Abstract
Plumbagin inhibited activation, proliferation, cytokine production, and graft-versus-host disease in lymphocytes and inhibited growth of tumor cells by suppressing nuclear factor-κB (NF-κB). Plumbagin was also shown to induce reactive oxygen species (ROS) generation in tumor cells via an unknown mechanism. Present report describes a novel role of cellular redox in modulation of immune responses in normal lymphocytes by plumbagin. Plumbagin depleted glutathione (GSH) levels that led to increase in ROS generation. The decrease in GSH levels was due to direct reaction of plumbagin with GSH as evinced by mass spectrometric and HPLC analysis. Further, addition of plumbagin to cells resulted in decrease in free thiol groups on proteins and increase in glutathionylation of proteins. The suppression of mitogen-induced T-cell proliferation and cytokine (IL-2/IL-4/IL-6/IFN-γ) production by plumbagin was abrogated by thiol antioxidants but not by non-thiol antioxidants confirming that thiols but not ROS play an important role in biological activity of plumbagin. Plumbagin also abrogated mitogen-induced phosphorylation of ERK, IKK, and degradation of IκB-α. However, it did not affect phosphorylation of P38, JNK, and AKT. Our results for the first time show that antiproliferative effects of plumbagin are mediated by modulation of cellular redox. These results provide a rationale for application of thiol-depleting agents as anti-inflammatory drugs.
Keywords: ANTI-INFLAMMATORY, THIOL, REDOX, IMMUNE, PLUMBAGIN, GLUTATHIONYLATION
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is found in the plants of Plumbaginaceae, Droseraceae, Ancestrocladaceae, and Dioncophyllaceae families. Plumbagin is also present along with a series of other structurally related naphthoquinones in the roots, leaves, bark, and wood of Juglans regia (English walnut, Persian walnut, and California walnut), Juglans cinerea (butternut and white walnut), and Juglans nigra (black walnut). Preparations derived from black walnut have been used as hair dyes and skin colorants in addition to being applied topically for the treatment of acne, inflammatory diseases, ring-worm, and fungal, bacterial, and viral infections. The root of Plumbago zeylanica (also called Chitrak), a major source of plumbagin, has been used in traditional Indian medicine since 750 BC as an antiatherogenic, cardiotonic, hepatoprotective, and neuroprotective agent [Padhye and Kulkarni, 1973; Tilak et al., 2004]. The active principle, plumbagin, was first isolated in 1829 [D’Astafort, 1829].
Plumbagin has been shown to exert several therapeutic biological effects including anticancer, antiproliferative, chemopreventive, chemotherapeutic, and radiosensitizing properties in experimental animals as well as in tumor cells in vitro [Naresh et al., 1996; Singh and Udupa, 1997; Hazra et al., 2002]. Earlier, we showed that plumbagin exerts its various activities through suppression of the transcription factor nuclear factor-κB (NF-κB). It suppressed constitutive as well as inducible NF-κB in various human tumor cell lines [Sandur et al., 2006]. Recently, we showed that plumbagin inhibited NF-κB activation in lymphocytes. The suppression of NF-κB by plumbagin resulted in inhibition of mitogen-induced activation and proliferation of lymphocytes [Checker et al., 2009]. It also inhibited lipopolysaccharide-induced activation and cytokine production in macrophages [Raghu et al., 2009]. We also showed that plumbagin suppressed homeostatic proliferation of autologous T cells in lymphopenic mice and graft-versus-host disease associated morbidity and mortality, which are known to require NF-κB activation [Checker et al., 2009; Sharma et al., 2009].
In addition to having anti-inflammatory and growth-modulatory effects, plumbagin exhibited antibacterial action through generation of pro-oxidants [Krishnaswamy and Purushothaman, 1980]. It generated reactive oxygen species (ROS) in tumor cells leading to DNA damage and cytotoxicity [Inbaraj and Chignell, 2004; Srinivas et al., 2004; Kawiak et al., 2007]. It was shown that plumbagin directly inhibited the binding of NF-κB to its consensus target sequence by modifying a critical cystine-38 residue on p65 in tumor cells. This suppressive effect of plumbagin was shown to be sensitive to thiol-containing antioxidant, dithiothreitol [Sandur et al., 2006]. Cellular redox status plays an important role in the biological effector functions of lymphocytes and leukocytes [Malmberg et al., 2001; Hildeman et al., 2003b; Klemke and Samstag, 2009]. Since oxidative stress has been shown to modulate signaling pathways through modulation of thiol groups present on proteins and glutathionylation of many proteins [Fratelli et al., 2002; Biswas et al., 2006; Winterbourn and Hampton, 2008; Dalle-Donne et al., 2009], we hypothesized that the anti-inflammatory effects of plumbagin may be due to its ability to perturb the redox balance in cells leading to modification of critical signaling molecules required for activation of lymphocytes. Further, the modulation of intracellular redox by plumbagin and its mechanism is not fully understood. To test this hypothesis, we investigated the effect of plumbagin on cellular redox status. We also examined the effects of different antioxidants (thiol/non-thiol) on immunosuppressive and anti-inflammatory effects of plumbagin. The biochemical and signaling mechanisms responsible for novel redox dependent anti-inflammatory action of plumbagin are described herein.
MATERIALS AND METHODS
REAGENTS
Plumbagin (practical grade), RPMI 1640 medium, HEPES, ethylenediaminetetraacetic (EDTA), ethylene glycol tetraacetic acid (EGTA), phenylmethylsulfonyl fluoride (PMSF), leupeptin, aprotinin, benzamidine, dithiothreitol (DTT), glutathione (GSH), N-acetyl cysteine (NAC), nonidet P-40, propidium iodide (PI), Hoechst, monochlorobimane, maleic acid diethyl ester (DEM), dimethyl sulfoxide (DMSO), rotenone, allopurinol, diphenyleneiodonium, and antibodies against BCL-2, BCL-xL, Cyclin A, and β-actin were purchased from Sigma Chemical Co. (USA). A 100mM solution of plumbagin was prepared in DMSO, stored as small aliquots at −20°C, and then diluted as needed in cell culture medium. Carboxy fluorescein diacetate succinimidyl ester (CFSE), 5-(and-6)-carboxy-2,7-dichlorofluorescein diacetate (DCF-DA), streptavidin agarose, biotin C2-iodoacetamide, glutathione ethyl ester biotin amide (Bio-GEE) were procured from Molecular Probes, Invitrogen. Fetal calf serum (FCS) was obtained from Gibco BRL. Concanavalin A (con A), Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), Trolox, Catalase Assay Kit, and Superoxide dismutase enzyme assay kit were purchased from Calbiochem (USA). ELISA sets for detection of cytokines (IL-2, IL-4, IL-6, and IFN-γ) were procured from BD Pharmingen (USA). Antibodies against p-ERK, ERK, p-IKKα/β, IKKα, IKKβ, IκB-α, p-P38, P38, p-JNK, JNK, p-AKT, and AKT were obtained from Cell Signaling Technologies (USA).
ANIMAL MAINTENANCE
Six- to eight-week-old inbred Swiss male mice, weighing approximately 20–25 g, reared in the animal house of Bhabha Atomic Research Centre were used. They were housed at 23 ± 3°C with a 12:12 h light/dark cycle and were given mouse chow and water ad libitum. The guidelines issued by the Institutional Animal Ethics Committee of Bhabha Atomic Research Centre, Government of India, regarding the maintenance and dissections of small animals were strictly followed.
PROLIFERATION ASSAY
Lymphocytes were obtained by squeezing the spleen through a nylon mesh in a petri plate containing RPMI medium. The RBCs were lysed by brief hypotonic shock. Lymphocytes were stained with CFSE (20 µM, 5 min, 37°C) and washed three times using ice-cold RPMI medium containing 10% FCS, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Two million lymphocytes were treated with different antioxidants for 2 h followed by plumbagin (1 µM, 2 h) and were stimulated with con A (5 µg/ml) for 72 h at 37°C in 2ml RPMI with 10% FCS in a 95% air/5% CO2 atmosphere. Vehicle (DMSO)-treated cells served as a control. Cell proliferation was measured by dye dilution in a flowcytometer (BD FACSAria). Percent daughter cells that showed a decrease in CFSE fluorescence intensity were calculated using FCS express (Version 3) software.
MEASUREMENT OF CYTOKINE SECRETION
The concentration of IL-2, IL-4, IFN-γ, and IL-6 in the supernatant of control vehicle-treated cells and cells stimulated with con A (5 µg/ml) for 24 h after plumbagin (1 µM) treatment was estimated using cytokine ELISA sets (BD Pharmingen).
