly, from 30 minutes to 2 hours after indomethacin administration, the DA-9601-pretreated rats showed a significant increase in serum and gastric mucosal ghrelin concentrations, whereas placebo-pretreated rats showed only a mild increase.
Conclusions
DA-9601 potentiates the endogenous production and secretion of ghrelin in acute gastric injury models induced by ethanol or indomethacin.
Keywords: DA-9601, Ghrelin, Acute gastric injury
INTRODUCTION
The gastric mucosa defends its structural integrity against many noxious substances such as alcohol and nonsteroidal anti-inflammatory drugs (NSAIDs) via pre-epithelial, epithelial and sub-epithelial factors that include the mucus barrier, bicarbonate secretion, epithelial tight junctions, prostaglandin (PG) synthesis, cell restitution, and mucosal blood flow.1 Alcohol ingestion may lead to gastric mucosal lesions by penetrating the gastric mucosa and causing mucosal inflammation, erosions and ulcer formation.2-4 Alcohol-induced gastric injury is mediated by free radicals, which is supported by the fact that ethanol administration depletes cells of major antioxidants such as superoxide dismutase, catalase, and glutathione peroxidase. Gastric injury induced by NSAIDs such as indomethacin is mediated by nonselective inhibition of the cyclooxygenase pathways, which results in decreased PG synthesis.
DA-9601, an extract of Artemisia asiatica, has been reported to have anti-inflammatory, antioxidative and cytoprotective effects and therefore to play a protective role in experimentally-induced gastrointestinal damage and hepatic and pancreatic lesions.5-9 However, the underlying mechanisms of its gastric mucosal protective effects have not been fully elucidated.
Another gastro-protective agent, ghrelin, which is made endogenously, has been recently found as one of the gut hormones. It affects the secretory and motor functions of gut as well as energy homeostasis by controlling appetite and food intake.8,10 It is a peptide hormone that binds to growth hormone secretagogue receptor (GHS-R).7 It can be produced in the small intestine, the large bowel, the kidney, and the placenta,11,12 as well as in the stomach, the main site of its production and secretion.13 In addition to modulating gastric acid secretion and accelerating gastric emptying,8,9 it has been reported to protect the gastric mucosa from acute gastric injury.5,14-17 Thus, ghrelin and DA-9601 seem to have similar gastro-protective effects.
The aim of this study was to examine possible interaction between DA-9601 and ghrelin in acute gastric injury induced by ethanol or indomethacin. We investigated serum ghrelin levels and gastric mucosal ghrelin expressions after pretreatment with DA-9601 or placebo in rat models of acute gastric injury.
MATERIALS AND METHODS
1. Animals and materials
The use of animals in the present study was approved by the Institutional Animal Care and Use Committee of Hanyang University. Male Sprague Dawley (SD) rats, weighing 240-260 g, were obtained from Charles River (Chiba, Japan). The animals were kept at a constant temperature (24±1℃) and 40-60% relative humidity and were acclimatized for 7 days before experiments. Prior to the experiments all rats were fasted for 24 hours but were allowed access to water ad libitum.
DA-9601, a standardized extract of Artemisia asiatica (Dong-A Pharmaceutical Research Institute, Yongin, Korea) was dissolved in 5% hydroxypropyl methylcellulose and given at concentrations of 100 mg/kg body weight (BW). Absolute ethanol was diluted 3:1 (vol/vol) in distilled water and given in a volume of 1.5 mL/rat by oral gavage. Indomethacin (40 mg/kg) was suspended in 1.0% carboxymethylcellulose in water (6 mg/mL) and administered orally at a dose of 40 mg/kg BW. One hundred and forty animals were divided into two groups at random: one group was pretreated with 100 mg/kg DA-9601, the other with placebo. Then all the rats were administered either ethanol or indomethacin to induce acute gastric mucosal injury.
2. Study design
1) Measurement of serum ghrelin levels
Twenty SD rats were fasted for 24 hours, and blood samples were taken to measure a baseline ghrelin level. Then DA-9601 (100 mg/kg) and placebo were administered orally to half the rats each. Thirty minutes later, acute gastric mucosal injuries were induced by oral administration of ethanol (1.5 mL) to half the rats in each group, and by indomethacin (40 mg/kg) in the other half of each group. Blood samples were also taken at 0.5, 1, 2, 4, and 6 hours after the induction of acute gastric mucosal injury.
