Abstract
Background/Aims
An epidemiologic shift of hepatitis A virus (HAV) seroprevalence is expected due to an improvement in socioeconomic status in young adults in Korea. We investigated the age-specific seroprevalence and socioeconomic factors associated with HAV seropositivity in young, healthy Korean adults.
Methods
Between March 2009 and February 2010, a total of 5,051 persons from 20 to 49 years of age presenting for a health check-up were included and responded to a questionaire. The seroprevalence of HAV was investigated by measuring immunoglobulin G (IgG) anti-HAV. A total of 984 pairs of cases and age- and sex-matched controls were analyzed for associated socioeconomic factors.
Results
The prevalence of seropositive HAV was 6.2% in the 20 to 29 age range, 33.1% in the 30 to 39 range and 82.4% in the 40 to 49 range (p<0.001). There were no significant differences in any group according to gender. A multivariate analysis for paired cases indicated that HAV seropositivity was significantly higher in the low monthly income (below five million won, approximately 4,300 dollars) group and the Helicobacter pylori (H. pylori)-positive group (odds ratio [OR], 1.65; 95% confidence interval [CI], 1.27-2.14; p<0.001; OR, 1.45; 95% CI, 1.19-1.76; p<0.001, respectively).
Conclusions
HAV seropositivity in young adults presenting for a health checkup appears to be decreasing, and the prevalence was significantly higher in the low monthly income group and the H. pylori-positive group.
Keywords: Hepatitis A virus, Seropositivity, Helicobacter pylori, Socioeconomic status
INTRODUCTION
Hepatitis A virus (HAV) is a common cause of hepatitis worldwide. The Korea Centers for Disease Control and Prevention reported that symptomatic HAV infections have remarkably been increased in the past four years; 15.3 per 100,000 in 2002, 27.4 per 100,000 in 2006, and 62.4 per 100,000 in 2008.1,2 Since most people in hyperendemic areas have protective anti-HAV antibodies as a result of subclinical exposure during childhood, the increasing incidence of symptomatic HAV infection might be associated with a rapid decrease in the anti-HAV prevalence.
Hepatitis A virus is transmitted by the fecal to oral route and transmission is enhanced by contaminated food or drink.3 Thus, the epidemiology of hepatitis A is associated with poor hygiene, accessibility to clean drinking water and socioeconomic status (SES).4 In countries with high endemicity such as Africa, Central and South America, most inhabitants become infected early in life, and the infection remains asymptomatic or is not recognized. Individuals in areas with low endemicity such as northern and western Europe, North America and Australia have little contact with HAV during childhood, and older persons in this area are more likely to develop symptomatic hepatitis A.5 However, due to the improvements in SES and living conditions, many developing countries have an epidemiological shift of HAV infection.6-8 During the past decades, public hygiene and the environmental conditions have improved in Korea, as a result, an epidemiological shift in HAV seroprevalence has occurred.9-13
Individuals that had HAV infection generally acquire lifelong immunity to all strains of HAV; the seroprevalence of HAV might reflect subclinical infection with HAV. In this study, we investigated the seroprevalence of HAV and associated socioeconomic factors for HAV seropositivity in young healthy Korean adults.
MATERIALS AND METHODS
1. Study population
A total of 5,051 subjects from 20 to 49 years of age that visited Seoul National University Hospital Gangnam Healthcare Center, Seoul, Korea for a routine health check-up between March 2009 and February 2010 were included in this study. All potential participants were asked to complete a structured questionnaire on dietary history as well as SES. To reduce the effects of confounding factors, the same number of normal healthy controls with negative immunoglobulin G (IgG) anti-HAV was randomly selected that matched the cases for age and sex. This study was approved by the Institutional Review Board of the Seoul National University Hospital.
2. Clinical and SES assessments
Alcohol drinking was classified into two categories based on the frequency: yes (three times a week or more than) and no (less than three times a week; reference group). Eating-out was classified into two categories based on the frequency: yes (three times a week or more than) and no (less than three times a week; reference group). Monthly family income was classified into two categories: high (more than five million won, approximately 4,300 dollars; reference group) and low (less than five million won). The education level was classified into two categories based on the highest level of education: high (college graduates or more than; reference group) and low (below college graduates). Information about occupational history was collected. The occupational history was classified into two groups: group A including employed or self-employed professionals such as judges, officers, directors, doctors, and educators, and group B unskilled manual workers, and assistant non manual employees such as farmers, students, soldiers, guards, employees, and retailers.