WESTERN BLOT ANALYSIS
To determine the levels of protein expression, whole cell lysates were prepared [Takada and Aggarwal, 2003] and fractionated by SDS–PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, probed with the appropriate antibodies, and detected by enhanced chemiluminescence (Roche, Germany).
INTRACELLULAR GSH ASSAY
To measure intracellular GSH, lymphocytes were incubated with the indicated concentrations of DEM or treated with plumbagin for 4 h at 37°C. Monochlorobimane (final concentration, 40 µM, 30 min at 37°C) was loaded into cells [Hedley and Chow, 1994]. Fluorescence emission from cellular sulfhydryl-reacted monochlorobimane was measured using a spectrofluorimeter (BMG Labtech Optima). Monochlorobimane is also known to react with small-molecular-weight thiols other than GSH but GSH forms the major monochlorobimane reactive thiol. Hence, MCB fluorescence is referred to as GSH levels in this manuscript. There are several reports in the literature measuring GSH levels using this dye [Bouzyk et al., 1997; Deas et al., 1997; Agrawal et al., 2007].
PRO-OXIDANT MEASUREMENTS
To detect intracellular ROS, lymphocytes were preincubated with 20 µM oxidation-sensitive DCF-DA for 25 min at 37°C before being treated with various concentrations of plumbagin. The oxidized form of the dye (DCF) acts as a control for changes in uptake, ester cleavage, and efflux [Checker et al., 2008]. After 1 h of incubation, the increase in fluorescence resulting from oxidation of H2DCF to DCF was measured using a spectrofluorimeter.
HPLC SEPARATION OF PRODUCTS OF REACTION OF PLUMBAGIN WITH GSH
A Waters (Milford, MA) Millenium32 chromatographic system equipped with model 510 pumps, a U6K injector, a system interface module, and a diode array detector was used for sample analyses. Separations were achieved on Waters Spherisorb S3 ODS2 reverse phase column (4.6mm × 150 mm). The column was equilibrated with HPLC grade water at a flow rate of 1 ml/min prior to injecting 25 µl of the reaction mixture. Elution was carried out using a gradient of acetonitrile from 0% to 100% over 20 min, beginning 2 min after injection of the sample. Absorbance of the effluent was measured using a diode array detector.
LIQUID CHROMATOGRAPHY–MASS SPECTROMETRY
LC–MS was performed using a Varian ProStar 410 AutoSampler in combination with Varian 1200 L LC–MS equipment triple quadrupole mass (QqQ) spectrometer with ESI source (Varian, Inc., USA). The column was 3 µm C18, 100mm × 2.1mm (Phenomex). A gradient mobile phase was used at 0.2 ml/min. The ESI source operated in positive ionization mode.
CONFOCAL MICROSCOPY
Confocal laser microscopy was used to study the entry of plumbagin into lymphocytes. Lymphocytes were incubated with or without plumbagin (10 µM) in medium containing 10% FBS. At the end of 24 h, the cells were centrifuged onto coverslips, fixed with paraformaldehyde, stained with Hoechst stain for nuclear staining, and mounted onto glass slides. Slides were examined using a LSM510 scanning module (Carl Zeiss Microscopy, Jena GmbH, Germany) with a krypton–argon laser, coupled to an Orthoplan Zeiss photomicroscope using a 488nm laser line and a 510nm-band pass filter. Overlay images were recorded by superimposing simultaneous images from each channel.
LABELING, PULL DOWN, AND DETECTION OF PROTEIN THIOLATES
To detect protein thiolate anions, lymphocytes were preincubated with plumbagin for 4 h after which the cells were harvested and whole cell lysates were prepared. The lysates were incubated with BIAM (0.2 mM) for 30 min at 37°C and then incubated with streptavidin agarose beads (20µl/mg of protein) for 1 h at 4°C. The agarose beads were separated by centrifugation, washed four times with RIPA buffer (1% NP-40, 0.1% SDS, 0.5 mg/ml sodium deoxycholate, 150mM NaCl, and 50mM Tris–HCl, pH 7.5) and boiled in SDS sample buffer. Proteins in the eluent were resolved by SDS–PAGE and detected by Coomassie blue staining [Ying et al., 2007].
PURIFICATION OF PUTATIVE S-GLUTATHIONYLATED PROTEINS USING BIO-GEE
Lymphocytes were incubated with Bio-GEE (250 µM, 1 h) prior to the addition of plumbagin at the concentrations indicated for 4 h with cycloheximide (1 µg/ml). Incubations were terminated, and a soluble protein extract was obtained as described above. The soluble proteins were then incubated with streptavidin agarose beads (20µl/mg of protein) for 30 min at 4°C. The agarose beads were separated by centrifugation, washed four times with RIPA buffer, and boiled in SDS sample buffer. Proteins in the eluent were resolved by SDS–PAGE and detected by Coomassie blue staining [Sullivan et al., 2000].
STATISTICAL ANALYSIS
The statistical significance of the differences in respect of all parameters studied between untreated and con A stimulated cells in presence or absence of plumbagin or antioxidants was assessed by Student’s “t”-test.
RESULTS
The aim of the present studies was to investigate the role of cellular redox status on immunomodulation by plumbagin. The antioxidants and pharmacological inhibitors used in these experiments did not affect viability of lymphocytes.
PLUMBAGIN MODULATES CELLULAR REDOX BY CHANGING ROS LEVELS, GSH CONTENT, AND ANTIOXIDANT ENZYME ACTIVITY
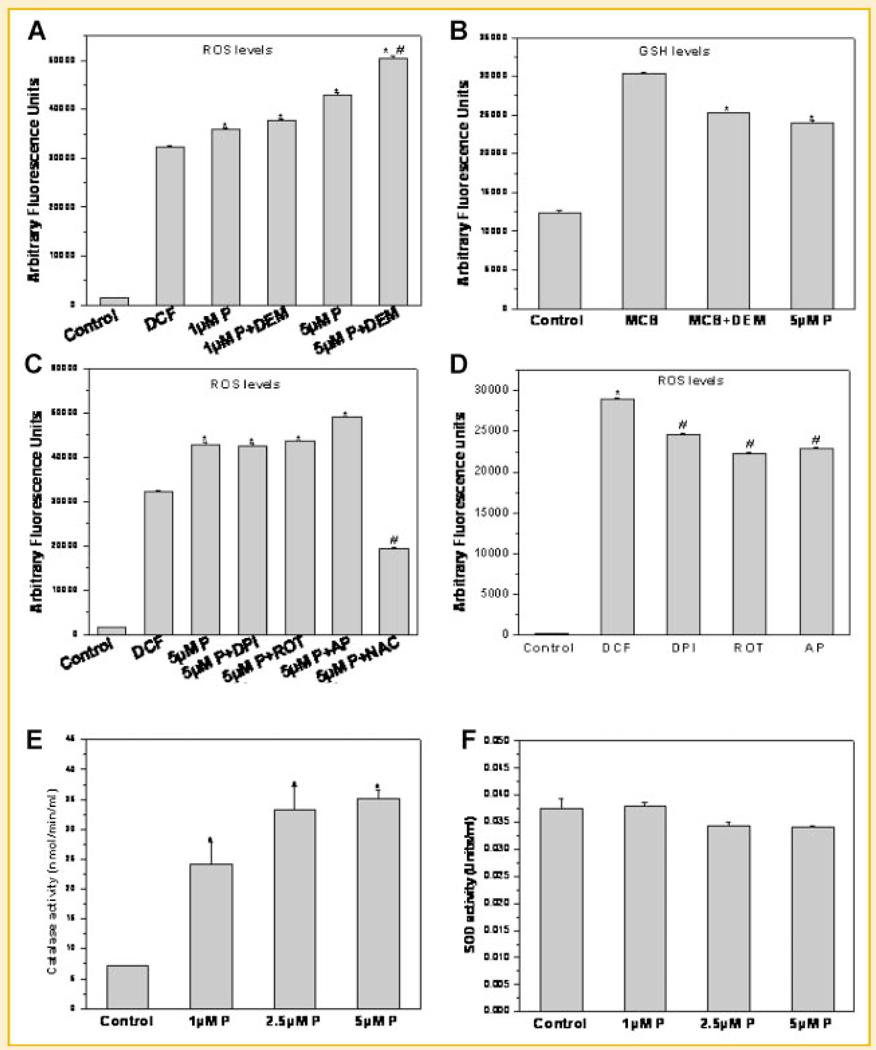
Figure 1A shows the levels of ROS in control and plumbagin-treated lymphocytes. Plumbagin (5 µM)-induced increase in ROS levels in lymphocytes was further augmented in presence of diethymaleate, a GSH depleting agent (Fig. 1A). To estimate the effect of plumbagin on intracellular GSH levels, lymphocytes were incubated with plumbagin or DEM for 4 h. Plumbagin-treated cells showed significantly lower levels of intracellular GSH as compared to untreated cells (Fig. 1B). The positive control, DEM showed depletion of GSH to a similar extent as plumbagin (Fig. 1B). Further, to identify the intracellular source of ROS in plumbagin-treated cells, lymphocytes were incubated with inhibitors of xanthine oxidase (allopurinol, AP, 1 mM), NADPH oxidase (DPI, 50 µM), or mitochondrial complex I inhibitor (rotenone, 10 µM). These inhibitors did not abrogate the plumbagin-induced increase in ROS levels in lymphocytes (Fig. 1C). Incubation of lymphocytes with NAC (10 mM) prior to addition of plumbagin, on the other hand, led to decrease in ROS production (Fig. 1C). All the inhibitors of ROS generation used in our experiment reduced the DCF fluorescence suggesting that basal ROS levels decreased upon addition of these inhibitors to lymphocytes (Fig. 1D). The common groups in Figure 1A,C (Control, DCF, 5µM P) are from the same experiment.