2) Measurement of gastric mucosal ghrelin expression
One hundred and twenty SD rats were divided into two groups of 60 rats per group for receipt of ethanol- or indomethacin-induced gastric mucosal injury. Each group was further divided into two groups of 30 rats per group for DA-9601 pretreatment and placebo pretreatment, respectively. After 24 hours of fasting, five SD rats in each group were sacrificed to measure a baseline gastric mucosal ghrelin expression and DA-9601 (100 mg/kg) or placebo was then administered orally to all the remaining rats. After 30 minutes, gastric mucosal injury was induced by administration of ethanol or indomethacin to the allocated groups. At 0.5, 1, 2, 4, and 6 hours after induction of gastric mucosal injury, five SD rats in each group were killed for gastric mucosal sampling.
3. Processing of specimens
Blood samples (more than 400 µL) were obtained from every animal and stored in EDTA test tubes with 500 IU heparin (3 µL). The samples were centrifuged at 3,000 rpm for 5 minutes. The serum was collected and stored at -70℃. Serum ghrelin levels were measured quantitatively with an ELISA kit (Linco Research, St. Charles, MO, USA) using 50 µL samples of serum. The gastric mucosae obtained were stored at -70℃, and gastric mucosal ghrelin mRNA was measured by RT-PCR as described previously.18 Briefly, rat gastric mucosae were homogenized and total RNA was extracted using a commercial RNA isolation kit (Easy-Blue; iNtRON, Seoul, Korea). Total RNA was then transcribed to cDNA using 10UofMMV-RT (iNtRON), 5 pmol of dNTP, 10 pmol of oligo(dT), 0.2 µmol of dithiothreitol, 2 µL of 10×reaction buffer, and distilled deionized water in a 20 µL reaction volume. Quantitative real-time RT-PCR was performed using an ABI prism 7700 (Applied Biosystems, Foster City, CA, USA).
4. Statistical analysis
Data for the DA-9601-pretreated and control groups were analyzed with the Mann-Whitney test. Paired values of the same variables before and after administration of a given regimen were analyzed by the Wilcoxon signed rank test. p-values less than 0.05 were considered statistically significant.
RESULTS
1. Change in serum ghrelin levels after DA-9601 administration in rats with ethanol-induced acute gastric injury
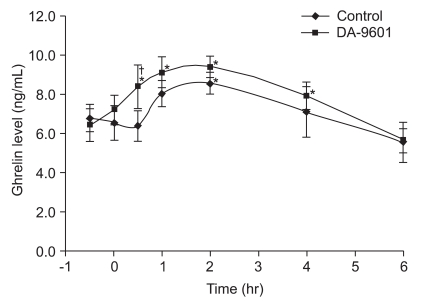
DA-9601 (100 mg/kg) or placebo was administered orally to rats according to the previously allocated schedule. Thirty minutes later, acute gastric mucosal injuries were induced by oral administration of ethanol (1.5 mL) or indomethacin (40 mg/kg) to half the rats each. Blood samples were taken at 0.5, 1, 2, 4, and 6 hours after the induction of acute gastric mucosal injury. In the group of rats not receiving DA-9601 treatment serum ghrelin levels increased from their baseline values 2 hours after ethanol administration (from 6.8±0.6 ng/mL to 8.2±0.5 ng/mL; p<0.05) whereas serum ghrelin levels increased by 30 minutes after ethanol administration in the DA-9601-pretreated group (6.5±0.8 ng/mL vs 8.8±0.6 ng/mL; p<0.05). Serum ghrelin levels were significantly higher in the DA-9601-pretreated group than in the control group at 30 minutes after ethanol administration (6.5±0.8 ng/mL vs 8.6±1.2 ng/mL; p<0.05) (Fig. 1).
Fig. 1.
Effects of DA-9601 pretreatment on serum ghrelin concentrations in rats receiving ethanol-induced acute gastric injury. Rats received DA-9601 or placebo at -30 minutes, and all were then exposed to alcohol at zero minutes. Vertical bars indicate means±SD. *p<0.05, compared with baseline, as determined by nonparametric Mann-Whitney unpaired U test; †p<0.05, compared with the control, as determined by nonparametric Wilcoxon signed rank test.