3. Laboratory assessments
The presence of IgG antibodies for HAV was tested using commercially available kits. The kit used was a radio-immunoassay kit, Hepavidine A (General Biological Corp., Hsinchu, Taiwan). Anti-Helicobacter pylori (H. pylori) IgG was determined using an enzyme-linked immunosorbent assay (H. pylori-EIA-Well; Randim, Rome, Italy). The cut-off values were established according to the manufacturer's instructions.
4. Statistical analysis
All statistical analyses were performed using the SPSS statistical package (version 17.0; SPSS Inc., Chicago, IL, USA). The Pearson chi-square test was used to examine the association between HAV and the variables included in the study, and p value <0.05 was considered statistically significant. Multiple logistic regression was performed for risk factors. The dependent variable was HAV seropositivity, and the independent variables were all the other variables in this study. The odds ratio (OR) and relevant 95% confidence interval (CI) are presented for the potential risk factors concerned.
RESULTS
1. Age-specific seroprevalence of HAV
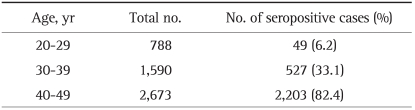
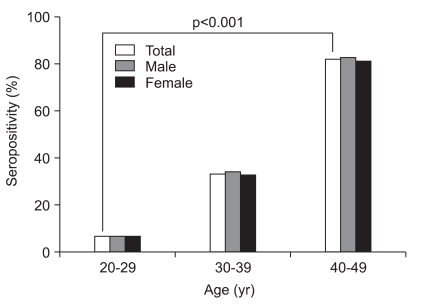
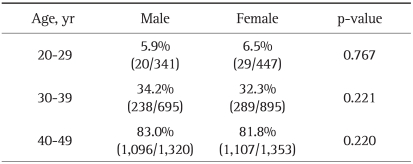
Among the 5,051 participants, 788 were 20 to 29 years of age, 1,590 were 30 to 39 years of age and 2,673 were 40 to 49 years of age. For IgG anti-HAV antibodies, 2,779 participants (55.0%) were seropositive. The seroprevalence rates for IgG anti-HAV antibodies according to age group are shown in Table 1. The seropositive results for HAV significantly increased with age: 6.2% (49/788) for 20-29 years of age, 33.1% (527/1,590) for 30-39, and 82.4% (2,203/2,673) for 40-49 years (p<0.001) (Fig. 1). There were no significant differences in seropositivity for IgG anti-HAV antibodies between male and female participants (Table 2).
Table 1.
Seroprevalence of IgG Anti-HAV according to Age
IgG, immunoglobulin G; HAV, hepatitis A virus.
Fig. 1.
Seropositivity of IgG anti-hepatitis A virus antibodies according to age and sex.
Table 2.
Comparison of the Seroprevalence of IgG Anti-HAV according to Sex
IgG, immunoglobulin G; HAV, hepatitis A virus.
2. Socioeconomic factors for HAV seropositivity
Among the 984 pairs of cases and controls, subjects with alcohol drinking accounted for 61.5% (1,211/1,968) of the participants and subjects that eating-out more three times a week or more than accounted for 54.5% (1,070/1,968). A high monthly income was documented in 70.0% (1,377/1,968) and a high education level in 79.4% (1,562/1,968). The number of participants in occupational group B was 59.1% (1,164/1,968). Subjects that had positive anti-H. pylori antibodies and those that reported taking H. pylori eradication medication were included in the H. pylori-positive group. The H. pylori-positive group had 1,022 subjects (51.9%).