Fig. 1.
A: Plumbagin increased ROS levels in lymphocytes. Lymphocytes were stained with DCF-DA (20 µM, 30 min, 37°C) and were treated with plumbagin (1 and 5 µM) in presence or absence of DEM. Each bar shows mean DCF-DA fluorescence ± SEM from four replicates and three such independent experiments were carried out. *P < 0.01 as compared to untreated cells, #P < 0.01 as compared to plumbagin (5 µM)-treated cells. B: Plumbagin depleted intracellular thiols. Lymphocytes were treated with plumbagin or DEM (90 µM) for 4 h at 37°C and stained with MCB (final concentration 40 µM) for 30 min. Fluorescence emission was measured at 490 nm following excitation at 394 nm. Each bar shows mean MCB–GSH adduct fluorescence ± SEM from four replicates and three such independent experiments were carried out. *P < 0.01 as compared to untreated cells stained with MCB. C: Effect of different inhibitors of ROS generation or N-acetylcysteine (NAC, 10 mM) on plumbagin-mediated increase in ROS. Lymphocytes were incubated with inhibitors of NADPH oxidase, xanthine oxidase, or mitochondrial complex I (diphenyl iodonium, DPI, 50 µM or allopurinol, AP, 1mM or rotenone, ROT, 10 µM, respectively) or NAC (10 mM) for 2 h and stained with DCF-DA. The cells were treated with plumbagin for 1 h at 37°C and fluorescence emission was measured at 520 nm following excitation at 488 nm. Each bar shows mean DCF fluorescence ± SEM from four replicates and two such independent experiments were carried out. *P < 0.01 as compared to untreated cells stained with DCF-DA. #P < 0.01 as compared to plumbagin-treated cells. D: Effect of different inhibitors of ROS generation on basal ROS levels. Lymphocytes were incubated with inhibitors of NADPH oxidase, xanthine oxidase, or mitochondrial complex I (diphenyl iodonium, DPI, 50 µM or allopurinol, AP, 1mM or rotenone, ROT, 10 µM, respectively) for 2 h and stained with DCF-DA. Fluorescence emission was measured at 520 nm following excitation at 488 nm. Each bar shows mean DCF fluorescence ± SEM from four replicates and two such independent experiments were carried out. *P < 0.01 as compared to untreated control cells. #P < 0.01 as compared untreated cells stained with DCF-DA. E: Plumbagin enhanced catalase activity but not SOD activity. Lymphocytes were incubated with plumbagin for 4 h and the activity of catalase (E) and SOD (F) enzymes was measured according to manufacturer's protocol. Each bar represents catalase activity (E) and SOD activity (F). *P < 0.01 as compared to vehicle-treated cells. Each bar shows mean ± SEM from three replicates and two such independent experiments were carried out.
Since plumbagin induced increase in ROS levels, the activities of antioxidant enzymes like catalase and SOD in lymphocytes treated with plumbagin were measured. Plumbagin-treated cells showed significantly higher catalase activity as compared to untreated cells (Fig. 1E) without altering the SOD activity in lymphocytes (Fig. 1F). These results suggest that plumbagin regulates redox status of lymphocytes by modulation of thiols, mainly GSH levels.
IMMUNOSUPPRESSIVE EFFECTS OF PLUMBAGIN IN LYMPHOCYTES WERE ABROGATED BY THIOL ANTIOXIDANTS
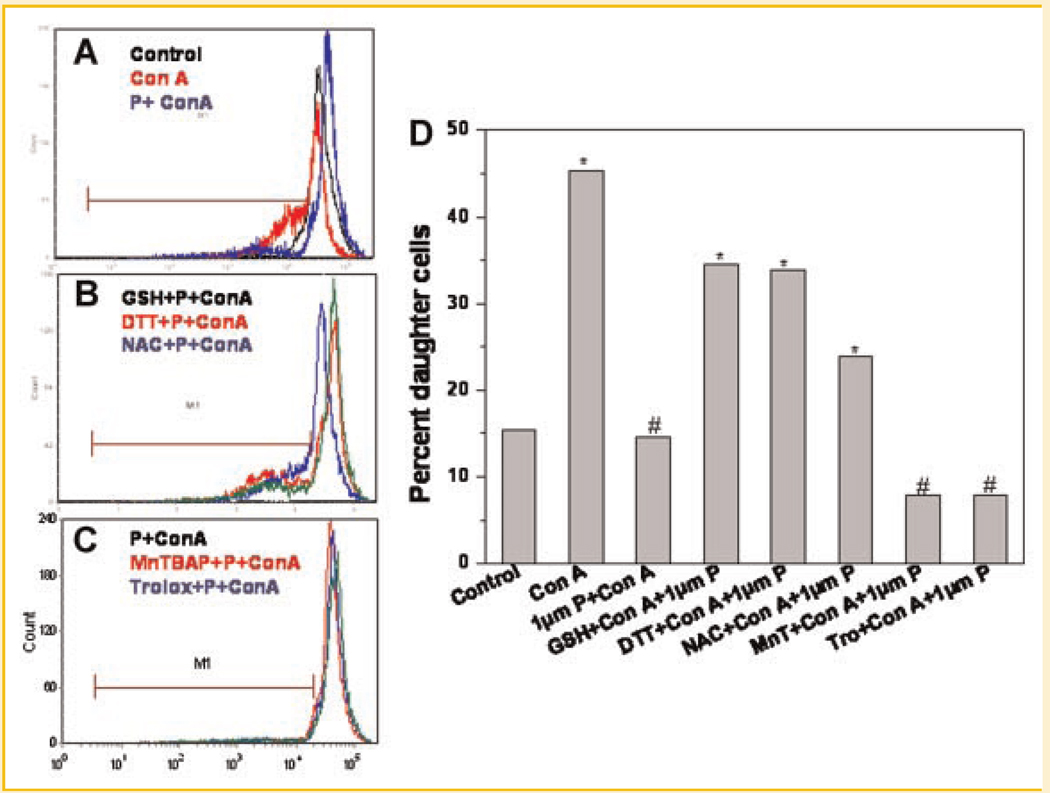
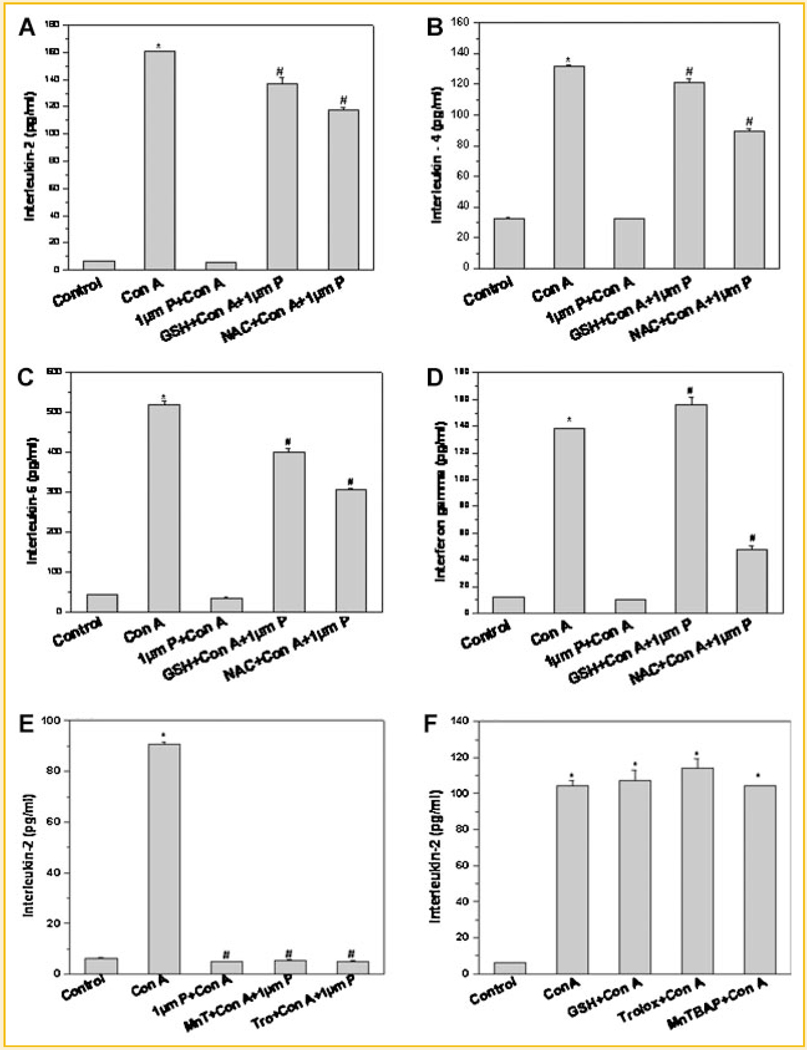
Figure 2 shows the modulation of immunosuppressive effects of plumbagin by different antioxidants. Thiol-containing antioxidants (DTT, GSH, and NAC) abrogated the antiproliferative effect of plumbagin in mitogen-activated lymphocytes (Fig. 2A–C). However, antioxidants that do not contain a thiol group (MnTBAP and trolox) did not restrain the antiproliferative action of plumbagin (Fig. 2D). Similarly, anti-inflammatory action of plumbagin as seen by suppression of mitogen-induced cytokines was also curbed by thiol-containing antioxidants, GSH and NAC, (Fig. 3A–D) but not by non-thiol antioxidants (Fig. 3E). Both thiol and non-thiol antioxidants per se did not affect con A induced IL-2 secretion (Fig. 3F).