2. Change in gastric mucosal ghrelin mRNA after DA-9601 administration in rats with ethanol-induced acute gastric injury
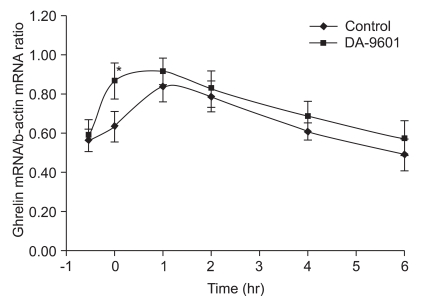
Gastric mucosal ghrelin mRNA expressions increased in both the DA-9601-pretreated group and in the control group. However, the increase occurred more rapidly in the DA-9601- pretreated rats and total mucosal mRNA expressions was higher than in the controls not receiving DA-9601-pretreatment (0.87±0.09 vs 0.62±0.08; p<0.01) (Fig. 2).
Fig. 2.
Effects of DA-9601 on gastric mucosal ghrelin in rats receiving ethanol-induced acute gastric injury. Rats received DA-9601 or placebo at -30 minutes and were then all exposed to alcohol. Vertical bars indicate means±SD. *p<0.05, compared to control, as determined by nonparametric Wilcoxon signed rank test.
3. Change in serum ghrelin levels after DA-9601 administration in rats with indomethacin-induced acute gastric injury
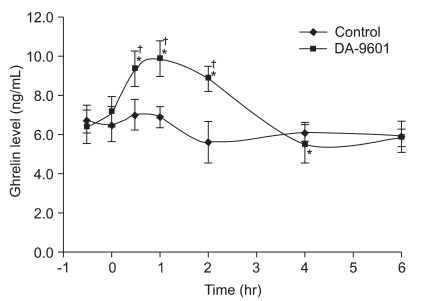
Serum ghrelin levels did not increase significantly from baseline values in the control group not receiving DA-9601-pretreatment. In the DA-9601-pretreated and indomethacin-induced rats, serum ghrelin levels increased significantly from 30 minutes to 2 hours after indomethacin administration (from 7.3±0.6 to 9.3±0.8; p<0.05). Thus, from 30 minutes to 2 hours after indomethacin administration, the DA-9601-pretreated rats had higher serum ghrelin levels than the control group (9.5±0.4 ng/mL vs 6.2±0.6 ng/mL; p<0.01) (Fig. 3).
Fig. 3.
Effects of DA-9601 on serum ghrelin levels in rats receiving indomethacin-induced acute gastric injury. Rats received DA-9601 or placebo at -30 minutes and were then all exposed to indomethacin at zero minutes. Compared with baseline, serum ghrelin levels significantly increased after indomethacin administration only in DA-960-pretreated rats. Vertical bars indicate means±SD. *p<0.05, compared with baseline, as determined by nonparametric Mann-Whitney unpaired U test; †p<0.05, compared with the control, as determined by nonparametric Wilcoxon signed rank test.
4. Change in gastric mucosal ghrelin mRNA after DA-9601 administration in rats with indomethacin-induced acute gastric injury
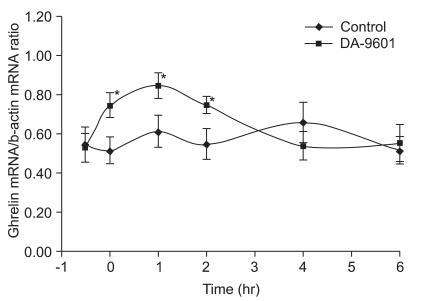
Transcription of gastric mucosal ghrelin mRNA increased significantly in the DA-9601-pretreated and indomethacin-treated rats compared to the control not receiving DA-9601-pretreatment from the start of the experiment to 2 hours after indomethacin administration (0.79±0.04 vs 0.56±0.04; p<0.01) (Fig. 4).
Fig. 4.
Effects of DA-9601 on gastric mucosal ghrelin concentrations in rats receiving indomethacin-induced acute gastric injury. Rats received DA-9601 or placebo at -30 minutes and were then all exposed to indomethacin at zero minutes. Gastric mucosal ghrelin mRNA increased significantly in DA-9601-pretreated rats compared with the placebo pretreated group. Vertical bars indicate means±SD. *p<0.05 compared to the control, as determined by nonparametric Wilcoxon signed rank test.