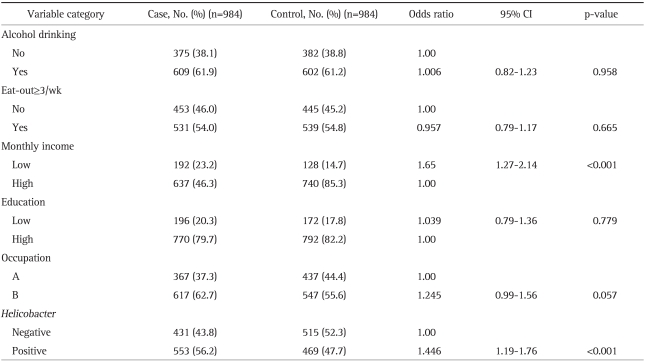
As shown in Table 3, the univariate analysis revealed that HAV seropositivity was significantly higher in the low monthly income group, in occupational group B and the H. pylori-positive group (p<0.001, p=0.002, and p<0.001, respectively). The multivariate analysis showed that HAV seropositivity was significantly higher in the low monthly income group and the H. pylori-positive group. The OR for HAV seropositivity in the low monthly income group was 1.65 times higher than that of the high monthly income group (95% CI, 1.27-2.14). According to H. pylori infection, the OR of H. pylori-positive group was 1.45 times higher than that of the H. pylori-negative group (95% CI, 1.19-1.76; Table 3).
Table 3.
The Relative Risk of Seropositivity of IgG Anti-HAV in the Matched Case-Controls
Occupation group A included employed or self-employed professionals, and group B unskilled manual workers, and assistant non manual employees.
IgG, immunoglobulin G; HAV, hepatitis A virus; CI, confidence interval.
DISCUSSION
Since the prevalence of HAV seropositivity in the young adults has declined and this age group has a high risk for HAV infection as a result, identification of associated factors for HAV seropositivity has significant clinical implications for managing hepatitis A.
In Korea, seropositivity of HAV in children under 10 years of age has been reported to be about 45% during 1982, while it was more than 90% in adults over 20 years.13 There has been a marked epidemiological shift in age-specific HAV seroprevalence and the current prevalence of anti-HAV seropositivity is thought to be in a transitional status that occurs as an underdeveloped country evolves to a developed country. In our study, seropositivity for HAV was identified in 6.2% of all subjects in the 20-29 age group, 33.1% for the 30-39 group, and 82.4% for the 40-49 group. In 2006, seropositivity for HAV was reported in 16-34% of 20 year old participants and 72-89% of 30 year old participants.14-16 Another study reported in 2006 showed a 2% seropositivity for HAV in 20 year old subjects and 72% in 30 year old subjects12; the seropositive findings of HAV in the 20 year old subjects seemed to be deviated from other data. Although the subjects from studies differed in terms of characteristics, geographic and/or socioeconomic status, the seropositivity of anti-HAV among young adults in their 20s and 30s appears to be markedly decreased. In addition, the finding that the HAV seroprevalence is declining among older ages suggests that the frequency of cases with clinical hepatitis A infection might increase in older adults and as a consequence, severe acute hepatitis might increase. Lee et al.2 reported outbreaks of hepatitis A among adults older than 20 years of age in 91.7% of cases.
To prevent HAV infection, it is essential to find the source and route of HAV transmission and the risk factors associated with HAV infection. Although HAV is known to be transmitted by the fecal-oral route, it is difficult to identify the source of an infection. The most important risk factor for HAV infection reported by the Centers for Disease Control and Prevention of United States is personal contact.17 In our study, alcohol drinking and frequent eating-out were evaluated as potential sources of food borne hepatitis A infection, however, no significant association was found. Since H. pylori is transmitted from person-to person suggesting fecal to oral transmission,18 several investigators have studied a possible link between seropositive H. pylori and HAV.19-21 In our study, HAV seropositivity was significantly higher in the H. pylori-positive group suggesting the possible association between both infections. The socioeconomic status is presumed to play an important role in H. pylori infection,22 which was one of the associated factors with HAV-seropositivity in our study.
Socioeconomic variables are known to be associated with HAV seropositivity. The rapid improvement of living conditions and sanitation due to economic growth has been associated with a rapid decrease in anti-HAV prevalence. It was reported that the proportion of the population with accessibility to clean water, the value of the human development index and per capita gross domestic product were negatively associated with HAV infection rates.4 Indeed, Japan, Australia, the United states, and most European nations have low anti-HAV rates while Latin America, Asia, and Middle Eastern nations have relatively high anti-HAV seroprevalence.6 In this study, HAV seropositivity was significantly higher in the group of a low monthly income. This result suggests that socioeconomic development at the individual level as well as at the national level is associated with HAV seropositivity.