Fig. 2.
Antiproliferative effects of plumbagin were abrogated by thiol-containing antioxidants. Lymphocytes were stained with CFSE and were incubated with different antioxidants (GSH 10mM or NAC 10mM or DTT 100 µM or MnTBAP 100 µM or trolox 100 µM) for 2 h. The cells were stimulated with con A in presence or absence of plumbagin for 92 h at 37°C in a 5%CO2/99% air atmosphere. Cell proliferation was measured from CFSE dye dilution using a flowcytometer. Percent daughter cells were calculated using FCSexpress3 software. 95 Representative flowcytometric histograms showing effect of plumbagin on T-cell proliferation (A) and its modulation by thiol-containing antioxidants (B) and non-thiol antioxidants (C). The percentage of daughter cells in each group is shown in (D). Each bar shows mean ± SEM from three replicates and three such independent experiments were carried out. *P < 0.01 as compared to untreated cells and #P < 0.01 as compared to untreated cells stimulated with con A.
Fig. 3.
Effect of thiol-containing antioxidants on cytokine production in plumbagin-treated lymphocytes stimulated with con A. Lymphocytes were incubated with different antioxidants (GSH or NAC) for 2 h. The cells were stimulated with con A in presence or absence of plumbagin for 24 h at 37°C in a 5%CO2/95% air atmosphere. The concentration of IL-2 (A), IL-4 (B), IL-6 (C), and IFN-γ (D) in the culture supernatant was estimated by ELISA. Each bar shows mean ± SEM from three replicates and three such independent experiments were carried out. *P < 0.01 as compared to control untreated cells. #P < 0.01 as compared to plumbagin-treated cells stimulated with con A. E: Effect of non-thiol antioxidants on IL-2 production in lymphocytes stimulated with con A in presence of plumbagin. Lymphocytes were incubated with different antioxidants (MnTBAP or trolox) for 2 h. The cells were stimulated with con A in presence or absence of plumbagin for 24 h at 37°C in a 5%CO2/95% air atmosphere. The concentration of IL-2 (E) in the culture supernatant was estimated by ELISA. Each bar shows mean ± SEM from three replicates and three such independent experiments were carried out. *P < 0.01 as compared to control untreated cells. #P < 0.01 as compared to cells stimulated with con A. F: Effect of thiol and non-thiol antioxidants on con A induced IL-2 production. Each bar represents mean ± SEM from three replicates and two such independent experiments were carried out. *P < 0.01 as compared to control untreated cells.
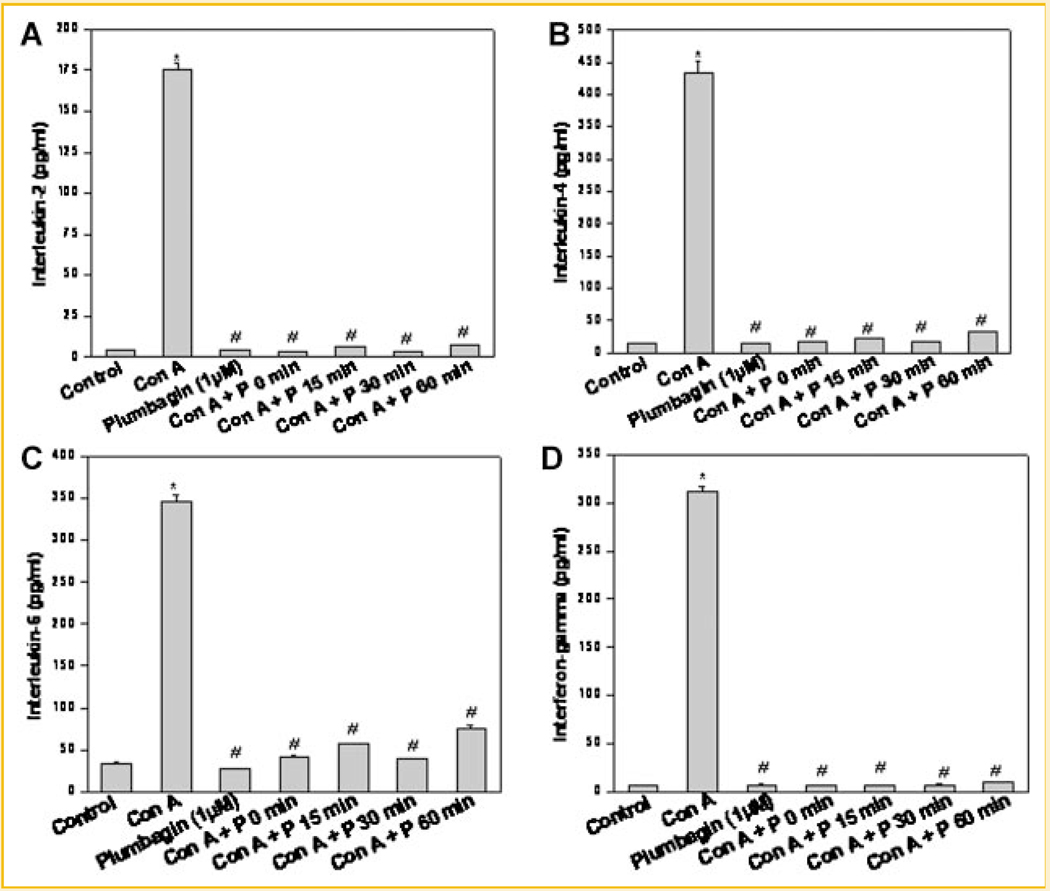
PLUMBAGIN INHIBITED CYTOKINE PRODUCTION IN ACTIVATED LYMPHOCYTES
Our present results pointed toward a novel redox-dependent immunosuppressive action of plumbagin. Our previous reports have shown effective immunosuppressive action of plumbagin in naïve T cells and B cells. Experiments were carried out to determine if plumbagin could inhibit effector functions in T cells when it was added after stimulation with mitogen. Figure 4 clearly shows that plumbagin effectively suppressed cytokine production in T cells even when it was added up to 60 min after their activation with a mitogen. To see the reversibility of plumbagin-mediated immunosuppression, splenic lymphocytes were treated with plumbagin (4 h) and cells were washed twice with medium. Cells were then rested for 24 h before stimulating with con A. Despite resting cells for 24 h, plumbagin suppressed con A induced IL-2 production. These results showed that immunosuppressive effects of plumbagin in lymphocytes are not reversible (Fig. S2).
Fig. 4.
Inhibition of cytokine production in activated lymphocytes by plumbagin. Lymphocytes were stimulated with con A (5 µg/ml) following which plumbagin was added at the indicated time points and the cells were cultured for 24 h at 37°C. Vehicle-treated cells served as control. The concentration of cytokines in the supernatant was estimated using ELISA. Each bar represents concentration of (A) IL-2, (B) IL-4, (C) IL-6, and (D) IFN-γ. Data points represent mean ± SEM from three replicates and two such independent experiments were carried out. *P < 0.01, as compared to vehicle-treated cells and #P < 0.01, as compared to con A stimulated cells.