DISCUSSION
We found that ghrelin increases after acute gastric injury in rats. In the rats in which gastric injury was induced by alcohol, the increase of ghrelin was greater than in those in which gastric injury was induced by indomethacin. In addition, serum and gastric ghrelin concentrations were higher in both the ethanol- and indomethacin-treated groups pretreated by DA-9601 than in those pretreated with placebo. These results suggest that there is an association between ghrelin and DA-9601 in these acute gastric injury models.
Acute gastric lesions caused by alcohol consumption or the use of NSAIDs have been demonstrated to be induced by reactive oxygen species.19 Free radical-induced mucosal damage can be prevented by administration of free radical scavengers.20,21 DA-9601 is reported to inhibit xanthine oxidase and thereby suppress oxidative stress and to prevent acute gastrohemorrhagic injury by ethanol.6 It has been used to treat erosive gastritis and atrophic gastritis,22 and its efficacy against erosive gastritis was reported to be higher than that of cetrexate.23 Even the susceptibility of patients using NSAIDs to gastric mucosal damage by alcohol was also shown to be reduced by DA-9601.22 The present study demonstrated that the production and secretion of ghrelin increased more in DA-9601-pretreated rats subsequently exposed to alcohol or indomethacin than in the placebo-pretreated rats suggesting that increased production of ghrelin may be responsible for the gastro-protective effect of DA-9601.
Ghrelin has beneficial effects on various inflammatory conditions. It was shown to inhibit the expression and production of pro-inflammatory cytokines such as interleukin (IL)-1b, IL-6, and tumor necrosis factor (TNF)-α,24 and protects against oxidative tissue injury caused by burns.25 In addition, patients with risk factors for severe acute pancreatitis were shown to have higher ghrelin concentrations than the low risk group, and ghrelin concentrations significantly increased after recovery from acute pancreatitis,26,27 indicating that ghrelin has the potential to protect against the inflammatory responses in acute pancreatitis. Protecting effects against oxidative damage have been also noted in the kidney,28 the hepatobiliary system,29,30 and the stomach.31
Protection of gastric mucosa was reported to involve sensory nerves and endogenous nitric oxide release after acute gastric mucosal damage induced by ethanol.17 In a study investigating the effects of ghrelin in fasted rats suffering acute gastric injury induced by ethanol, intraperitoneal injection of ghrelin was reported to decrease gastric erosions and to increase gastric blood flow and gastric mucosal ghrelin expressions, suggesting that exogenous ghrelin protects the gastric mucosa, and that release of endogenous ghrelin is induced by ethanol ingestion.14 Similar results were found in rats exposed to gastric injury caused by water immersion and restraint stress. The acute gastric lesions induced by the stressors were attenuated by exogenous ghrelin, and the exogenous ghrelin significantly increased plasma ghrelin levels, gastric mucosal blood flow and endogenously secreted ghrelin concentrations measured by RT-PCR. These effects of exogenous ghrelin were thought to be mediated by a combination of the the cyclooxygenase-prostaglandin system, the NO synthase-NO system, and activation of the vagus nerve and sensory nerves because the gastroprotective effects were abolished by indomethacin, L-NNA (NG-nitro-L-arginine), vagotomy, and capsaicin.5
This is the first study to demonstrate an association between DA-9601 and ghrelin in the established rat model of acute gastric injury. However, there are some limitations to the present study. Since we did not examine the acute gastric mucosal lesions induced by ethanol or indomethacin, we could not compare the extent of mucosal injury in the rats damaged by ethanol and by indomethacin, in the DA-9601-pretreated and placebo-pretreated groups. Also we did not assess levels of nitric oxide and PG which are known to mediate gastro-protection by ghrelin.14,17 Although some of these limitations may be alleviated by the fact that ethanol- or indomethacin-induced injury models are well-established rat models of acute gastric injury, the study results should be interpreted with caution.
In conclusion, the present study shows that serum and gastric ghrelin increased more and longer in response to gastric injury in DA-9601-pretreated rats than in placebo-pretreated rats. Therefore, ghrelin may be considered a gastro-protective agent in acute mucosal injury caused by ethanol or indomethacin exposure, and DA-9601 is able to augment production and secretion of ghrelin.
ACKNOWLEDGEMENTS
Financial disclosure: None.