In addition to income and wealth, other markers of SES are associated with HAV risk, including the educational level and occupation. Seroprevalence of HAV in children increases with lower levels of parental education;6 physicians, dentists, therapists and paramedical workers were reported to have a high risk of hepatitis A in a previous study.23 In the current study, a low educational level and participants in occupational group B such as farmers, students, soldiers, guards, employees, and retailers were at high risk for HAV-seropositivity, however, these differences were not statistically significant. The different criteria used for educational and occupational classification in studies might explain the inconsistent results.
Since highly effective and safe vaccines are now available, active immunization of subjects that are vulnerable to HAV infection is needed for prevention of outbreaks of HAV infection. The data reported here shows a decreasing trend and reflects HAV epidemiology: the findings suggest that an increasing number of young adults do not have protective antibodies against HAV. Currently, active immunization is selectively recommended for the susceptible adults at high risk for hepatitis A, including travelers to regions where HAV is endemic, healthcare workers or child-care providers as well as family members of patients.24 Based on the analysis performed in this study, vaccination should be recommended for the 20-29 age group because seropositivity for HAV was identified in only 6.2% of all subjects. Considering socioeconomic factors, young adults in high income groups and H. pylori-negative groups are candidates to screening for protective antibodies against HAV and vaccination. Although further studies regarding cost-effectiveness of nationwide hepatitis A vaccination should be performed, any immunization strategy should focus on the individuals at high risk, including low income groups. Frequent outbreaks of hepatitis A would be a serious health problem and especially, an economic burden to a low income group. Government sponsored vaccination against hepatitis A should be considered in young adults in a low income group.
The strengths of our study include the large sample size that allowed for assessment of age-specific seroprevalence and the associated socioeconomic factors of HAV seropositivity. In addition, to our best knowledge, there was no data to analysis the individual levels of socioeconomic factors, and it would be helpful to establish the strategy of vaccination.
The limitation of this study includes followings: 1) the history of hepatitis A vaccination and clinical hepatitis A could not be assessed; there is limited data regarding the vaccination rate for HAV in Korea. A recent study of a Korean army cohort reported that the HAV vaccination rate was 2% (2 of the 96 soldiers).25 Although the long-lasting protective effect of vaccination could have an influence on the seroprevalence of HAV, the effect of the vaccination rate in our study was likely minimal because of the large number of study subjects. 2) high income group was relatively a large proportion in our study. They are thought to be interested in health more than the lower income group. Thus, this can be a limitation in considering that the results from this study are representative data of current Korean general population.
In conclusion, HAV seropositivity in asymptomatic health young Korean adults presenting for a health check-up showed a decreasing trend. Thus, the catch-up vaccination for susceptible young adults should be considered. The risk of subclinical infection was significantly higher in the low monthly income group and H. pylori-positive group. Multi-center large trials for analysis of socioeconomic risk factors of clinically overt hepatitis A are needed to develop a targeted vaccination strategy.
ACKNOWLEDGEMENTS
We thank Medical Research Collaborating Center of Seoul National University Hospital for the statistical analysis performed for this study.