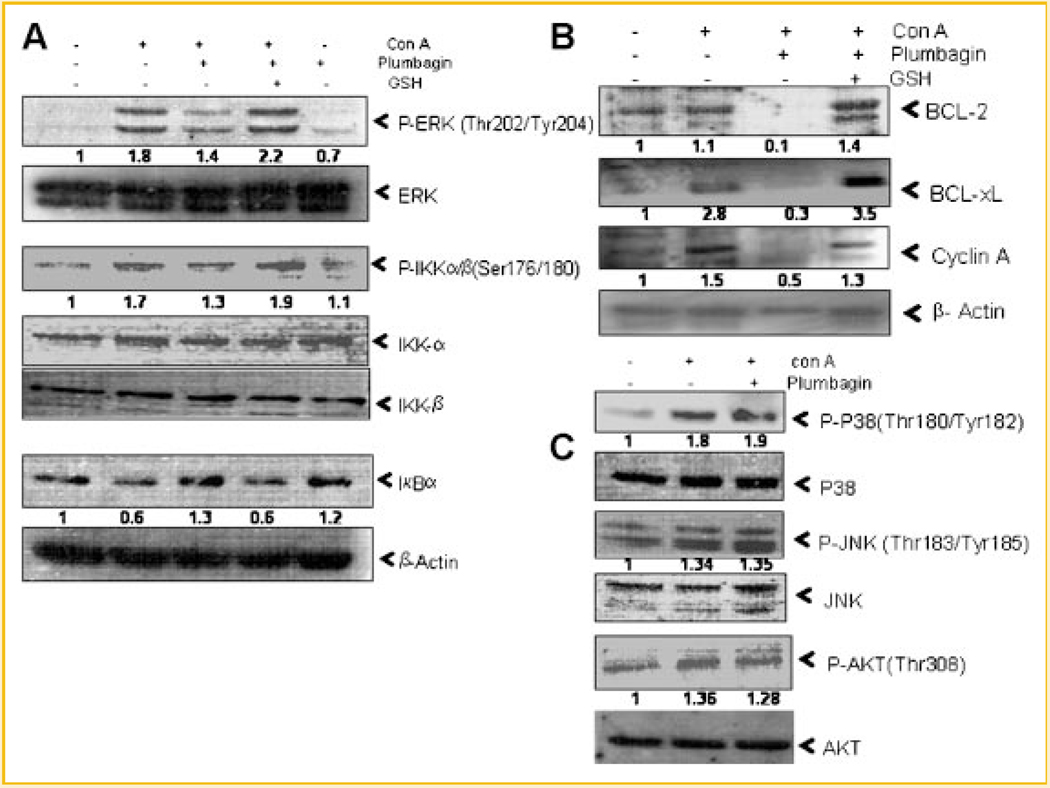
SUPPRESSIVE EFFECTS OF PLUMBAGIN ON MITOGEN-INDUCED SIGNALING EVENTS WERE SENSITIVE TO GSH
Figure 5A shows the activation of ERK and IKK and degradation of IκB-α in mitogen-activated lymphocytes as compared to unstimulated cells. Plumbagin inhibited mitogen-induced activation of ERK and IKK and degradation of IκB-α in lymphocytes. Plumbagin down-modulated the expression of NF-κB-dependent genes like Bcl-2, Bcl-xL, and cyclin A (Fig. 5B). The suppressive action of plumbagin in activated lymphocytes was completely abrogated by GSH (Fig. 5A,B). At the same time, plumbagin did not suppress mitogen-induced activation of redox-sensitive molecule c-Jun N-terminal kinase (JNK) (Fig. 5C). The mitogen-induced activation of P38MAPkinase and atypical kinase (AKT) was also not suppressed by plumbagin as shown in Figure 5C suggesting that it has specific targets in cells.
Fig. 5.
Inhibition of proliferation/survival associated signaling molecules by plumbagin and their modulation by GSH in activated T cells. Lymphocytes were incubated with plumbagin (1 µM, 4 h) in presence or absence of GSH and were stimulated with con A (5 µg/ml) for 1 h (A,C) or 24 h (B). Whole cell lysates were prepared, fractionated on 10% SDS–PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed using different antibodies specific for (A) P-ERK, ERK, P-IKK-α/β, IKK-α, IKK-β, IκB-α, (B) BCL-2, BCL-xL, Cyclin A, and (C) P-P38, P-38, P-JNK, JNK, P-AKT, AKT. β-Actin was used as loading control. Two such independent experiments were carried out.
PLUMBAGIN INTERACTED WITH GSH
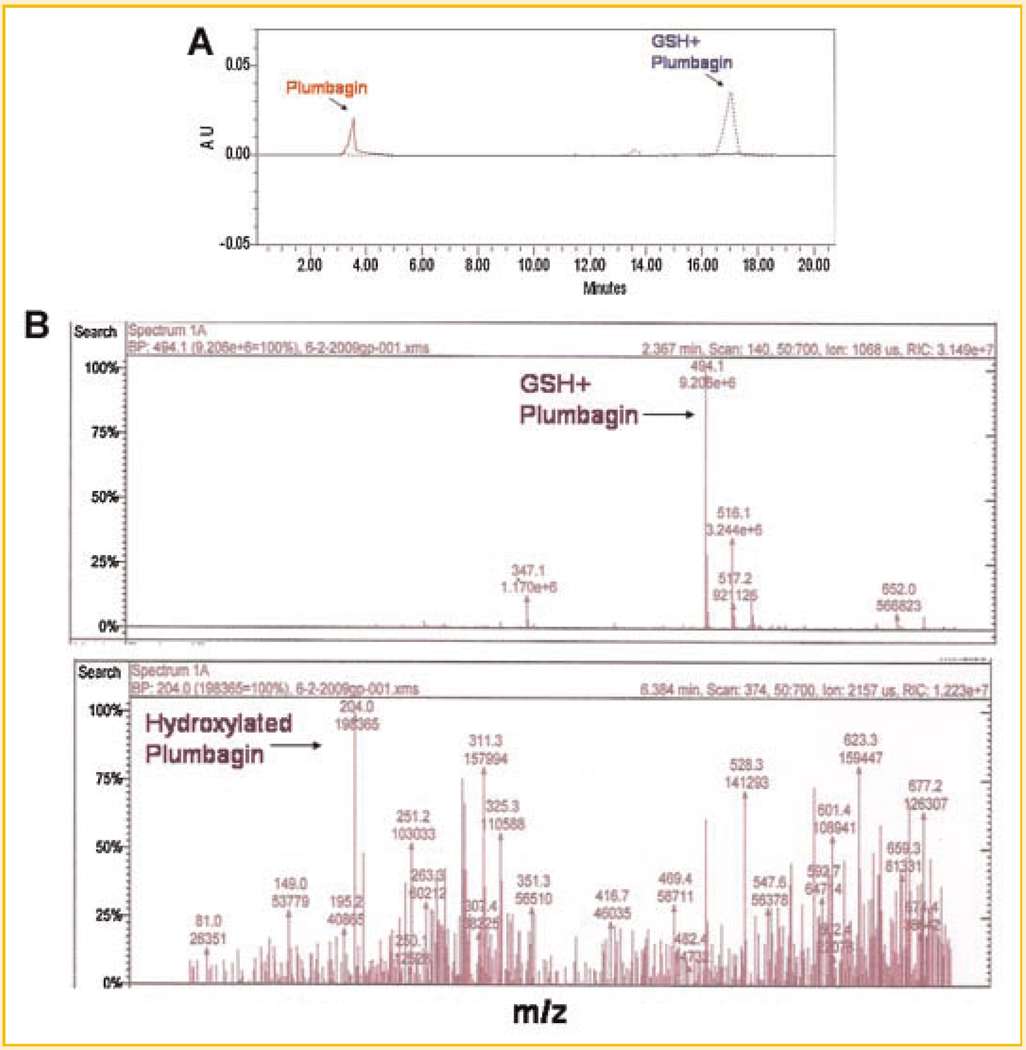
Since antiproliferative and anti-inflammatory effects of plumbagin were sensitive to presence of thiol antioxidants, experiments were carried out to determine whether plumbagin physically interacted with thiol groups. Plumbagin was incubated with GSH and subjected to HPLC separation. Retention time of pure plumbagin on C18 column was 3.5 min (Fig. 6A, red line). This peak disappeared when plumbagin was preincubated with GSH and a new peak appeared at 17.5 min (Fig. 6A, blue dotted line). A minor peak also appeared at 13.5 min. These results indicated that the reaction of plumbagin with GSH formed a single major product which could be adduct of plumbagin with GSH. To characterize this adduct, LC–MS analysis was carried out. Molecular mass analysis revealed that the major peak corresponded to plumbagin–GSH adduct (M = 494) (Fig. 6B, upper panel). In addition to plumbagin–GSH complex, plumbagin also induced GSH to GSSG conversion. A peak corresponding to molecular mass 204 also appeared which may be hydroxylated plumbagin (Fig. 6B, lower panel). There were other minor peaks appearing at different molecular masses, which could not be characterized. However, these products did not appear in HPLC separation. This could be due to similar polarity of the products making them separate at the same retention time.