References
- 1.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Taylor B, Rehm J. Moderate alcohol consumption and diseases of the gastrointestinal system: a review of pathophysiological processes. Dig Dis. 2005;23:177–180. doi: 10.1159/000090164. [DOI] [PubMed] [Google Scholar]
- 3.Laine L, Weinstein WM. Histology of alcoholic hemorrhagic "gastritis": a prospective evaluation. Gastroenterology. 1988;94:1254–1262. doi: 10.1016/0016-5085(88)90661-0. [DOI] [PubMed] [Google Scholar]
- 4.Tarnawski A, Stachura J, Hollander D, Sarfeh IJ, Bogdal J. Cellular aspects of alcohol-induced injury and prostaglandin protection of the human gastric mucosa. Focus on the mucosal microvessels. J Clin Gastroenterol. 1988;10(Suppl 1):S35–S45. doi: 10.1097/00004836-198812001-00008. [DOI] [PubMed] [Google Scholar]
- 5.Brzozowski T, Konturek PC, Konturek SJ, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39–51. doi: 10.1016/j.regpep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Huh K, Kwon TH, Shin US, et al. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88:269–273. doi: 10.1016/s0378-8741(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 7.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 8.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 9.Sibilia V, Pagani F, Guidobono F, et al. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology. 2002;75:92–97. doi: 10.1159/000048225. [DOI] [PubMed] [Google Scholar]
- 10.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 11.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Lee HM, Englander E, Greeley GH., Jr Ghrelin: not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 13.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 14.Konturek PC, Brzozowski T, Pajdo R, et al. Ghrelin: a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol. 2004;55:325–336. [PubMed] [Google Scholar]
- 15.Konturek PC, Burnat G, Rau T, Hahn EG, Konturek S. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53:597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- 16.Peeters TL. Ghrelin: a new player in the control of gastrointestinal functions. Gut. 2005;54:1638–1649. doi: 10.1136/gut.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 18.Jun DW, Lee OY, Lee YY, Choi HS, Kim TH, Yoon BC. Correlation between gastrointestinal symptoms and gastric leptin and ghrelin expression in patients with gastritis. Dig Dis Sci. 2007;52:2866–2872. doi: 10.1007/s10620-006-9651-x. [DOI] [PubMed] [Google Scholar]
- 19.Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–1401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- 20.La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 21.Cantürk NZ, Cantürk Z, Ozbilim G, Yenisey C. Protective effect of vitamin E on gastric mucosal injury in rats with biliary obstruction. Can J Gastroenterol. 2000;14:499–503. doi: 10.1155/2000/641671. [DOI] [PubMed] [Google Scholar]
- 22.Oh TY, Shin CY, Sohn YS, et al. Therapeutic effect of DA-9601 on chronic reflux gastritis induced by sodium taurocholate in rats. World J Gastroenterol. 2005;11:7430–7435. doi: 10.3748/wjg.v11.i47.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004;10:2379–2382. doi: 10.3748/wjg.v10.i16.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sehirli O, Sener E, Sener G, Cetinel S, Erzik C, Yeğen BC. Ghrelin improves burn-induced multiple organ injury by depressing neutrophil infiltration and the release of pro-inflammatory cytokines. Peptides. 2008;29:1231–1240. doi: 10.1016/j.peptides.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Kim YD, Kong YH, et al. The relevance of serum ghrelin concentration to severity of acute pancreatitis. Gut Liver. 2010;4:234–240. doi: 10.5009/gnl.2010.4.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Liu X, Tang C. Change of plasma ghrelin level in acute pancreatitis. Pancreatology. 2006;6:531–535. doi: 10.1159/000096976. [DOI] [PubMed] [Google Scholar]
- 28.Takeda R, Nishimatsu H, Suzuki E, et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol. 2006;17:113–121. doi: 10.1681/ASN.2004080626. [DOI] [PubMed] [Google Scholar]
- 29.Işeri SO, Sener G, Saglam B, Ercan F, Gedik N, Yeğen BC. Ghrelin alleviates biliary obstruction-induced chronic hepatic injury in rats. Regul Pept. 2008;146:73–79. doi: 10.1016/j.regpep.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Kasımay O, Işeri SO, Barlas A, et al. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res. 2006;36:11–19. doi: 10.1016/j.hepres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Işeri SO, Sener G, Yüksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol. 2005;187:399–406. doi: 10.1677/joe.1.06432. [DOI] [PubMed] [Google Scholar]