References
- 1.Jeong SH. Current status and vaccine indication for hepatitis A virus infection in Korea. Korean J Gastroenterol. 2008;51:331–337. [PubMed] [Google Scholar]
- 2.Lee D, Cho YA, Park Y, et al. Hepatitis a in Korea: epidemiological shift and call for vaccine strategy. Intervirology. 2008;51:70–74. doi: 10.1159/000127428. [DOI] [PubMed] [Google Scholar]
- 3.Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43(2 Suppl 1):S164–S172. doi: 10.1002/hep.21052. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34:600–609. doi: 10.1093/ije/dyi062. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek T, Nothdurft HD. Changing epidemiology of hepatitis A: time for vaccination in childhood. J Travel Med. 2000;7:142–148. doi: 10.2310/7060.2000.00046. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–1022. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CS, Kwon KS, Koh DH, Youm JH, Gwack J, Lee JH. Declining hepatitis A antibody seroprevalence in the Korean military personnel. Jpn J Infect Dis. 2010;63:192–194. [PubMed] [Google Scholar]
- 8.Broman M, Jokinen S, Kuusi M, et al. Epidemiology of hepatitis A in Finland in 1990-2007. J Med Virol. 2010;82:934–941. doi: 10.1002/jmv.21759. [DOI] [PubMed] [Google Scholar]
- 9.Kwon SY. Current status of liver diseases in Korea: hepatitis A. Korean J Hepatol. 2009;15(Suppl 6):S7–S12. doi: 10.3350/kjhep.2009.15.S6.S7. [DOI] [PubMed] [Google Scholar]
- 10.Jung YK, Kim JH. Epidemiology and clinical features of acute hepatitis A: from the domestic perspective. Korean J Hepatol. 2009;15:438–445. doi: 10.3350/kjhep.2009.15.4.438. [DOI] [PubMed] [Google Scholar]
- 11.Kim YJ, Lee HS. Increasing incidence of hepatitis A in Korean adults. Intervirology. 2010;53:10–14. doi: 10.1159/000252778. [DOI] [PubMed] [Google Scholar]
- 12.Song YB, Lee JH, Choi MS, et al. The age-specific seroprevalence of hepatitis A virus antibody in Korea. Korean J Hepatol. 2007;13:27–33. [PubMed] [Google Scholar]
- 13.Sohn YM, Lee JS, Park JH, et al. Immunizing children to protect against the increasing risk of hepatitis A in adolescents and young adults in South Korea. Southeast Asian J Trop Med Public Health. 2004;35:954–958. [PubMed] [Google Scholar]
- 14.Kim JH, Kang JH, Lee SY, et al. A study for seroprevalence of antibody to hepatitis A in Korea. Korean J Hepatol. 2007;13(3 Suppl):S27. [Google Scholar]
- 15.Yoo HJ, Yoon BC, Han BH, et al. The clinical feature of acute hepatitis A and Ig G anti-HAV seroprevalence of the Busan: single center experience. Korean J Hepatol. 2007;13(3 Suppl):S173. [Google Scholar]
- 16.Kim do Y, Ahn SH, Lee HW, et al. Anti-hepatitis A virus seroprevalence among patients with chronic viral liver disease in Korea. Eur J Gastroenterol Hepatol. 2007;19:923–926. doi: 10.1097/MEG.0b013e3282efa432. [DOI] [PubMed] [Google Scholar]
- 17.Fiore AE. Hepatitis A transmitted by food. Clin Infect Dis. 2004;38:705–715. doi: 10.1086/381671. [DOI] [PubMed] [Google Scholar]
- 18.Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami SQ. Helicobacter pylori infection in children: population-based age-specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99:279–282. doi: 10.1111/j.1651-2227.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 19.BinSaeed AA. Is there a link between seropositivity to Helicobacter pylori and hepatitis A virus? A systematic review. Int J Infect Dis. 2010;14:e567–e571. doi: 10.1016/j.ijid.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Bizri AR, Nuwayhid IA, Hamadeh GN, Steitieh SW, Choukair AM, Musharrafieh UM. Association between hepatitis A virus and Helicobacter pylori in a developing country: the saga continues. J Gastroenterol Hepatol. 2006;21:1615–1621. doi: 10.1111/j.1440-1746.2006.04268.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin HY, Chuang CK, Lee HC, Chiu NC, Lin SP, Yeung CY. A seroepidemiologic study of Helicobacter pylori and hepatitis A virus infection in primary school students in Taipei. J Microbiol Immunol Infect. 2005;38:176–182. [PubMed] [Google Scholar]
- 22.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(Suppl 2):33–39. [PubMed] [Google Scholar]
- 23.Lerman Y, Chodik G, Aloni H, Ribak J, Ashkenazi S. Occupations at increased risk of hepatitis A: a 2-year nationwide historical prospective study. Am J Epidemiol. 1999;150:312–320. doi: 10.1093/oxfordjournals.aje.a010004. [DOI] [PubMed] [Google Scholar]
- 24.Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA. 2005;294:194–201. doi: 10.1001/jama.294.2.194. [DOI] [PubMed] [Google Scholar]
- 25.Shin DH, Han SK, Choi PC, Lim SW, Kim KM, Sinn DH. Vaccination rate and seroepidemiology of hepatitis a in chronic-hepatitis-B-infected individuals in the Korean army. Gut Liver. 2010;4:207–211. doi: 10.5009/gnl.2010.4.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]