Fig. 6.
A: HPLC separation of products of reaction of plumbagin with GSH. Plumbagin (100 µM) was mixed with GSH (10 mM) in 10mM potassium phosphate buffer. After 1 h incubation at 37°C, 25 µl of each sample was subjected to HPLC. Effluent was monitored using a diode array detector. Red line (solid) indicates plumbagin alone and blue (dotted) line indicates reaction product(s) of plumbagin with GSH. B: Identification of products of reaction of plumbagin with GSH by mass spectrometry. Sample containing 100 µM plumbagin in 10mM GSH was subjected to LC–MS analysis using ESI ion trap detector. Two major peaks corresponding to plumbagin–GSH adduct (mol. wt 494, upper panel) and hydroxylated product of plumbagin (mol. wt 204, lower panel) were observed following ESI-MS analysis. Two such independent experiments were carried out.
PLUMBAGIN ENTERED CELLS AND INDUCED GLUTATHIONYLATION OF PROTEINS IN LYMPHOCYTES
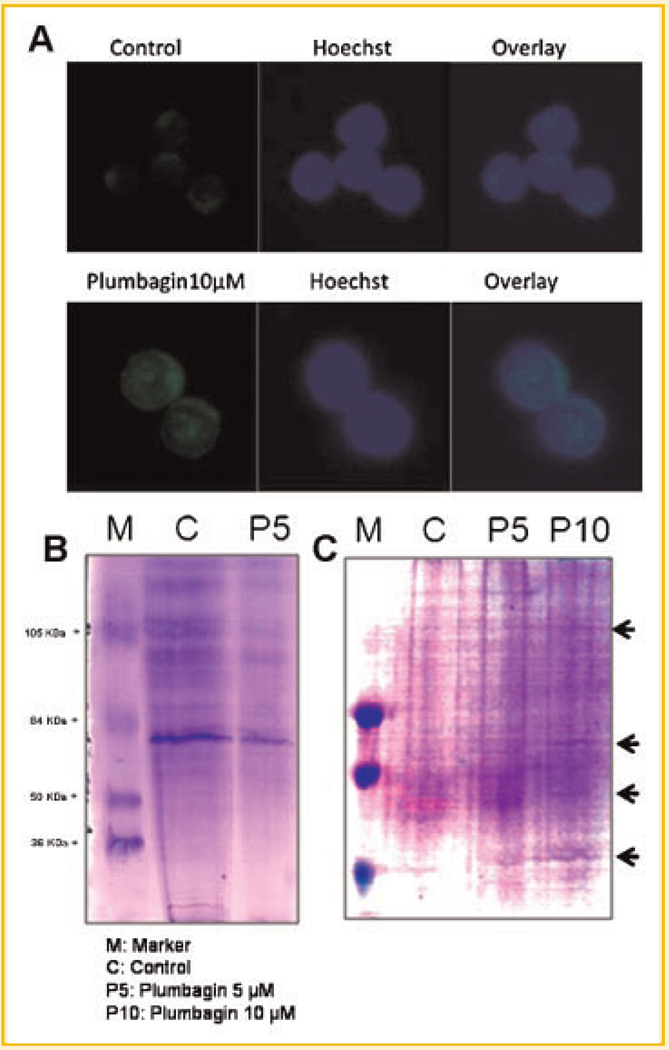
Figure 7A shows the confocal images of lymphocytes treated with plumbagin for 24 h and stained with Hoechst. Plumbagin entered lymphocytes and majority of it was localized in the nucleus (Fig. 7A). Since plumbagin reacted with GSH and also induced conversion of GSH to GSSG in cell-free systems and also depleted cellular GSH levels, experiments were carried out to determine whether plumbagin induces protein glutathionylation in cells. Lymphocytes were treated with plumbagin and cell lysates were incubated with BIAM. Using equal amount of protein in each group, non-glutathionylated proteins conjugated with BIAM were pulled down using streptavidin agarose beads and were resolved on SDS–PAGE. Lymphocytes treated with plumbagin showed marked decrease in free thiol groups on proteins (Fig. 7B). To assess the effect of plumbagin on protein glutathionylation, cells were incubated with cell permeable biotinylated-glutathione-ethyl ester (Bio-GEE). Bio-GEE loaded cells were treated with vehicle or plumbagin and cell lysates were prepared. Equal amount of protein in each group was used for precipitation of glutathionylated proteins using streptavidin agarose beads and resolved on SDS–PAGE. Coomassie staining showed a dose-dependent increase in specific glutathionylated proteins in plumbagin-treated samples as compared to that in untreated control cells (Fig. 7C).
Fig. 7.
A: Plumbagin enters lymphocytes. Lymphocytes were incubated with plumbagin for 24 h and fixed with paraformaldehyde. The cells were stained with Hoechst and observed under confocal microscope. B: Enrichment of iodoacetamide reactive sulfhydryl groups on proteins. Iodoacetamide (IAM) binds selectively to protein thiolate anions. The lysates of lymphocytes incubated with 5 µM plumbagin for 4 h were treated with BIAM (0.2 mM) for 30min at 37°C. Streptavidin agarose beads (20 µl/mg of protein) were added to the lysates and incubated for 1 h at 4°C. The agarose beads were separated by centrifugation, washed four times with RIPA buffer, and boiled in SDS sample buffer. Proteins in the eluent were resolved by SDS–PAGE and detected by Coomassie blue staining. C: Purification of putative S-glutathionylated proteins. Protein extract was obtained as described under the Materials and Methods Section from Bio-GEE loaded lymphocytes incubated with plumbagin (0 or 5 or 10 µM, 4 h). Biotin-labeled proteins were extracted from total protein using streptavidin agarose beads. The beads were washed with RIPA buffer and boiled in SDS sample buffer. The eluted proteins were resolved by SDS–PAGE and were detected by Coomassie blue staining of the gel. Two such independent experiments were carried out.
DISCUSSION
Cellular redox status is determined by balance between levels of pro-oxidants (ROS and RNS) and antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) and rate of cellular respiration/metabolism. The ratio of GSH to GSSG is also considered to be intrinsic determinant of the cellular redox status. Cellular redox has been shown to play a critical role in lymphocyte activation, survival, and proliferation [Flescher et al., 1994, 1998; Lahdenpohja et al., 1998; Hildeman et al., 2003b]. Exposure of T cells to ROS scavenging agents like MnTBAP and chlorophyllin has been shown to increase cellular survival via upregulation of antiapoptotic gene expression [Hildeman et al., 2003a; Sharma et al., 2007a,b]. On the contrary, exposure of T cells to oxidizing agents like H2O2, xanthine/xanthine oxidase has been shown to suppress T cell activation, proliferation, and cytokine production via suppression of NF-κB [Pahlavani and Harris, 1998]. The anti-inflammatory and immunosuppressive effects of plumbagin are known to be mediated via inhibition of NF-κB activation in lymphocytes [Checker et al., 2009; Sharma et al., 2009].
In the present report we found that plumbagin disrupted cellular redox homeostasis (Fig. 1A,B). The increased levels of ROS in plumbagin-treated cells could be due to (i) an increase in the activities of NADPH oxidase or (ii) xanthine oxidase or (iii) influx from mitochondrial electron transport chain or (iv) modulation of free thiol levels. Since plumbagin-induced increase in intracellular ROS was not affected by specific inhibitors of NADPH oxidase, xanthine oxidase, and mitochondrial complex I (Fig. 1C), the role of first three of the above possibilities toward plumbagin-induced disruption of cellular redox was not apparent. Therefore, it may be concluded that plumbagin exerted its effects by depleting GSH in lymphocytes. This was supported by decrease in MCB–GSH adduct fluorescence in plumbagin-treated cells (Fig. 1B). DEM, a well-known depletor of GSH [Plummer et al., 1981], accentuated ROS levels in plumbagin-treated lymphocytes compared to that by plumbagin alone (Fig. 1A). Further, cell permeable thiol antioxidant NAC significantly lowered ROS levels in plumbagin-treated cells (Fig. 1C). These data clearly suggested that depletion of intracellular thiols by plumbagin could be responsible for increased ROS levels in lymphocytes [Henry and Wallace, 1996]. Although plumbagin was previously reported to induce ROS generation in tumor cells, the mechanism of this phenomenon was not known. In this report, for the first time we show GSH depletion as a source of ROS generation in normal lymphocytes following plumbagin treatment.
In normal cells, ROS homeostasis is maintained by antioxidant enzymes like superoxide dismutase, catalase, and glutathione peroxidase. The changes in ROS levels by exogenous agents have also been shown to affect the expression/activity of these enzymes. It was observed that the activity of catalase was significantly higher in plumbagin-treated cells (Fig. 1E). This may be due to cellular response to increased levels of H2O2 following treatment with plumbagin. However, the activity of SOD enzyme was not altered (Fig. 1F) indicating that plumbagin may preferentially induce hydrogen peroxide and not superoxide anions in lymphocytes. The DCF-DA dye used in our experiments to detect intracellular ROS levels is known to be relatively specific for hydrogen peroxide which confirms the production of this pro-oxidant. Earlier reports by several investigators have shown that agents that increase oxidative stress are capable of activating redox-dependent transcription factor NF-E2-related factor-2 (Nrf-2) [Kang et al., 2005]. In agreement with this, a recent report showed that plumbagin is able to increase nuclear localization and transcriptional activity of Nrf2 in human neuroblastoma cells [Son et al., 2010].
In order to determine whether increased ROS levels or decreased thiol content or both were responsible for immunosuppressive action of plumbagin, effect of thiol and non-thiol antioxidants was investigated on suppression of mitogen-induced T-cell activation by plumbagin. Addition of DTT or NAC would increase the intracellular thiols whereas GSH being cell impermeable may inhibit plumbagin’s action by extracellular interaction with it. It was observed that suppression of mitogen-induced cytokines and T-cell proliferation by plumbagin were ameliorated by thiol-containing antioxidants GSH, DTT, and NAC, but not by non-thiol antioxidants suggesting that modulation of cellular thiol levels was very critical for immunosuppression by plumbagin (Figs. 2 and 3).
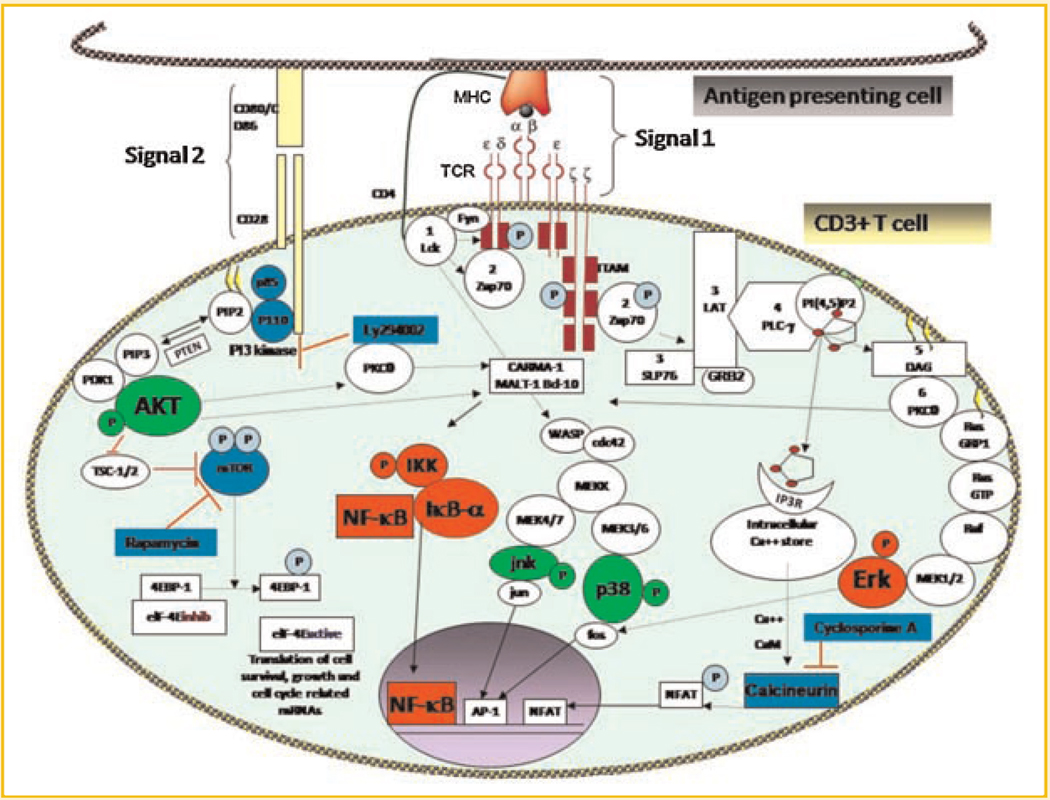
Changes in cellular thiol levels have been shown to affect multiple signaling pathways in different cell lines. Many of the proteins and transcription factors like P65, IKK, thioredoxin reductase, PI3kinase/AKT, and Keap1 have been shown to be highly sensitive to changes in cellular redox status [Zhang and Hannink, 2003; Smart et al., 2004; Sandur et al., 2006; Gloire and Piette, 2009; Harikumar et al., 2009; Naughton et al., 2009]. Our present results show that disruption of cellular redox by plumbagin affected specific signaling events following TCR ligation with mitogen (including NF-κB, ERK, BCL-2, BCL-xL, and cyclin A) which are involved in cellular survival and proliferation (Fig. 5A,B). However, plumbagin did not inhibit signaling events required for activation of transcription factor AP-1 (P38MAPkinase and JNK) and signals emanating from costimulatory molecule (AKT) in activated T cells (Fig. 5C). These results were in agreement with our earlier report showing that plumbagin did not suppress AP-1 in tumor cells [Sandur et al., 2006]. However, the mechanism of suppression of certain signaling events by plumbagin without affecting other activation signals in T cells is not completely understood. These novel targets identified for immunosuppressive action of plumbagin differ from the targets of pharmacological drugs like rapamycin, FK506, and cyclosporine which inhibit mTOR and calcineurin, respectively. Scheme 1 highlights the signaling events that are unaffected by plumbagin (shown in green) and blocked by plumbagin (shown in red), or rapamycin, cyclosporine, and the PI3kinase inhibitor, Ly294002 (shown in blue).
Scheme. 1.
T-cell receptor signaling and its inhibition by plumbagin and other pharmacological inhibitors. The signaling molecules highlighted in red were specifically inhibited by plumbagin and the molecules highlighted in green were not affected by plumbagin in activated T cells. Targets of pharmacological inhibitors (rapamycin, Ly294002, and cyclosporine) are highlighted in blue.
Most quinones mediate their cellular effects through redox cycling [Henry and Wallace, 1996]. The modulation of thiol groups on proteins could be in terms of direct reaction with plumbagin or glutathionylation of proteins or formation of disulfide bridge between proteins. Our results for the first time demonstrate that plumbagin indeed formed an adduct with GSH and also converted GSH to GSSG in cell-free systems (Fig. 6B). Further, it was shown that plumbagin modulates thiol groups present on the proteins as evinced from decrease in iodoacetamide reactive thiol groups of the proteins (Fig. 7B). The results using Bio-GEE for the first time demonstrated formation of PSSG (protein–glutathione adduct) in plumbagin-treated cells (Fig. 7C). Earlier reports have shown that IKK and IκB-α, whose activation is suppressed by plumbagin in lymphocytes, is regulated by glutathionylation suggesting that NF-κB activation is regulated by redox modulation [Reynaert et al., 2006; Kil et al., 2008]. Such reactions have been shown to occur in cells exposed to oxidative stress [Sullivan et al., 2000].
Any possible therapeutic use of plumbagin for amelioration of inflammation or immunosuppression would require inhibition of effector functions in activated lymphocytes. Our earlier published results had shown that prior incubation of cells with plumbagin before stimulation with mitogen resulted in complete suppression of effector functions like cytokine production, proliferation, and GVHD [Checker et al., 2009; Raghu et al., 2009; Sharma et al., 2009]. Present results showed an efficient suppression of proinflammatory cytokine production in mitogen-activated lymphocytes by plumbagin even when it was added to the cells 1 h after stimulation (Fig. 4). The use of a phytochemicals with a favorable toxicity profile is particularly helpful in chronic inflammatory conditions such as autoimmune diseases where protracted use of traditional immunosuppressants such as corticosteroids and cytotoxic agents (methotrexate, azathioprine, cyclosporine, etc.) is associated with cumulative long-term toxicities. Thus, plumbagin may find potential clinical application as an anti-inflammatory or immunosuppressive agent for the treatment of disease conditions mediated by activated and memory lymphocytes, even when used after the triggering event has occurred.
Present report for the first time shows a ROS-independent mechanism of anti-inflammatory action of plumbagin. Modulation of cellular thiols played a more significant role than increased ROS levels in biological actions of plumbagin. For the first time, evidence for a role for glutathionylation of cellular proteins as a mechanism of anti-proliferative action of plumbagin is provided. Further, mechanistic basis for potential therapeutic application of plumbagin as an immunosuppressive or anti-inflammatory drug is highlighted.
ACKNOWLEDGMENTS
The authors wish to acknowledge Mr. Manu Tiwari, IIT-Bombay for help in flowcytometry. We thank Dr. Suprasanna, Mr. Ashish Srivastava, and Mr. Radha Krishna, FTD, BARC for facilitating HPLC analysis and Dr. Mayuri N. Gandhi, SAIF, IIT-Bombay, for help in mass spectrometry. We also thank Mr. Deepak Kathole, Mr. Narendra Sidnalkar, and Mr. Kashinath Munankar for their technical assistance.
Grant sponsor: Department of Atomic Energy, India.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Agrawal A, Kaushal P, Agrawal S, Gollapudi S, Gupta S. Thimerosal induces TH2 responses via influencing cytokine secretion by human dendritic cells. J Leukoc Biol. 2007;81:474–482. doi: 10.1189/jlb.0706467. [DOI] [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Bouzyk E, Iwanenko T, Jarocewicz N, Kruszewski M, Sochanowicz B, Szumiel I. Antioxidant defense system in differentially hydrogen peroxide sensitive L5178Y sublines. Free Radic Biol Med. 1997;22:697–704. doi: 10.1016/s0891-5849(96)00388-7. [DOI] [PubMed] [Google Scholar]
- Checker R, Chatterjee S, Sharma D, Gupta S, Variyar P, Sharma A, Poduval TB. Immunomodulatory and radioprotective effects of lignans derived from fresh nutmeg mace (Myristica fragrans) in mammalian splenocytes. Int Immunopharmacol. 2008;8:661–669. doi: 10.1016/j.intimp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Checker R, Sharma D, Sandur SK, Khanam S, Poduval TB. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int Immunopharmacol. 2009;9:949–958. doi: 10.1016/j.intimp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- D’Astafort D. Chemische Versuche mit dem Plumbagin, der scharfen Materie aus der Wurzel von Plumbago europaea. Archiv der Pharmazie. 1829;29:245–250. [Google Scholar]
- Deas O, Dumont C, Mollereau B, Metivier D, Pasquier C, Bernard-Pomier G, Hirsch F, Charpentier B, Senik A. Thiol-mediated inhibition of FAS and CD2 apoptotic signaling in activated human peripheral T cells. Int Immunol. 1997;9:117–125. doi: 10.1093/intimm/9.1.117. [DOI] [PubMed] [Google Scholar]
- Flescher E, Ledbetter JA, Schieven GL, Vela-Roch N, Fossum D, Dang H, Ogawa N, Talal N. Longitudinal exposure of human T lymphocytes to weak oxidative stress suppresses transmembrane and nuclear signal transduction. J Immunol. 1994;153:4880–4889. [PubMed] [Google Scholar]
- Flescher E, Tripoli H, Salnikow K, Burns FJ. Oxidative stress suppresses transcription factor activities in stimulated lymphocytes. Clin Exp Immunol. 1998;112:242–247. doi: 10.1046/j.1365-2249.1998.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Piette J. Redox regulation of nuclear post-translational modifications during NF-κB activation. Antioxid Redox Signal. 2009;11:2209–2222. doi: 10.1089/ars.2009.2463. [DOI] [PubMed] [Google Scholar]
- Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, Aggarwal BB. Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra B, Sarkar R, Bhattacharyya S, Ghosh PK, Chel G, Dinda B. Synthesis of plumbagin derivatives and their inhibitory activities against Ehrlich ascites carcinoma in vivo and Leishmania donovani Promastigotes in vitro. Phytother Res. 2002;16:133–137. doi: 10.1002/ptr.867. [DOI] [PubMed] [Google Scholar]
- Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- Henry TR, Wallace KB. Differential mechanisms of cell killing by redox cycling and arylating quinones. Arch Toxicol. 1996;70:482–489. doi: 10.1007/s002040050302. [DOI] [PubMed] [Google Scholar]
- Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci USA. 2003a;100:15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Mitchell T, Kappler J, Marrack P. T cell apoptosis and reactive oxygen species. J Clin Invest. 2003b;111:575–581. doi: 10.1172/JCI18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem Res Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Kim SG. Molecular mechanism of Nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- Kawiak A, Piosik J, Stasilojc G, Gwizdek-Wisniewska A, Marczak L, Stobiecki M, Bigda J, Lojkowska E. Induction of apoptosis by plumbagin through reactive oxygen species-mediated inhibition of topoisomerase II. Toxicol Appl Pharmacol. 2007;223:267–276. doi: 10.1016/j.taap.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Kil IS, Kim SY, Park JW. Glutathionylation regulates IkappaB. Biochem Biophys Res Commun. 2008;373:169–173. doi: 10.1016/j.bbrc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Klemke M, Samstag Y. Molecular mechanisms mediating oxidative stress-induced T-cell suppression in cancer. Adv Enzyme Regul. 2009;49:107–112. doi: 10.1016/j.advenzreg.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy M, Purushothaman KK. Plumbagin: A study of its anticancer, antibacterial & antifungal properties. Indian J Exp Biol. 1980;18:876–877. [PubMed] [Google Scholar]
- Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-kappa B-dependent transcription in T lymphocytes. J Immunol. 1998;160:1354–1358. [PubMed] [Google Scholar]
- Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, Lenkei R, Masucci G, Pettersson S, Kiessling R. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J Immunol. 2001;167:2595–2601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- Naresh RA, Udupa N, Devi PU. Niosomal plumbagin with reduced toxicity and improved anticancer activity in BALB/C mice. J Pharm Pharmacol. 1996;48:1128–1132. doi: 10.1111/j.2042-7158.1996.tb03907.x. [DOI] [PubMed] [Google Scholar]
- Naughton R, Quiney C, Turner SD, Cotter TG. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- Padhye SB, Kulkarni BA. Studies on the root constituents of Plumbago zeylanica. J Univ Poona Sci Technol. 1973;44:27. [Google Scholar]
- Pahlavani MA, Harris MD. Effect of in vitro generation of oxygen free radicals on T cell function in young and old rats. Free Radic Biol Med. 1998;25:903–913. doi: 10.1016/s0891-5849(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Plummer JL, Smith BR, Sies H, Bend JR. Chemical depletion of glutathione in vivo. Methods Enzymol. 1981;77:50–59. doi: 10.1016/s0076-6879(81)77010-1. [DOI] [PubMed] [Google Scholar]
- Raghu R, Sharma D, Ramakrishnan R, Khanam S, Chintalwar GJ, Sainis KB. Molecular events in the activation of B cells and macrophages by a non-microbial TLR4 agonist, G1–4A from Tinospora cordifolia. Immunol Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kumar SS, Raghu R, Khanam S, Sainis KB. Differential modulation of mitogen driven proliferation and homeostasis driven proliferation of T cells by rapamycin, Ly294002 and chlorophyllin. Mol Immuno. 2007a;l44:2831–2840. doi: 10.1016/j.molimm.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kumar SS, Sainis KB. Antiapoptotic and immunomodulatory effects of chlorophyllin. Mol Immunol. 2007b;44:347–359. doi: 10.1016/j.molimm.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kumar SS, Checker R, Raghu R, Khanam S, Krishnan S, Sainis KB. Spatial distribution, kinetics, signaling and cytokine production during homeostasis driven proliferation of CD4+ T cells. Mol Immunol. 2009;46:2403–2412. doi: 10.1016/j.molimm.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UV, Udupa N. Reduced toxicity and enhanced antitumor efficacy of betacyclodextrin plumbagin inclusion complex in mice bearing Ehrlich ascites carcinoma. Indian J Physiol Pharmacol. 1997;41:171–175. [PubMed] [Google Scholar]
- Smart DK, Ortiz KL, Mattson D, Bradbury CM, Bisht KS, Sieck LK, Brechbiel MW, Gius D. Thioredoxin reductase as a potential molecular target for anticancer agents that induce oxidative stress. Cancer Res. 2004;64:6716–6724. doi: 10.1158/0008-5472.CAN-03-3990. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu Q-S, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/RAE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004;40:201–211. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- Sullivan DM, Wehr NB, Fergusson MM, Levine RL, Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: Abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–3286. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004;9:219–227. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. Thiol oxidation in signaling and response to stress: Detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]